Introduction
Osteosarcoma (OS) is the most common primary
malignant neoplasm in adolescents. The estimated worldwide
incidence rate is 4 million per year, with a peak incidence at the
age of 15–19 years (1). By
combining surgery with multiagent chemotherapy, the 5-year
cumulative survival rate of primary OS has significantly improved
to 60–90% in the past three decades (2–6).
Unfortunately, as a result of approximately 80% of patients
eventually developing metastatic disease following surgical
treatment (7), pulmonary metastasis
in OS patients is a major cause of fatal outcome (8). Therefore, it is essential to determine
the mechanisms contributing to invasion and metastasis in OS.
Fatty acid metabolic pathways play an important role
in carcinogenesis (9). Fatty acid
synthase (FASN) is an enzyme crucial to endogenous lipogenesis in
mammals and is responsible for catalyzing the synthesis of
long-chain fatty acids. FASN is critical in sustaining the
biological features of cancer cells (10). FASN is expressed at high levels in a
variety of human tumors (11–14),
but exists at low levels in normal tissues. Various studies have
reported that the inhibition of FASN expression suppresses cancer
cell proliferation in vitro and in vivo (15–20).
Thus, FASN is considered a novel promising target for anticancer
therapy. Recent studies (21,22)
have revealed that FASN may also contribute to cancer cell
metastasis.
microRNAs (miRNAs) are endogenous small RNAs
averaging 20 to 24 nucleotides, transcribed from non-protein-coding
genes or introns, which mediate translational suppression or
cleavage of their target mRNAs by binding to complementary sites in
their 3′-UTR (23–25). A large number of miRNAs are located
inside or close to chromosomal fragile sites that are frequently
lost or amplified in cancers (26).
miRNAs have been characterized as oncogenes, tumor suppressors or
as components of regulatory pathways critical for tumorigenesis,
therefore miRNAs play an important role in tumorigenesis and
metastasis.
Recently, miR-195 has been reported to be
deregulated in certain types of cancer, including upregulation in
chronic lymphocytic leukemia and breast cancer but downregulation
in hepatocellular carcinoma, adrenocortical carcinoma, and squamous
cell carcinoma of the tongue (27–31).
miR-195 was also suggested to be correlated with lymph node
metastasis and poor prognosis in colorectal cancer (27). However, the role of miR-195 in OS
migration remains elusive. In our previous study, the prediction
was performed by microRNA.org and TargetScan.human6.0.
The results revealed that FASN may be a target of miR-195.
Therefore, we speculate that miR-195 may suppress OS invasion and
metastasis by targeting FASN.
In the present study, we found that miR-195
functions as a tumor suppressor in OS, upregulates miR-195
expression in the OS cell line U2OS and reduces cell migration and
invasion in vitro. Additionally, it was revealed that FASN
may be directly targeted by miR-195. Together, our data indicate
that miR-195 plays an important role in regulating OS cell
metastasis by targeting FASN.
Materials and methods
Cell culture and transfection
The human OS cell line U2OS (Shanghai Cell Bank,
Chinese Academy of Sciences) was cultured in Dulbecco’s modified
Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and
incubated at 37˚C in 5% CO2. U2OS cells were seeded in
six-well plates at 30% confluence one day prior to transfection.
Transfection with miR-195 or negative miRNA was performed using
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA).
Transfection complexes were prepared according to the
manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA from cells treated with miR-195 or the
negative control miRNA was isolated using a TRIzol reagent
(Tiangen, Beijing, China) and reverse transcribed using a reverse
transcription kit (Tiangen) according to the manufacturer’s
instructions. Reactions were performed and analyzed using an ABI
7300 system (Applied Biosystems, Carlsbad, CA, USA). β-actin was
used as the internal control to quantify initial cellular
transcripts. Details of the primers and probes used in this study
are summarized in Table I. All
qRT-PCR were performed six times according to the manufacturer’s
instructions. The relative expression level of FASN was normalized
to that of β-actin by the 2−ΔΔCt cycle
threshold method. The ΔCt data were collected automatically. The
average ΔCt of each group was calculated by the following formula:
ΔCt = average miR-195 Ct - average of β-actin Ct. ΔΔCt was
calculated by ΔΔCt = ΔCt of miR-195 group - ΔCt of the negative
control group. The fold-change in FASN expression level was
calculated using 2−ΔΔCt.
 | Table IPrimers and probes. |
Table I
Primers and probes.
| Primers and
probes | Sequences
5′-3′ |
|---|
| FASN sense |
5′-AAGCAGGCACACACGATGG-3′ |
| FASN antisense |
5′-TCGGAGTGAATCTGGGTTGATG-3′ |
| FASN probe |
5′-CTGCGGCTGCTGCTGGAAGTCACC-3′ |
| β-actin sense |
5′-TGCCCATCTACGAGGGGTATG-3′ |
| β-actin
antisense |
5′-CTCCTTAATGTCACGCACGATTTC-3′ |
| β-actin probe |
5′-CCTGCGTCTGGACCTGGCTGGC-3′ |
Luciferase activity assay
Primers were designed in accordance with the Genbank
query FASN gene mRNA (NM_004104.4) sequence. A fragment of the
3′-UTR of FASN was amplified from U2OS cells by PCR using the
forward primer 5′-CCCCTCGAGCCTGCCACCGGAGGTCACT-3′ and the reverse
primer 5′-CGGGCGGCCGCGTGGGAG GCTGAGAGCAGCA-3′. Following digestion
of the PCR product by XhoI and NotI, the FASN 3′-UTR
was cloned into pSiCHECK2 (Promega, Madison, WI, USA) at the
XhoI and NotI sites. All PCR products were verified
by DNA sequencing. U2OS cells were cotransfected with the pSiCHECK2
vectors containing the 3′-UTR variants and miR-195 or the negative
control miRNA. Luciferase activity was measured 36 h after
transfection. The firefly luciferase activity was then normalized
to the Renilla luciferase activity.
Transwell invasion assay in vitro
Invasion assays were performed in triplicate using
Transwell invasion chambers (Costar 3422, Corning Inc., NY, USA)
coated with Matrigel (50 μl per filter) (BD, USA) as described in
the manufacturer’s instructions. U2OS cells were transfected with
either miR-195 or the negative control oligonucleotide, cultured
for 48 h and then transferred to the top of the Matrigel-coated
invasion chambers in 1% fetal calf serum DMEM/F12 (2x104
cells/well). DMEM/F12 containing 10% fetal calf serum was added to
the lower chambers. Following incubation for 24 h, cells that
remained on the top of the filter were removed and cells that
migrated to the lower surface were fixed in 90% alcohol followed by
crystal violet staining. The values for invasion were obtained by
counting three fields per membrane and represented as the average
of six independent experiments made over multiple days.
Wound healing migration assay
When U2OS cells transfected with miR-195 or negative
control oligonucleotides were grown to confluence, a scratch in the
cell monolayer was made with a micropipette tip. Following
incubation of the cells under standard conditions for 24 h, the
plates were washed twice with fresh medium and images were captured
at different times. The migration potential was estimated by
counting the cells that migrated from the wound edge. The cell
migration rate was obtained by counting three fields per area and
represented as the average of six independent experiments made over
multiple days.
Western blot analysis
U2OS cells in the exponential growth phase were
transfected with miR-195 for 48 h. Total proteins were isolated
from U2OS cells. Protein concentrations were measured using a Micro
BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins were
resolved by 10% SDS-PAGE gel, transferred to the nitrocellulose
membrane and blocked in 5% non-fat dry milk in Tris-buffered saline
pH 7.4, containing 0.05% Tween-20. They were subsequently blotted
with a rabbit polyclonal antibody against FASN (1:1000, Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) and goat anti-rabbit IgG
(1:3000, Santa Cruz), with β-actin used as a loading control.
Signals were detected by secondary antibodies labeled with
horseradish peroxidase (HRP). All western blot analyses were
performed six times.
Statistical analysis
Data were expressed as the means ± SD of at least
three experiments. A one-way analysis of variance (ANOVA) test and
a least significant difference (LSD) test were used for statistical
analysis. A value of p<0.05 was considered to indicate a
statistically significant result. All analyses were performed using
SPSS version 13.0 (Statistical Package for the Social Sciences,
Chicago, IL, USA).
Results
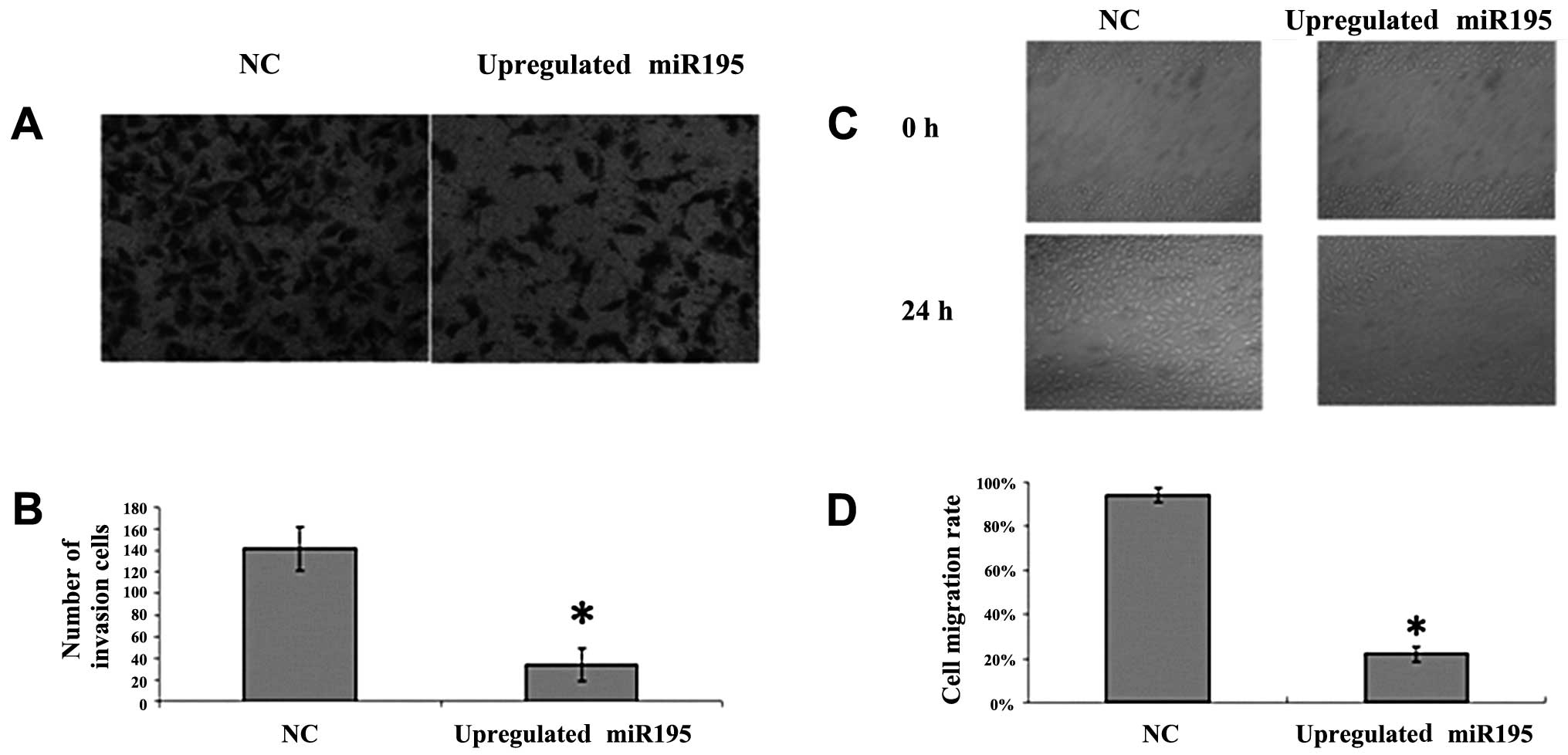
miR-195 inhibits cell invasion and
migration in vitro
To further investigate the effect of miR-195 on U2OS
cell invasion and migration, we employed the Transwell invasion
assay and wound healing assay. U2OS cells were transfected with
miR-195 or negative miRNA. We observed a significant inhibition of
invasion into Matrigel in miR-195-transfected cells (Fig. 1A and B; P<0.05). We also noted
that the number of migrated cells transfected with miR-195 was
significantly fewer than the number transfected with negative miRNA
(Fig. 2C and D; P<0.05). These
results suggest that the upregulation of miR-195 inhibits the
invasion and migration of U2OS cells.
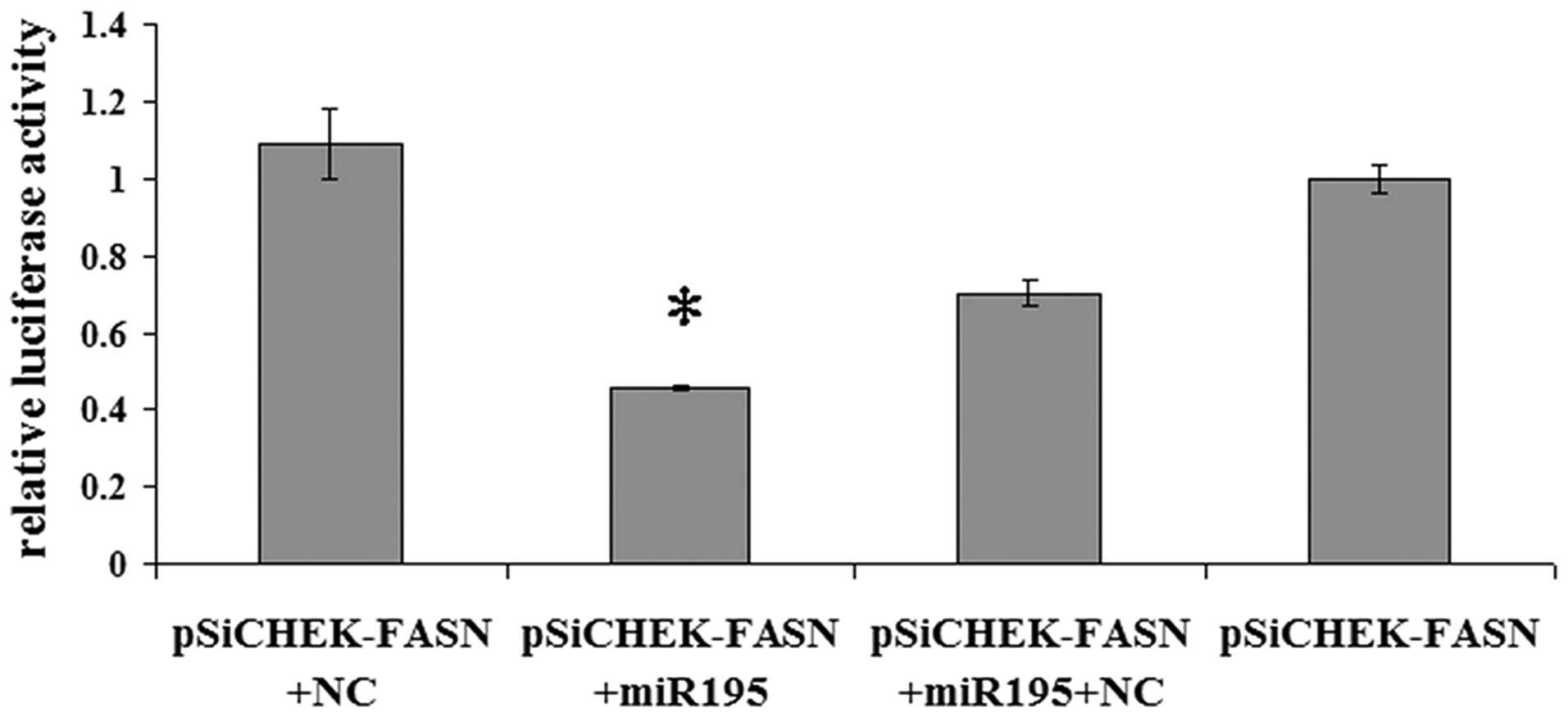
FASN is a direct target of miR-195
To validate whether miR-195 regulates FASN directly
through a putative binding site in U2OS cells, we cloned FASN
3′-UTR in the predicted miRNA binding site into the luciferase gene
(pSiCHECK2; Promega). Following cotransfection with the pSiCHECK2
vectors and miR-195 or the negative control miRNA, the upregulation
of miR-195 in U2OS cells transfected with miR-195 resulted in a
significant decrease in the luciferase activity of the wild-type
FASN 3′-UTR (Fig. 2; P<0.05).
The results indicate that FASN is a direct target of miR-195.
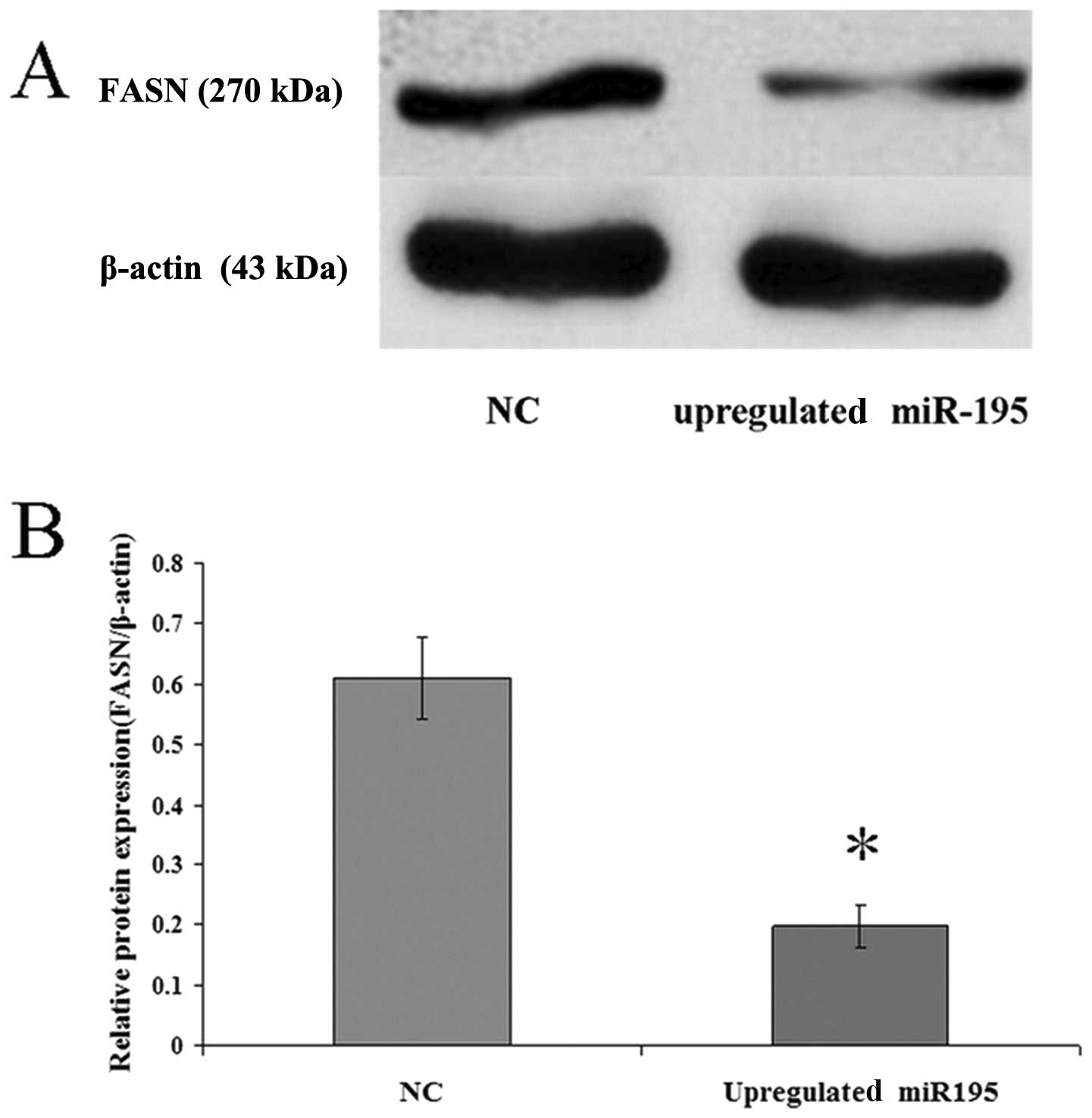
miR-195 negatively regulates FASN
expression in U2OS cells
To investigate the correlation between miR-195 and
FASN, miR-195 was upregulated in U2OS cells by treatment with
miR-195 for 36 h. The expression levels of FASN mRNA and protein
were measured by qRT-PCR and western blot analysis. The data
(2−ΔΔCt = 0.162±0.01179) revealed that the
FASN mRNA expression in cells transfected with the negative control
vector was six-fold higher than the expression in the cells
transfected with miR-195. Western blot analysis revealed that
upregulated miR-195 in cells leads to a corresponding decrease in
endogenous FASN protein (Fig. 3).
These data suggest that miR-195 may negatively regulate FASN
expression.
Discussion
miR-195, one of the miR-16/15/195/424/497 family
members, has been shown to play an important role in tumorigenesis.
Recently, miR-195 has been reported to be downregulated in
hepatocellular carcinoma, adrenocortical carcinoma and squamous
cell carcinoma of the tongue (29–34).
However, in chronic lymphocytic leukemia and breast cancer, miR-195
expression is reported to be upregulated (35–39).
Hence, the deregulation of miR-195 may be different in different
types of cancer, and the role of miR-195 in carcinogenesis and
progression cannot simply be concluded to be that of a tumor
suppressor or an oncogene. The roles of miR-195 deregulation in
cancer development remain to be further investigated. In the
present study, we found that miR-195 is downregulated in the human
OS cell line U2OS. The inhibition of migration and invasion due to
the upregulation of miR-195 in the human OS cell line U2OS was also
observed. This suggests that miR-195 functions as a tumor
suppressor and inhibits U2OS cell migration and invasion, which is
consistent with its roles in gastric cancer, colorectal cancer and
hepatocellular carcinoma (27,29,40).
Our previous study reported that the inhibition of
FASN causes a decrease in OS cell invasion and migration. The
prediction of the FASN gene was performed using Targetscan software
(microRNA.org and TargetScan.human6.0). This revealed
that miR-195 may be targeting FASN. In the present study, RT-PCR
and western blotting were performed to investigate the molecular
mechanisms that inhibit the migration and invasion by restoring
miR-195 in U2OS and to detect the expression levels of FASN mRNA
and protein in U2OS cells. The results revealed that FASN
expression was significantly inhibited in cells transfected with
miR-195 when compared with the control group (Fig. 3). This suggests that the restoration
of miR-195 expression may inhibit FASN expression in U2OS cells.
Furthermore, to identify whether miR-195 can genuinely regulate the
expression of FASN, the FASN 3′-UTR was cloned into the pSiCHECK2,
placing the 3′-UTR with the majority of potential miRNA binding
sites downstream of the coding sequence of luciferase (pMiR-Report;
Promega). U2OS cells were cotransfected with the pSiCHECK2 vector
containing the 3′-UTR and miR-195 or negative miRNA. We found that
the overexpression of miR-195 significantly reduced the luciferase
activity from the reporter construct containing the FASN 3′-UTR.
This indicates that FASN is a direct miR-195 target. However, as
there were hundreds of predicted targets of miR-195 revealed in the
TargetScan prediction and a single miRNA has been proven to target
multiple mRNAs in order to regulate gene expression (41), it is probable that other targets of
miR-195 may also participate in OS migration and invasion and
miR-195 may also target different molecules in different types of
cancer. Additionally, the tumor microenvironment may influence
tumor progression, invasion and migration. Therefore, further
studies are needed to identify the entire role of miR-195 in OS
metastasis.
Our present study indicated that the expression of
the oncogene FASN is negatively regulated by miR-195 through a
special binding site in the FASN 3′-UTR. Moreover, miR-195 inhibits
cell invasion and migration in U2OS cells. These results suggest
that miR-195 may serve as a target in the discovery of effective
therapies for OS.
References
|
1.
|
L MirabelloRJ TroisiSA SavageOsteosarcoma
incidence and survival rates from 1973 to 2004: data from the
Surveillance, Epidemiology, and End Results
ProgramCancer11515311543200910.1002/cncr.2412119197972
|
|
2.
|
VO LewisWhat’s new in musculoskeletal
oncologyJ Bone Joint Surg Am91154615562009
|
|
3.
|
PA MeyersCL SchwartzM KrailoOsteosarcoma:
a randomized, prospective trial of the addition of ifosfamide
and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose
methotrexateJ Clin
Oncol2320042011200510.1200/JCO.2005.06.03115774791
|
|
4.
|
Y ChoGH JungSH ChungJY KimY ChoiJD
KimLong-term survivals of stage IIb osteosarcoma: a 20-year
experience in a single institutionClin Orthop
Surg34854201121369478
|
|
5.
|
H TsuchiyaK TomitaY MoriCaffeine-assisted
chemotherapy and minimized tumor excision for nonmetastatic
osteosarcomaAnticancer Res1865766619989584049
|
|
6.
|
T BollingP SchullerB
DistelmaierPerioperative high-dose rate brachytherapy using a bendy
applicator (flab): treatment results of 74 patientsAnticancer
Res2838853890200819192645
|
|
7.
|
N MarinaM GebhardtL TeotR GorlickBiology
and therapeutic advances for pediatric
osteosarcomaOncologist9422441200410.1634/theoncologist.9-4-42215266096
|
|
8.
|
T WadaK IsuN TakedaM UsuiS IshiiS
YamawakiA preliminary report of neoadjuvant chemotherapy NSH-7
study in osteosarcoma: preoperative salvage chemotherapy based on
clinical tumor response and the use of granulocyte
colony-stimulating factorOncology53221227199610.1159/000227564
|
|
9.
|
CS YehJY WangTL ChengCH JuanCH WuSR
LinFatty acid metabolism pathway play an important role in
carcinogenesis of human colorectal cancers by
Microarray-Bioinformatics analysisCancer
Lett233297308200610.1016/j.canlet.2005.03.05015885896
|
|
10.
|
D HessRA IgalGenistein downregulates de
novo lipid synthesis and impairs cell proliferation in human lung
cancer cellsExp Biol Med
(Maywood)236707713201110.1258/ebm.2011.01026521565896
|
|
11.
|
PL AloM AminiF PiroImmunohistochemical
expression and prognostic significance of fatty acid synthase in
pancreatic carcinomaAnticancer Res2725232527200717695548
|
|
12.
|
K WalterSM HongS NyhanSerum fatty acid
synthase as a marker of pancreatic neoplasiaCancer Epidemiol
Biomarkers
Prev1823802385200910.1158/1055-9965.EPI-09-014419723916
|
|
13.
|
Y OkawaT HideshimaH IkedaFatty acid
synthase is a novel therapeutic target in multiple myelomaBr J
Haematol141659671200810.1111/j.1365-2141.2008.07114.x
|
|
14.
|
T MigitaS RuizA FornariFatty acid
synthase: a metabolic enzyme and candidate oncogene in prostate
cancerJ Natl Cancer
Inst101519532200910.1093/jnci/djp03019318631
|
|
15.
|
H OritaJ CoulterE TullyFP KuhajdaE
GabrielsonInhibiting fatty acid synthase for chemoprevention of
chemically induced lung tumorsClin Cancer
Res1424582464200810.1158/1078-0432.CCR-07-417718413838
|
|
16.
|
DT ColemanR BigelowJA CardelliInhibition
of fatty acid synthase by luteolin post-transcriptionally
down-regulates c-Met expression independent of
proteosomal/lysosomal degradationMol Cancer
Ther8214224200910.1158/1535-7163.MCT-08-072219139131
|
|
17.
|
GE SaatiMC ArcherInhibition of fatty acid
synthase and Sp1 expression by 3,3′-diindolylmethane in human
breast cancer cellsNutr Cancer637907942011
|
|
18.
|
M NotarnicolaS PisantiV TutinoEffects of
olive oil polyphenols on fatty acid synthase gene expression and
activity in human colorectal cancer cellsGenes
Nutr66369201110.1007/s12263-010-0177-721437031
|
|
19.
|
M NotarnicolaC MessaMG RefoloV TutinoA
MiccolisMG CarusoPolyunsaturated fatty acids reduce fatty acid
synthase and hydroxy-methyl-glutaryl CoA-reductase gene expression
and promote apoptosis in HepG2 cell lineLipids Health
Dis1010201110.1186/1476-511X-10-1021244676
|
|
20.
|
KG ZecchinFA RossatoHF RaposoInhibition of
fatty acid synthase in melanoma cells activates the intrinsic
pathway of apoptosisLab
Invest91232240201110.1038/labinvest.2010.15720805790
|
|
21.
|
MA CarvalhoKG ZecchinF SeguinFatty acid
synthase inhibition with Orlistat promotes apoptosis and reduces
cell growth and lymph node metastasis in a mouse melanoma modelInt
J Cancer12325572565200810.1002/ijc.2383518770866
|
|
22.
|
S MurataK YanagisawaK FukunagaFatty acid
synthase inhibitor cerulenin suppresses liver metastasis of colon
cancer in miceCancer
Sci10118611865201010.1111/j.1349-7006.2010.01596.x20491775
|
|
23.
|
VN KimJ HanMC SiomiBiogenesis of small
RNAs in animalsNat Rev Mol Cell
Biol10126139200910.1038/nrm263219165215
|
|
24.
|
DP BartelMicroRNAs: target recognition and
regulatory
functionsCell136215233200910.1016/j.cell.2009.01.00219167326
|
|
25.
|
MA Valencia-SanchezJ LiuGJ HannonR
ParkerControl of translation and mRNA degradation by miRNAs and
siRNAsGenes Dev20515524200610.1101/gad.139980616510870
|
|
26.
|
GA CalinC SevignaniCD DumitruHuman
microRNA genes are frequently located at fragile sites and genomic
regions involved in cancersProc Natl Acad Sci
USA10129993004200410.1073/pnas.030732310114973191
|
|
27.
|
X WangJ WangH MaJ ZhangX
ZhouDownregulation of miR-195 correlates with lymph node metastasis
and poor prognosis in colorectal cancerMed Oncol201121390519
|
|
28.
|
X BaiD MaA LiuRheb activates mTOR by
antagonizing its endogenous inhibitor,
FKBP38Science318977980200710.1126/science.114737917991864
|
|
29.
|
T XuY ZhuY XiongYY GeJP YunSM
ZhuangMicroRNA-195 suppresses tumorigenicity and regulates G1/S
transition of human hepatocellular carcinoma
cellsHepatology50113121200910.1002/hep.2291919441017
|
|
30.
|
PS SoonLJ TaconAJ GillmiR-195 and
miR-483-5p identified as predictors of poor prognosis in
adrenocortical cancerClin Cancer
Res1576847692200910.1158/1078-0432.CCR-09-158719996210
|
|
31.
|
TS WongXB LiuBY WongRW NgAP YuenWI
WeiMature miR-184 as potential oncogenic microRNA of squamous cell
carcinoma of tongueClin Cancer
Res1425882592200810.1158/1078-0432.CCR-07-066618451220
|
|
32.
|
L LiuL ChenY XuR LiX DumicroRNA-195
promotes apoptosis and suppresses tumorigenicity of human
colorectal cancer cellsBiochem Biophys Res
Commun400236240201010.1016/j.bbrc.2010.08.04620727858
|
|
33.
|
D LiY ZhaoC LiuAnalysis of MiR-195 and
MiR-497 expression, regulation and role in breast cancerClin Cancer
Res1717221730201110.1158/1078-0432.CCR-10-180021350001
|
|
34.
|
B BrennerMB HoshenO PurimMicroRNAs as a
potential prognostic factor in gastric cancerWorld J
Gastroenterol1739763985201110.3748/wjg.v17.i35.397622046085
|
|
35.
|
HM HeneghanN MillerR KellyJ NewellMJ
KerinSystemic miR-195 differentiates breast cancer from other
malignancies and is a potential biomarker for detecting noninvasive
and early stage
diseaseOncologist15673682201010.1634/theoncologist.2010-010320576643
|
|
36.
|
HM HeneghanN MillerAJ LoweryKJ SweeneyJ
NewellMJ KerinCirculating microRNAs as novel minimally invasive
biomarkers for breast cancerAnn
Surg251499505201010.1097/SLA.0b013e3181cc939f20134314
|
|
37.
|
BN HannafonP SebastianiA de las MorenasJ
LuCL RosenbergExpression of microRNA and their gene targets are
dysregulated in preinvasive breast cancerBreast Cancer
Res13R24201110.1186/bcr283921375733
|
|
38.
|
H ZhangSB SuQM ZhouYY LuDifferential
expression profiles of microRNAs between breast cancer cells and
mammary epithelial cellsAi Zheng28493499200919624877
|
|
39.
|
DL ZanetteF RivadaviaGA MolfettamiRNA
expression profiles in chronic lymphocytic and acute lymphocytic
leukemiaBraz J Med Biol
Res4014351440200710.1590/S0100-879X200700110000317934639
|
|
40.
|
WY WuXY XueZJ ChenPotentially predictive
microRNAs of gastric cancer with metastasis to lymph nodeWorld J
Gastroenterol1736453651201110.3748/wjg.v17.i31.364521987613
|
|
41.
|
M SelbachB SchwanhausserN ThierfelderZ
FangR KhaninN RajewskyWidespread changes in protein synthesis
induced by
microRNAsNature4555863200810.1038/nature0722818668040
|