Introduction
Lung cancer, one of the most common malignancies, is
a major cause of morbidity and mortality throughout the world.
Approximately 80% of diagnosed lung cancers are non-small cell lung
cancers (NSCLCs). In approximately 20–40% of NSCLC cases,
metastasis to the brain occurs (1–3).
Pemetrexed, a multi-targeted antifolate drug, has
been demonstrated in vitro to inhibit at least three
different enzymes involved in the human folate pathway including
thymidylate synthase (TS), dihydrofolate reductase (DHFR) and
glycinamide ribonucleotide formyl transferase (4,5). These
enzymes are involved in the synthesis of nucleotides, and
ultimately pemetrexed is capable of interfering with RNA and DNA
synthesis procedures (6). It was
revealed that pemetrexed had a slightly higher brain penetration
capability than methotrexate by the analysis of arterial blood and
frontal cortex microdialysis samples obtained simultaneously
(7,8). However, it remains unclear whether
penetrexed is effective in NSCLC patients with progressive brain
metastases. In the present study we report a successful case of
pemetrexed treatment. Patient consent was obtained from the patient
and the patient’s family.
Case report
A 46-year-old male patient presented with persistent
pain in the left side of his neck, accompanied by low fever. An
enlarged lymph node in the left side of the neck was subsequently
discovered, which cervical magnetic resonance imaging (MRI)
revealed to be a metastatic tumor. A mass (2.8x1.2 cm) was further
visualized by chest computed tomography (CT), and was found to be
localized in the posterior segment of the upper right lung.
Multiple enlarged lymph nodes in the mediastinum were also
detected. Multiple bone and brain metastases were detected by ECT
and PET-CT, respectively. The primary lung cancer was diagnosed by
histopathologic analysis, electronic bronchoscopy and lung biopsy
which revealed a poorly differentiated adenocarcinoma. Although the
patient had limited mobility in his neck, the rest of the
examinations were normal. Potential mutations on the epithelial
growth factor receptor (EGFR) and K-ras genes, obtained from the
left cervical lymph node biopsy, were analyzed by DNA sequencing.
It was revealed that exon 21 of the EGFR gene contained a
heterozygous T to G mutation at nucleotide 2573. However, no
mutations were found in the K-ras gene.
To treat the detected tumors, 900 mg of pemetrexed
disodium (500 mg/m2; Eli Lilly, Indianapolis, IN, USA)
was administered to the patient on day 1, and 40 mg of cisplatin
(25 mg/m2; QILU Pharma., Jinan, China) was administered
on days 1–3, with an interval of 3 weeks. Cervical radiotherapy
(total dose, 4,200 cGy) was initiated simultaneously. Cetuximab
(Merck, Whitehouse Station, NJ, USA) was injected during the second
cycle of chemotherapy. Cetuximab (400 mg/m2) was
administered on the first day, with weekly injections of 250
mg/m2 cetuximab thereafter. The treatment was terminated
after 3 months.
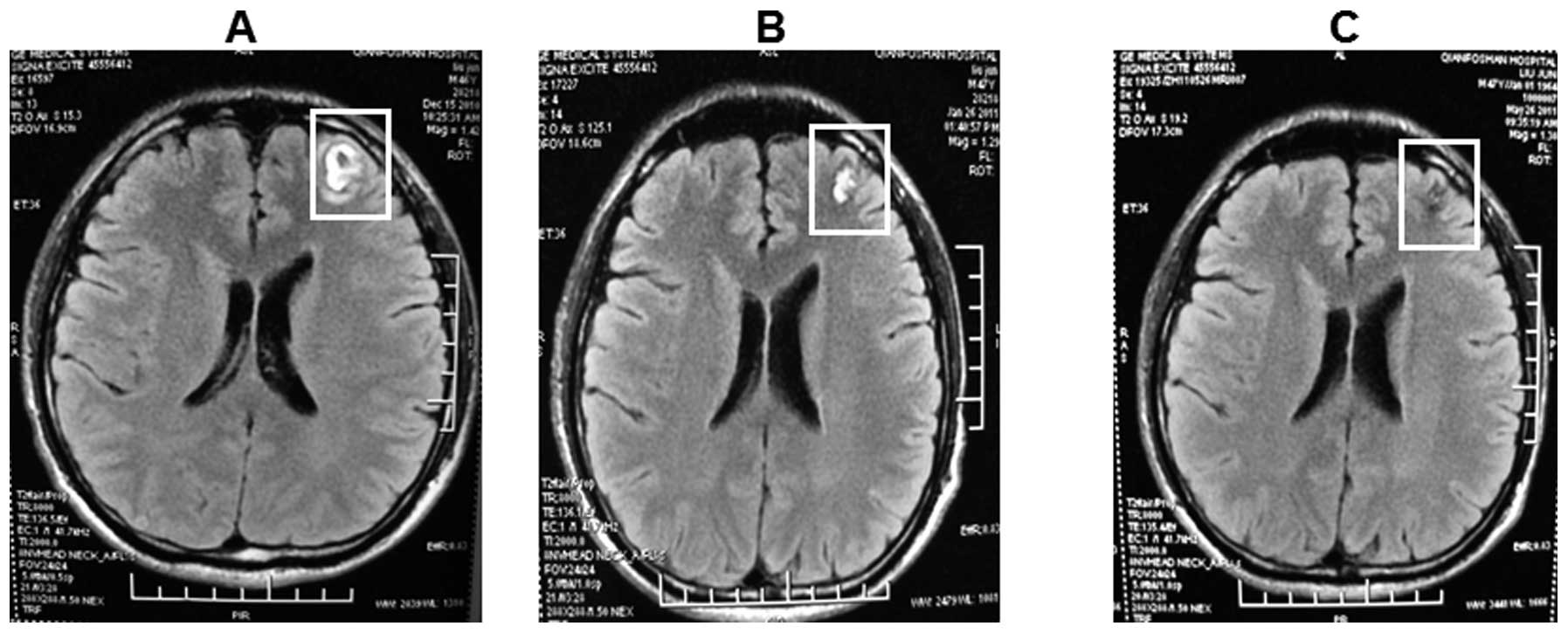
After two cycles of chemotherapy, the brain
metastases were reduced as revealed by brain MRI (Fig. 1). The lesion in the lung was reduced
as determined by chest CT scans (Fig.
2). The systemic chemotherapy combined with the cetuximab
injection was continued. After six cycles of treatment, a PET-CT
scan was performed to examine the therapeutic efficacy. There was
no abnormal FDG uptake in the brain or the lung. As a result, 900
mg of pemetrexed (500 mg/m2; Eli Lilly) was administered
again every 3 weeks. Following the treatments described above, the
tumor sizes were significantly decreased (Figs. 1 and 2).
Discussion
Lung cancer, one of the most common malignant
tumors, is a major cause of morbidity and mortality. Approximately
80% of patients with lung cancers are diagnosed with non-small cell
lung cancers (NSCLCs). Approximately 20–40% of NSCLC eventually
metastasizes to the brain (1–3).
Pemetrexed, an antifolate drug, is known to inhibit
at least three different proteins involved in the human folate
pathway including thymidylate synthase (TS), dihydrofolate
reductase (DHFR), and glycinamide ribonucleotide formyl transferase
(4,5). These enzymes are involved in the
synthesis of nucleotides, and ultimately pemetrexed is capable of
interfering with RNA and DNA synthesis (6). Pemetrexed, in combination with
cisplatin, has recently been used in the treatment of metastatic
NSCLC patients (9–11). NSCLC patients with the exon 19
deletion have a distinctive pattern of brain metastases which
usually present as multiple small metastases with low levels of
brain edema (12,13). Certain studies have indicated that
gefitinib and erlotinib penetrate into the central nervous system
and elicit responses in NSCLC patients with brain metastases
(13–15). It was revealed that pemetrexed had a
slightly higher brain penetration capability compared to that of
methotrexate, by the analysis of arterial blood and frontal cortex
microdialysis samples obtained simultaneously (7,8). Bearz
et al (16) investigated the
therapeutic effect of pemetrexed on reducing brain metastases and
revealed that PR (partial remission) was observed in 11 patients
(28.2%) and SD (stable disease) was observed in 21 patients
(53.8%). The total beneficiary rate was 82% (16). In our case, pemetrexed was proven to
be effective even following the failure of radiotherapy. Our study
suggests that pemetrexed is an effective therapy for patients with
NSCLC (adenocarcinoma) and progressive brain metastases.
References
|
1.
|
V AdamoT FranchinaB AdamoG ScandurraA
ScimoneBrain metastases in patients with non-small cell lung
cancer: focus on the role of chemotherapyAnn
Oncol1711731175200610.1093/annonc/mdj93016608991
|
|
2.
|
T KawabeJH PhiM YamamotoTreatment of brain
metastasis from lung cancerProg Neurol
Surg25148155201210.1159/00033118822236676
|
|
3.
|
P MulvennaR BartonP WilsonSurvival of
patients with non-small cell lung cancer and brain metastasesClin
Oncol (R Coll
Radiol)23375376201110.1016/j.clon.2011.01.50821392952
|
|
4.
|
C ShihVJ ChenLS GossettSB GatesWC
MacKellarLL HabeckLY231514, a pirrolo [2,3-d] pyrimidine-based
antifolate that inhibits multiple folate-requiring enzymesCancer
Res57111611231997
|
|
5.
|
F BarlesiR GervaisH LenaJ HureauxH BerardD
PaillotinPemetrexed and cisplatin as first-line chemotherapy for
advanced non-small-cell lung cancer (NSCLC) with asymptomatic
inoperable brain metastases: a multicenter phase II trial (GFPC
07-01)Ann Oncol2224662470201110.1093/annonc/mdr00321321089
|
|
6.
|
RM SchultzVJ ChenJR BewleyEF RobertsC
ShihJA DempseyBiological activity of the multitargeted antifolate,
MTA (LY231514), in human cell lines with different resistance
mechanisms to antifolate drugsSemin Oncol266873199910598558
|
|
7.
|
H DaiY ChenWF ElmquistDistribution of the
novel antifolate pemetrexed to the brainJ Pharmacol Exp
Ther315222229200510.1124/jpet.105.09004315987831
|
|
8.
|
W OrtuzarN HannaE PennellaG PengC LangerM
MonbergG ScagliottiBrain metastases as the primary site of relapse
in two randomized phase III pemetrexed trials in advanced
non-small-cell lung cancerClin Lung
Cancer132430201210.1016/j.cllc.2011.05.00721831719
|
|
9.
|
W SchuetteH TeschH BüttnerT KrauseV
SoldatenkovaC StoffregenSecond-line treatment of stage III/IV
non-small-cell lung cancer (NSCLC) with pemetrexed in routine
clinical practice: Evaluation of performance status and
health-related quality of lifeBMC
Cancer1214201210.1186/1471-2407-12-1422244076
|
|
10.
|
CG AzzoliS TeminT AliffS Baker JrJ
BrahmerDH JohnsonJL Laskin2011 Focused update of 2009 American
Society of Clinical Oncology clinical practice guideline update on
chemotherapy for stage IV non-small-cell lung cancerJ Clin
Oncol2938253831201110.1200/JCO.2010.34.277421900105
|
|
11.
|
D GalettaS PiscontiS CinieriGL PappagalloV
GebbiaN BorsellinoInduction pemetrexed and cisplatin followed by
maintenance pemetrexed versus carboplatin plus paclitaxel plus
bevacizumab followed by maintenance bevacizumab: a quality of
life-oriented randomized phase III study in patients with advanced
non-squamous non-small-cell lung cancer (ERACLE)Clin Lung
Cancer124024062011
|
|
12.
|
A SekineT KatoE HagiwaraT ShinoharaT
KomagataT IwasawaMetastatic brain tumors from non-small cell lung
cancer with EGFR mutations: Distinguishing influence of exon 19
deletion on radiographic featuresLung
Cancer776469201210.1016/j.lungcan.2011.12.01722335887
|
|
13.
|
M Jamal-HanjaniJ SpicerEpidermal growth
factor receptor tyrosine kinase inhibitors in the treatment of
epidermal growth factor receptor-mutant non-small cell lung cancer
metastatic to the brainClin Cancer
Res18938944201210.1158/1078-0432.CCR-11-252922167408
|
|
14.
|
GA PesceD KlingbielK RibiA ZouhairR von
MoosM SchlaeppiOutcome, quality of life and cognitive function of
patients with brain metastases from non-small cell lung cancer
treated with whole brain radiotherapy combined with gefitinib or
temozolomide. A randomised phase II trial of the Swiss Group for
Clinical Cancer Research (SAKK 70/03)Eur J Cancer483773842012
|
|
15.
|
S HeonBY YeapGJ BrittDevelopment of
central nervous system metastases in patients with advanced
non-small cell lung cancer and somatic EGFR mutations treated with
gefitinib or erlotinibClin Cancer
Res1658735882201010.1158/1078-0432.CCR-10-158821030498
|
|
16.
|
A BearzI GarassinoM TiseoActivity of
Pemetrexed on brain metastases from non-small cell lung cancerLung
Cancer68264268201010.1016/j.lungcan.2009.06.01819632738
|