Introduction
Myoepithelial carcinoma is a very rare disease that
comprises only 2% of all salivary gland carcinomas. In the majority
of cases, it occurs in the parotid gland; however, it occasionally
occurs in the minor salivary gland (1).
Myoepithelial carcinoma may present with diverse
types of tumor cells including eosinophilic, epithelioid,
polygonal, spindle, stellate, hyaline and clear cells. Among them,
lesions that display clear cell-type tumor cells focally or
predominantly are referred to as clear cell myoepithelial
carcinomas (CCMC) (2,3). To date, only 17 cases of CCMC have
been reported worldwide. Until the present study, no case has
involved the tongue base (2–7). We
present a case of CMCC that developed in the base of the tongue.
Written informed consent for publication was obtained from the
patient.
Case report
A 52-year-old female visited Chosun University
Hospital (Gwangju, Korea) complaining of the sensation of foreign
bodies in the pharynx that had developed several months previously.
The patient’s history and family history were unremarkable. Upon
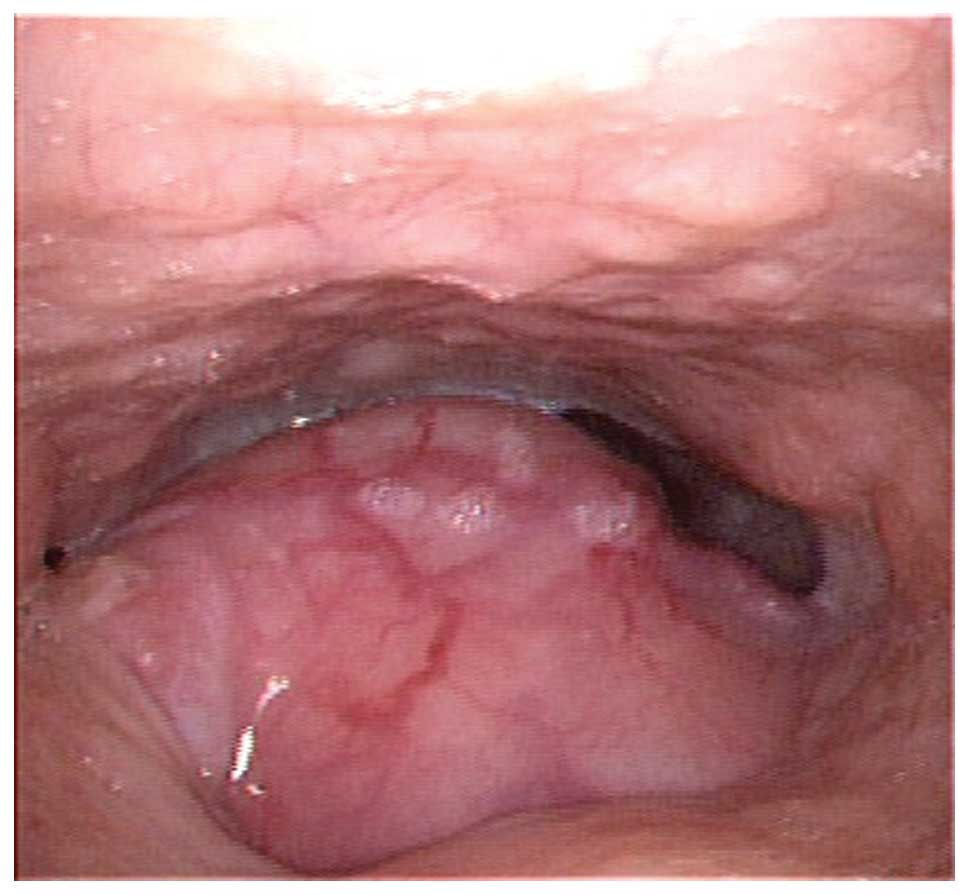
physical examination, a mass 3×3 cm in size, with an unclear
boundary and no associated ulcer, was discovered on the left side
of the tongue base (Fig. 1). No
other significant findings in the nasal cavity or the neck area
were observed. The results of the general blood tests were normal.
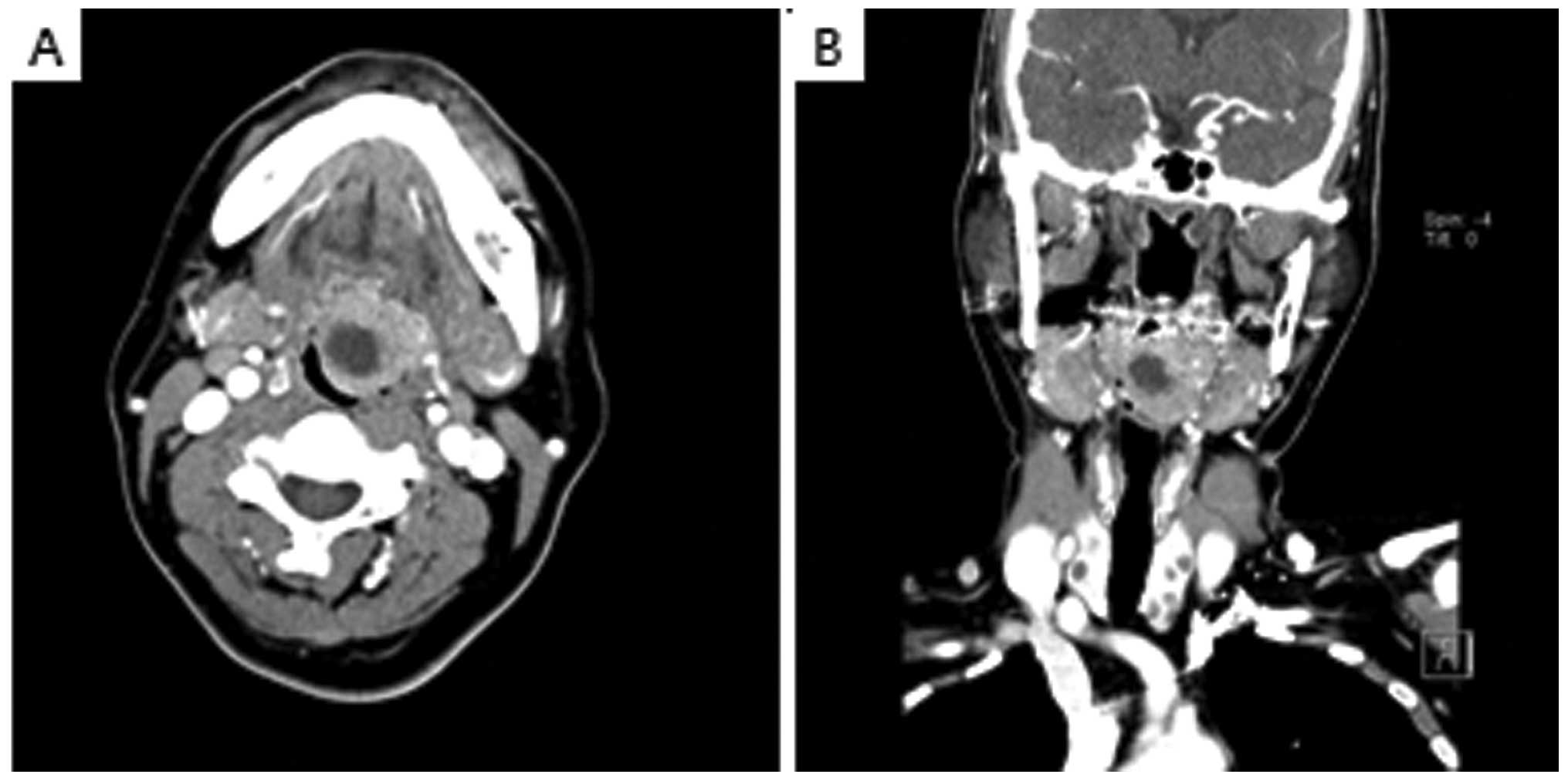
Computed tomography (CT) revealed necrosis in the central area of
the left side of the tongue base and a 2.9×2.6 cm circular lesion
showing contrast enhancement was also detected (Fig. 2). As several colloidal cysts were
detected in both thyroids, ultrasonography was performed. The
results were clinically insignificant.
A biopsy was performed under local anesthesia at our
outpatient clinic due to suspected adenoid cystic carcinoma. To
assess systemic metastasis and other associated diseases,
18F-fluorodeoxyglucose positron emission tomography/CT
was performed. There was no evidence of metastasis in the neck or
systemic metastasis.
Under general anesthesia, the mass was removed
through suprahyoid pharyngotomy. Tracheostomy and primary suture
were also performed. The patient was discharged 10 days after
surgery without any significant complications.
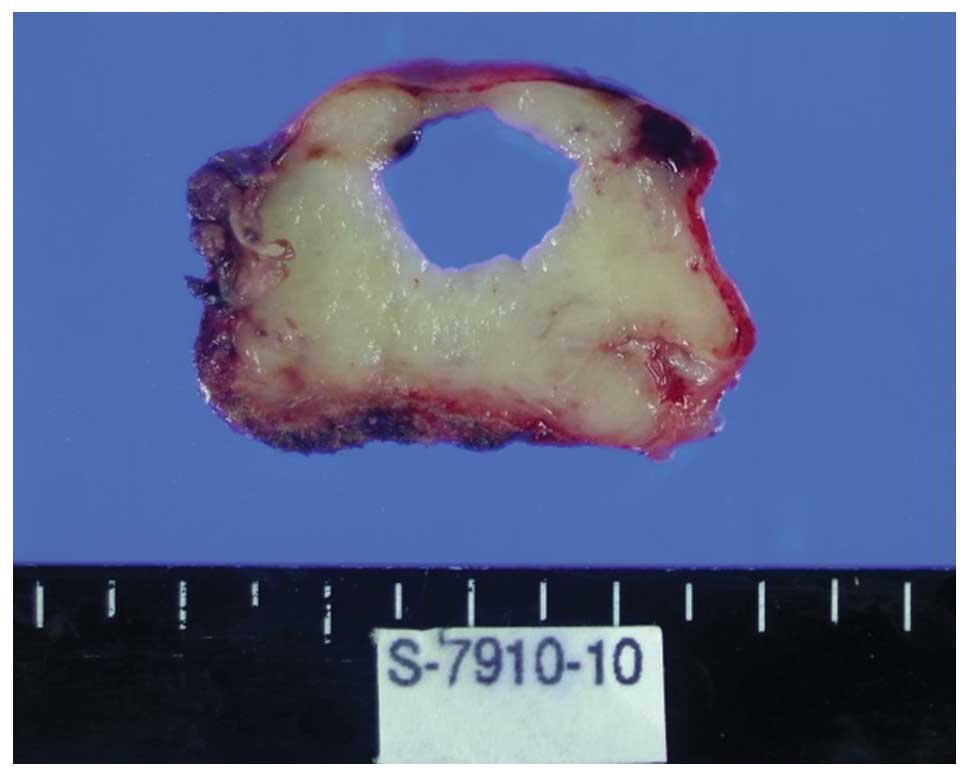
Macroscopically, the removed mass was light yellow
in color and 4.5×3 cm in size. At the time of resection, a 1.5×1.2
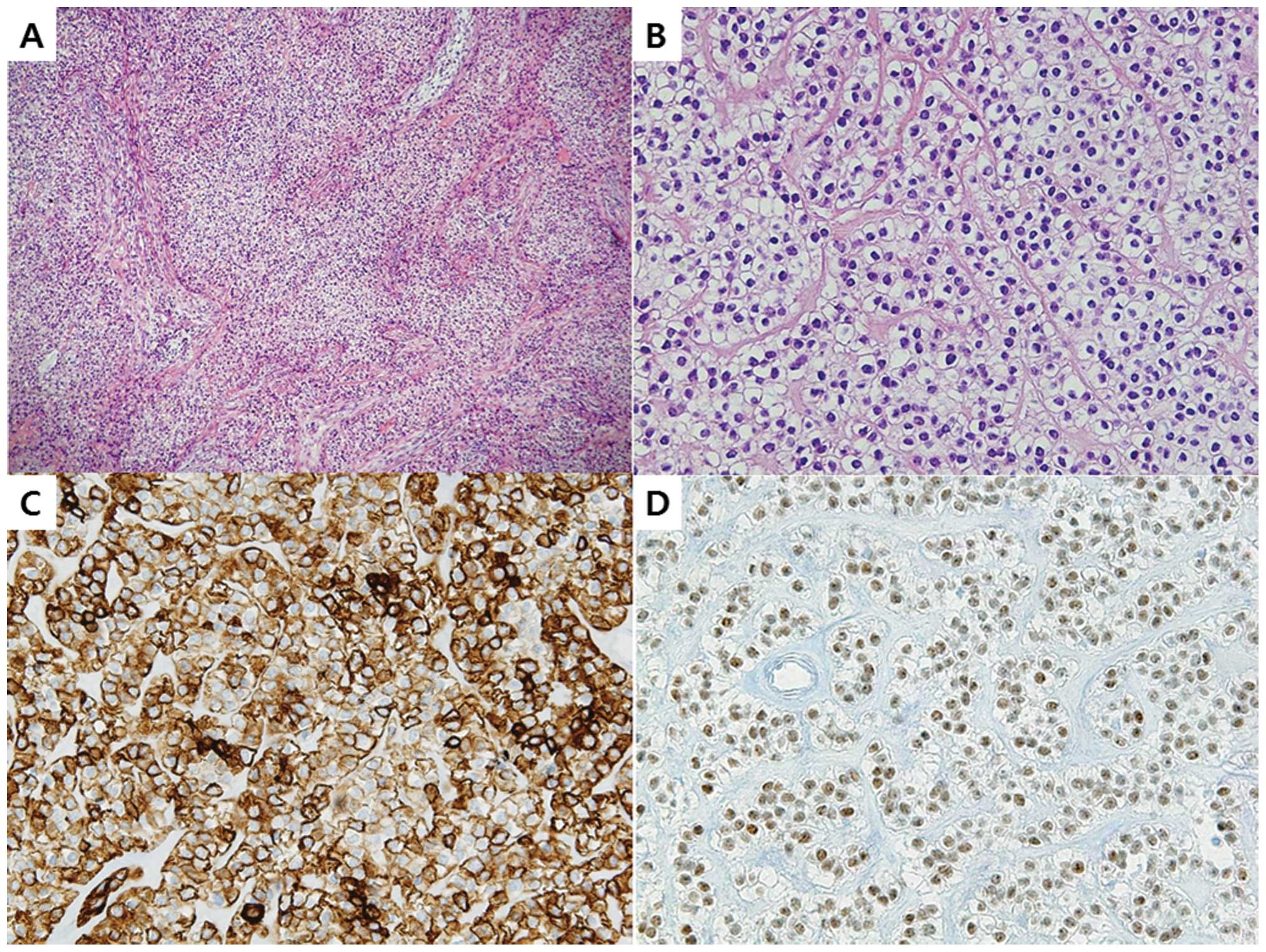
cm zone filled with red fluid was detected (Fig. 3). Under light microscopy, the mass
was partitioned by fibrous septa and contained clear cells that
formed a pattern of nests, sheets and cords. The clear cells were
polygonally shaped and contained abundant, clear cytoplasm. By
immunohistochemical staining, the tumor cells were revealed to be
positive for cytokeratin 7 (CK 7), p63 and high molecular weight
cytokeratin (HMWCK), and revealed focally weak responses to
cytokeratin (Fig. 4). Additionally,
the tumor cells revealed negative reactions to S-100 and smooth
muscle actin (SMA). As a result of the light microscopy examination
and immunohistochemical staining results, a diagnosis of CCMC was
made.
The patient has been under follow-up observation for
2 years without recurrence or local or distant metastasis.
Discussion
In salivary gland tumors, myoepithelial
differentiation is relatively common. However, cases in which the
tumors themselves are primarily formed by myoepithelial cells are
very rare. Furthermore, among salivary gland myoepithelial
carcinomas, CCMC which presents with almost exclusively the
phenotype of clear cells is also extremely rare. Hence, CCMC is a
clear cell neoplasm formed by tumor cells with myoepithelial
differentiation that presents with a epithelial phenotype and
smooth muscle phenotype only (6,8,9). To
date, only 17 cases of CCMC have been reported. By examination of
the site of tumor development, 9 cases involved the parotid gland,
4 cases involved the submandibular gland and one case involved the
maxillary sinus. Three cases occurred in the oral cavity, 2 cases
occurred in the palate and one case occurred in the retromolar
region (2–7). This is the first study to report a
case of CCMC in the base of the tongue.
CCMC displays diverse histological patterns that
include nests, cords, trabeculae and sheets. The central area is
paucicellular and the peripheral area is hypercellular. Vacuolated
clear cells display signet ring or lipoblast shapes (2,3). In
the majority of cases, clear tumor cells predominate. However,
certain components of myoepithelial carcinoma, spindle cells or
nonclear epithelioid cells may appear in certain areas.
Myoepithelial differentiation by immunohistochemical
staining is possible as the cells reveal positive reactions to
epithelial markers (epithelial membrane antigen and cytokeratin)
and at least two of the myoepithelial markers (S-100 protein,
calponin, p63, glial fibrillary acidic protein, maspin and actin).
Myoepithelial carcinoma reacts most sensitively to vimentin, S-100
and HMWCK (3,6). Furthermore, it has been recently
suggested that the p63 and maspin members of the p53 family of
nuclear proteins may be new myoepithelial markers (6). Our case was positive for CK7, p63 and
HMWCK and revealed focal weak reactions to CK.
CCMC should be differentiated from other salivary
gland tumors that contain clear cell components and from
odontogenic and metastatic tumors. Acinic cell carcinoma,
high-grade mucoepidermoid carcinoma, sebaceous carcinoma and clear
cell oncocytoma that develop in the salivary gland are negative for
myoepithelial markers and should therefore readily be capable of
being differentially diagnosed. Additionally, metastatic clear cell
renal cell carcinoma with the clear cell phenotype invading the
salivary gland, presents with prominent sinusoidal vascularity and
hemorrhage within tumors and is evident under light microscopy
examination, which allows for a differential diagnosis (2,7,10).
Based on the treatment for myoepithelial carcinoma,
the basic treatment for CCMC is wide surgical excision in the
primary tumor site. If metastasis in the cervical lymph nodes is
not detected, dissection of the lymph nodes is unnecessary
(3,11). Additional postsurgical radiotherapy
does not improve the overall prognosis. Nonetheless, it can be
administered for the purpose of local control in cases in which an
intact safety margin is not secured during surgery, or for cases in
which invasion into adjacent tissues is confirmed by microscopic
examination. Additionally, postsurgical chemotherapy is
administered to treat systemic metastasis, similar to other cancers
(3,11). In the present case, lymph node
metastasis in other areas, including the neck, was not detected in
presurgical tests and a tumor-free margin could be secured by wide
surgical excision. Thus, additional postsurgical treatments were
not administered.
The prognosis of CCMC remains unclear due to the
small number of reported cases. However, a study conducted on
myoepithelial carcinoma that reported a 47% metastatic rate and 29%
mortality rate revealed clinically aggressive patterns (5). Another study that was conducted on
CCMC alone reported a 50% recurrence rate, metastasis in the lung
and the scalp and a 40% metastatic rate (12).
A differential diagnosis of CCMC from other primary
tumors containing clear cells and metastatic lesions is essential.
When a case is diagnosed as CCMC, aggressive treatments including
surgical excision are required.
References
|
1.
|
A SkalovaKT JakelMyoepithelial
carcinomaWHO Classification of Tumours. Pathology and Genetics of
Head and Neck TumoursL BarnesJW EvesonP ReichartD SidranskyIARC
PressLyon2402412005
|
|
2.
|
M MichalA SkalovaRH SimpsonV RychterovaI
LeivoClear cell malignant myoepithelioma of the salivary
glandsHistopathology28309315199610.1046/j.1365-2559.1996.d01-439.x8732339
|
|
3.
|
AT SaveraA SlomanAG HuvosDS
KlimstraMyoepithelial carcinoma of the salivary glands: a
clinicopathologic study of 25 patientsAm J Surg
Pathol24761774200010.1097/00000478-200006000-0000110843278
|
|
4.
|
S YangM ZengJ ZhangX ChenClear cell
myoepithelial carcinoma of minor salivary gland: a case reportInt J
Oral Maxillofac
Surg39297300201010.1016/j.ijom.2009.10.01319939628
|
|
5.
|
NS LositoG BottiF IonnaG PasquinelliP
MinennaM BiscegliaClear-cell myoepithelial carcinoma of the
salivary glands: a clinicopathologic, immunohistochemical, and
ultrastructural study of two cases involving the submandibular
gland with review of the literaturePathol Res
Pract204335344200810.1016/j.prp.2007.11.006
|
|
6.
|
AT SaveraRJ ZarboDefining the role of
myoepithelium in salivary gland neoplasiaAdv Anat
Pathol116985200410.1097/00125480-200403000-0000115090843
|
|
7.
|
B WangM BrandweinR GordonR RobinsonM
UrkenRJ ZarboPrimary salivary clear cell tumors - a diagnostic
approach: a clinicopathologic and immunohistochemical study of 20
patients with clear cell carcinoma, clear cell myoepithelial
carcinoma, and epithelial-myoepithelial carcinomaArch Pathol Lab
Med1266766852002
|
|
8.
|
I DardickMJ ThomasAW van
NostrandMyoepithelioma - new concepts of histology and
classification: a light and electron microscopic studyUltrastruct
Pathol13187224198910.3109/019131289090574422544051
|
|
9.
|
I DardickMyoepithelioma: definitions and
diagnostic criteriaUltrastruct
Pathol19335345199510.3109/019131295090219067483010
|
|
10.
|
I OgawaH NikaiT TakataClear cell tumors of
minor salivary gland origin. An immunohistochemical and
ultrastructural analysisOral Surg Oral Med Oral
Pathol72200207199110.1016/0030-4220(91)90164-81717916
|
|
11.
|
G YuD MaK SunT LiY ZhangMyoepithelial
carcinoma of the salivary glands: behavior and managementChin Med J
(Engl)116163165200312775221
|
|
12.
|
RJ ZarboSalivary gland neoplasia: a review
for the practicing pathologistMod
Pathol15298323200210.1038/modpathol.388052511904344
|