Introduction
Multiple myeloma (MM) is an incurable B-cell
malignancy characterized by infiltrating, slow-growing plasma cells
in the bone marrow that produce monoclonal immunoglobulin molecules
(1). MM is more common than Hodgkin
disease and acute leukemia and accounts for 1% of all cancers and
slightly more than 10% of all hematological cancers. Few effective
therapies have existed until recently. Alkylating agents and
corticosteroids were the therapy of choice for several decades. A
number of novel agents for MM have now become available, including
the immunomodulatory drugs thalidomide and lenalidomide, as well as
the proteasome inhibitor bortezomib (2–5).
Although numerous therapeutic advances, including combined
chemotherapy and hematopotietic stem cell transplantation, have
been made to improve the survival of patients with MM, a high
proportion of patients are not able to expect long term remission
due to drug-resistant disease, minimal residual disease or
infection. In order to overcome these limitations, certain new
promising approaches are being widely studied (6,7).
As with unregulated proliferation, dysregulated
apoptosis is also an important event in carcinogenesis. MM cells
over-express the apoptotic antagonists bcl-2 and bcl-xL, although
they are not overtly hyperproliferative (8,9). The
protection against apoptosis may be significant in the expansion
and/or resistance of the MM cell clones to chemotherapy. Modulation
of apoptosis with adjuvant therapy may have the potential to
improve the clinical outcome of MM. In further studies, the safety
and efficacy of a combination therapy should be investigated
(10).
Cyclic adenosine monophosphate (cAMP), a well-known
and ubiquitous chemical messenger, has an antiproliferative effect
on the majority of cell types (11,12).
The best studied of its derivatives is 8-chloroadenosine
3′,5′-monophosphate (8-Cl-cAMP), which has demonstrated
antiproliferative effects in vitro and in vivo and
has been evaluated in phase I/II clinical trials (11,13,14).
8-Cl-cAMP is a site-selective analog of cAMP. It has been reported
that 8-Cl-cAMP shows a potent growth inhibitory effect and has
reverse-transforming activity in cancer cells (15–18).
In the current study, we investigated the ability of 8-Cl-cAMP to
induce apoptosis in two MM cell lines, RPMI-8226 and U266. Our
results indicate that 8-Cl-cAMP-induced cellular apoptosis occurred
in a concentration- and time-related manner with mitochondrial
transmembrane potential collapse, increased expression levels of
p27 and decreased expression levels of c-myc. p27 knockdown was
able to decrease the 8-Cl-cAMP-induced apoptosis of the MM cells,
indicating that the apoptotic action occured through a
p27-dependent pathway.
Materials and methods
The study was approved by the Independent Ethics
Committee of Shanghai Ninth People’s Hospital Affiliated to
Shanghai Jiao Tong University School of Medicine, Shanghai,
China.
Reagents and cell culture
8-Cl-cAMP, propidium iodide (PI), rhodamine 123
(Rh123), SB202190 and other reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA). p27 siRNA was designed by
Dharmacon (Lafayette, CO, USA). Fetal bovine serum (FBS), RPMI-1640
medium and penicillin-streptomycin were obtained from Gibco-BRL
(Gaithersburg, MD, USA). All antibodies were purchased from Santa
Cruz Biotechnology, (Santa Cruz, CA, USA. The ECL kit was purchased
from Amersham Pharmacia Biotech (Amersham, UK). An Annexin V-FITC
apoptosis detection kit, Oligofectamine and a mitochondrial
membrane potential detection kit were purchased from Invitrogen
(Eugene, OR, USA). The human myeloma cell lines RPMI-8226 and U266
(Shanghai Institute of Hematology, China) were cultured in
RPMI-1640 medium supplemented with 10% FBS in a humidified
atmosphere of 5% CO2 at 37°C.
Trypan blue exclusion assay
The effect of 8-Cl-cAMP on MM cell viability was
measured by the trypan blue exclusion assay (19). RPMI-8226 and U266 cells were
collected, mixed with an equal volume of PBS containing 0.4% trypan
blue dye and manually counted. Actual cell numbers were calculated
by multiplying by the dilution factor and were compared with the
initial cell numbers. Cell viability (%) = viable cell
numbers/total (viable + dead) cell numbers x 100.
Flow cytometric analysis of nuclear DNA
distribution
Cells (2x106) were collected, rinsed and
fixed overnight with 70% cold ethanol. They were then rinsed with
PBS, treated with 1 mg/ml RNase at 37°C for 30 min and stained with
250 μg/ml PI. The nuclear DNA contents were detected by flow
cytometry (Beckman Coulter, Miami, FL, USA). All data were
collected, stored and analyzed by MultiCycle software.
Flow cytometric analysis of mitochondrial
membrane potential
After washing with PBS twice, 1–2x105
RPMI-8226 cells were incubated with 10 μg/ml Rh123 at 37°C
for 30 min. Subsequently, 250 μg/ml PI was injected into
cells. Rh123 and PI staining intensities were determined by flow
cytometry.
Western blot analysis
At appropriate time-points following treatment with
10 μmol/l 8-Cl-cAMP, the RPMI-8226 cells were collected.
Protein extracts (100 μg) were loaded onto a 10%
SDS-polyacrylamide gel, electrophoresed and transferred onto
nitrocellulose membranes, which were subsequently stained with 0.2%
Ponceau red to assure equal protein loading and transfer. After
blocking with 10% non-fat milk powder, the membrane was incubated
with primary antibody overnight at 4°C. The membrane was then
washed with PBS and incubated with horseradish
peroxidase-conjugated secondary antibody for 60 min at room
temperature. The blots were again washed and the immunocomplex was
visualized using the ECL kit.
Transfection of p27 siRNA and cell
viability assay
The cells (1x104 cells/well) were seeded
in a 96-well plate, incubated for 24 h to enable them to attach to
the bottom of the well, and then transfected with 80 nM p27 siRNA
or control siRNA using Oligofectamine. Following transfection for
12 h, the cells were treated with 10 μmol/l 8-Cl-cAMP for 48
h. Cell growth was was then determined by measuring the MTT dye
absorbance of the living cells (20). Following exposure to 8-Cl-cAMP for
the indicated time, 20 μl MTT solution (5 mg/ml in PBS) was
added to each well and the plates were incubated for an additional
4 h at 37°C. The MTT solution in the medium was aspirated. To
achieve solubilization of the formazan crystals formed in viable
cells, 150 μl dimethylsulfoxide (DMSO) was added to each
well and the absorbance at 570 nm was measured using a microplate
reader (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Each result in this study is representative of at
least three separate experiments. Values represent the mean ±
standard deviation (SD) of these experiments. The statistical
significance was calculated with a Student’s t-test. P<0.05 was
considered to indicate a statistically significant result.
Results
8-Cl-cAMP induced MM cell growth
inhibition and apoptosis in a concentration- and time-related
manner
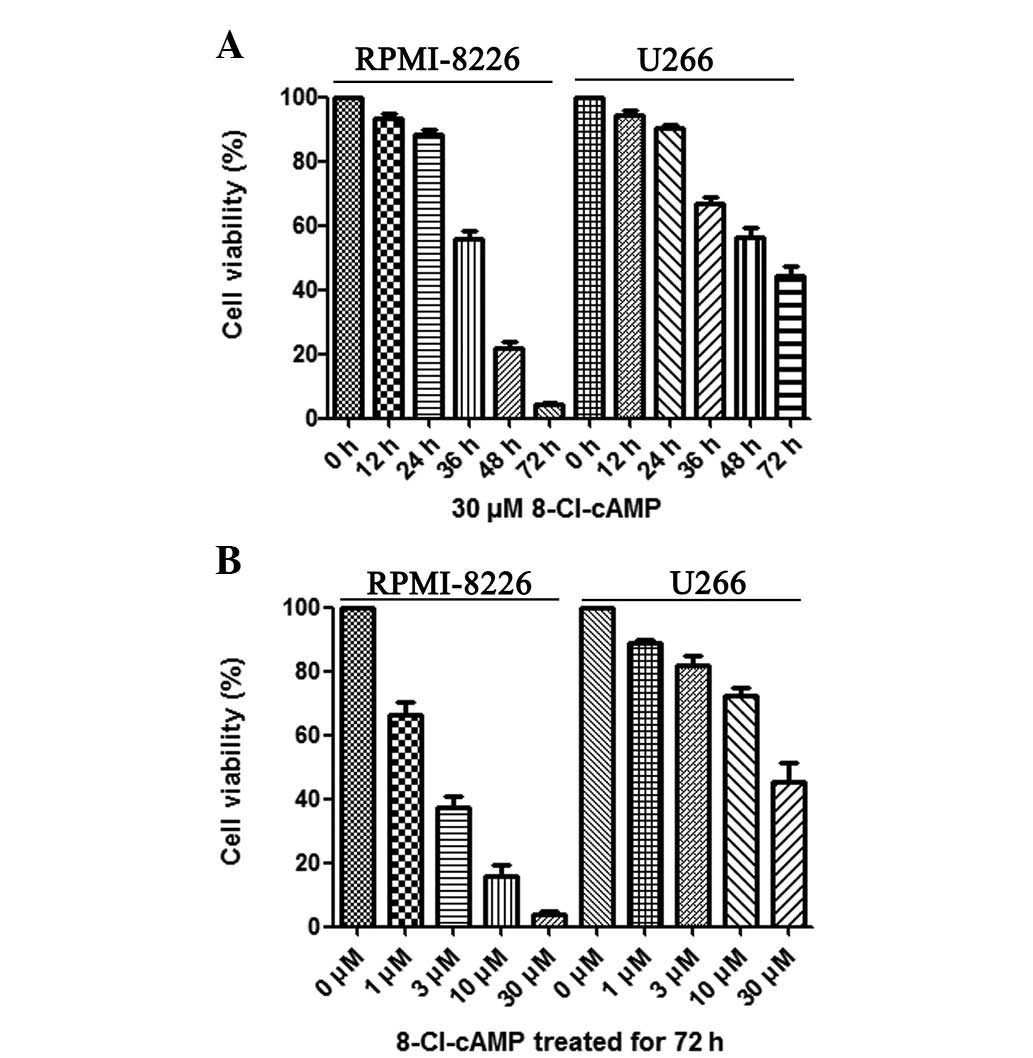
To investigate the cytotoxic effect of 8-Cl-cAMP on
RPMI-8226 and U266 cells, the cell viability following treatment
with 8-Cl-cAMP at various concentrations (1, 3, 10, 30 μM)
was determined by trypan blue exclusion assay. As shown in Fig. 1B, 8-Cl-cAMP reduced the cell
viability of the RPMI-8226 and U266 cells in a
concentration-dependent manner. Higher concentrations of 8-Cl-cAMP
yielded more effective inhibition. The RPMI-8226 cells were more
sensitive than the U266 cells to 8-Cl-cAMP. When treated with 30
μM 8-Cl-cAMP for 72 h, almost all RPMI-8226 cells died
(Fig. 1A). The results of Annexin
V-FITC and PI staining revealed that 8-Cl-cAMP induced apoptotic
and necrotic cell death. The percentage of cell death was 19.27% in
the RPMI-8226 cells treated with 30 μM 8-Cl-cAMP for 24 h
(Fig. 2A). Since the reduction of
mitochondrial transmembrane potential is often associated with
apoptosis, we further investigated the effect of 8-Cl-cAMP on
mitochondria. The treatment of RPMI-8226 cells with 8-Cl-cAMP
resulted in an increase in the percentage of cells with a reduced
mitochondrial transmembrane potential and increased apoptosis in a
time-dependent manner (Fig. 2B).
These features suggest that the 8-Cl-cAMP-induced MM cell death was
due to apoptosis.
8-Cl-cAMP induces cell cycle arrest and
apoptosis
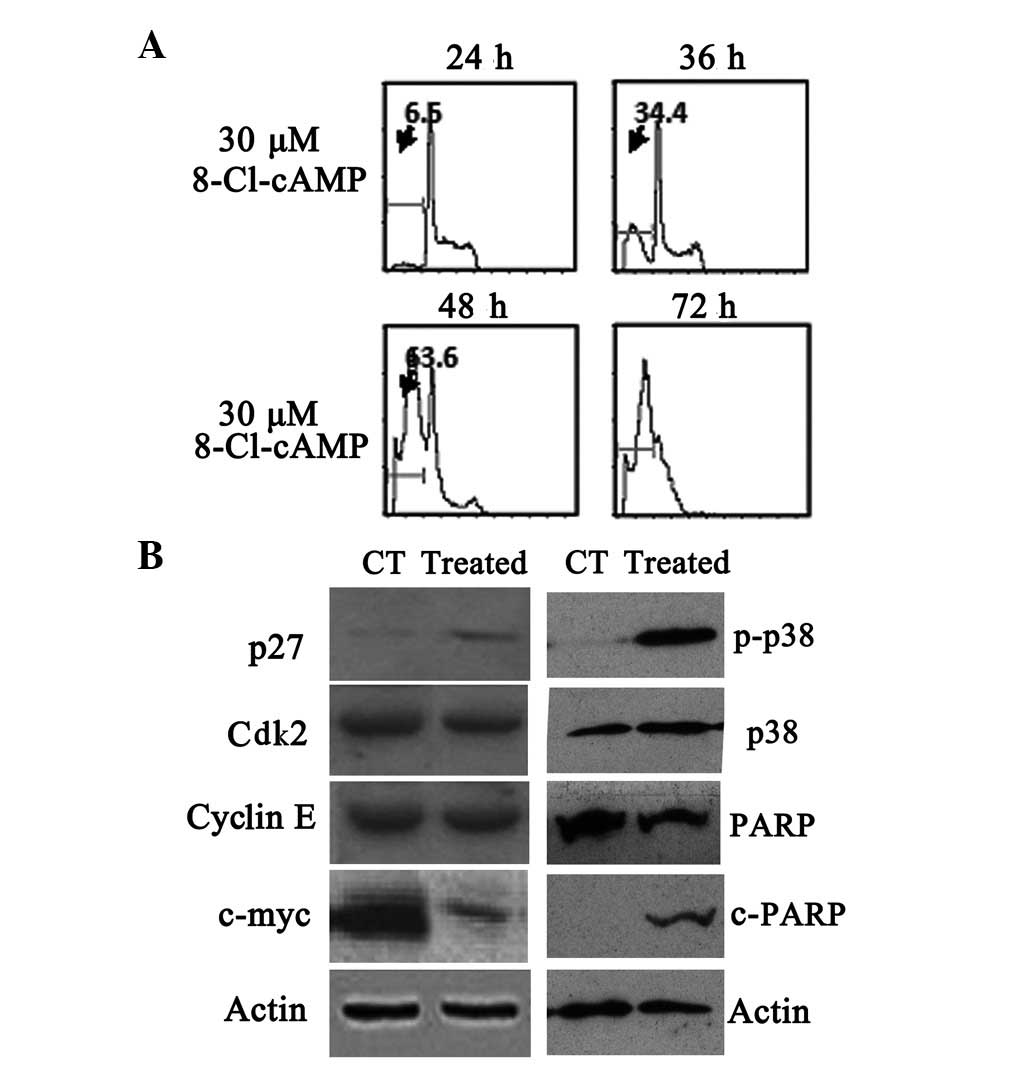
To elucidate whether the apoptosis induced by
8-Cl-cAMP was due to cell cycle arrest, we evaluated the cell cycle
distribution by flow cytometry. Flow cytometric analysis of the
cellular DNA contents revealed that the sub-G1 phase ratio rose
from 6.5 to 63.6% in the RPMI-8226 cells treated with 30 μM
8-Cl-cAMP for 48 h (Fig. 3A). The
expression of the cyclin-dependent kinase (Cdk) inhibitor p27,
which is involved in the arrest of the cell cycle in the G1 phase,
was also evaluated. p27 levels, not detectable under basal
conditions, were found to be increased following exposure to 10
μM 8-Cl-cAMP for 48 h (Fig.
3B). We also monitored the levels of other cell
cycle-associated proteins. The levels of Cdk2 and cyclin E almost
remained constant, but the expression levels of c-myc protein
decreased in the RPMI-8226 cells exposed to 10 μmol/l
8-Cl-cAMP for 48 h (Fig. 3B). These
results suggest that the 8-Cl-cAMP-induced cell cycle arrest was
mediated by the expression of cell cycle-associated proteins. We
also found that poly(ADP-ribose) polymerase (PARP) was cleaved
following treatment with 8-Cl-cAMP (Fig. 3B).
8-Cl-cAMP induces apoptosis in MM cells
through a p27-dependent pathway
Cell cycle associated proteins play a critical role
in mediating the cytotoxicity induced by 8-Cl-cAMP, but their
targets are unknown. To determine whether higher p27 levels were a
critical factor contributing to the 8-Cl-cAMP-induced MM cell
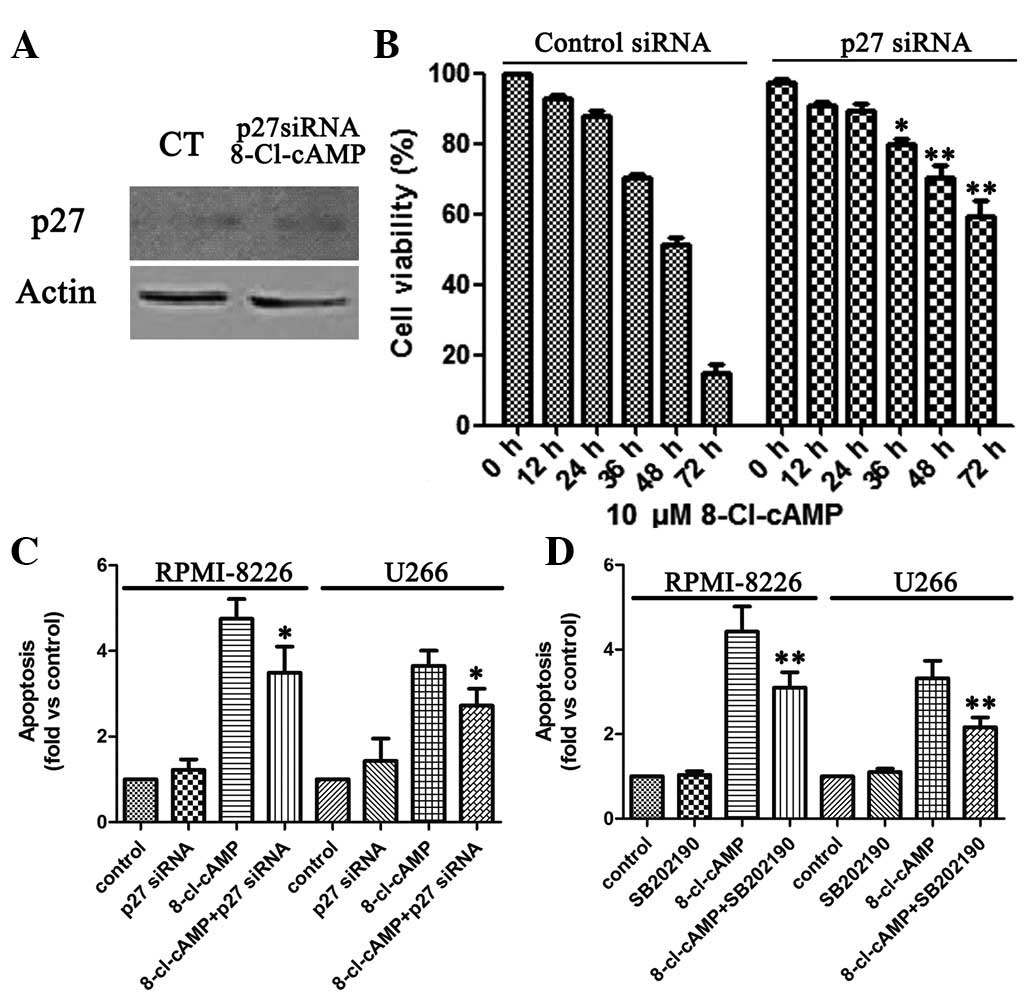
apoptosis, we knocked down p27 expression by RNA interference.
Immunoblotting revealed that the transient introduction of p27
siRNA into the RPMI-8226 cells markedly reduced p27 expression
levels compared with those in their control counterparts (Fig. 4A). The viability of the RPMI-8226
cells in the presence of 10 μM 8-Cl-cAMP was 14.7%, whereas
that of the p27-knocked-down RPMI-8226 cells was 74.1% (Fig. 4B). Similarly, flow cytometric
analysis revealed that the level of apoptosis of sub-G1 cells
increased in the RPMI-8226 cells treated with 10 μM
8-Cl-cAMP, whereas the apoptosis of sub-G1 cells was almost
undetectable in the p27-knocked--down RPMI-8226 cells (Fig. 4C). Thus, on the basis of the above
results, p27 is functionally linked to the sensitivity of the
RPMI-8226 cells to 8-Cl-cAMP-induced apoptosis.
Effect of 8-Cl-cAMP on p38
mitogen-activated protein kinase (MAPK) expression
8-Cl-cAMP has been shown to induce p38 MAPK
phosphorylation in HL60 and HeLa cells (15,21).
Therefore, we evaluated the effect of 8-Cl-cAMP on p38 MAPK
phosphorylation in the MM cells. We identified that the treatment
of RPMI-8226 and U266 cells with 8-Cl-cAMP was associated with a
progressive increase in p38 MAPK phosphorylation that started after
48 h of incubation (Fig. 3B).
Finally, we evaluated whether the pro-apoptotic effect of 8-Cl-cAMP
was dependent upon p38 MAPK activation. To this aim, we treated the
two cell lines with 8-Cl-cAMP for 48–72 h in the presence or
absence of a selective p38 MAPK inhibitor (SB 202190) and analyzed
the cell cycle distribution by flow cytometry as described
previously. Notably, the inhibition of p38 MAPK largely prevented
the pro-apoptotic effect of 8-Cl-cAMP (Fig. 4D). These data strongly suggest that
the pro-apoptotic effect of 8-Cl-cAMP on RPMI-8226 and U266 cells
is mediated by p38 MAPK.
Discussion
Following the identification of cAMP in 1958 by Rall
and Sutherland, research focused for more than a decade on
elucidating the role that this secondary messenger played in
regulating metabolic pathways, as well as identifying the enzymes
responsible for cAMP synthesis and catabolism (22–24).
By the 1970s, however, cAMP was implicated as a regulator of cell
growth (25–27), and several investigators reported
that the elevation of cAMP levels induced proliferation arrest or
cell death in susceptible normal or malignant lymphoid populations
(28–33). The cAMP signaling pathway has
emerged as a key regulator of hematopoietic cell proliferation,
differentiation and apoptosis. The 8-chloro derivative of cAMP is a
very potent cAMP analog that is under investigation as a potential
chemotherapeutic agent. Phase I clinical studies in patients with
solid tumors revealed its safety as well as evidence of clinical
response (34).
In our study, we identified that 8-Cl-cAMP induced
mitochondial transmembrane potential collapse and apoptosis
simultaneously in RPMI-8226 cells. Western blot analysis revealed
that 8-Cl-cAMP (10 μmol/l)-induced apoptosis was accompanied
by upregulation of p27 and downregulation of c-myc in RPMI-8226
cells. p27, also known as Cdk inhibitor 1B, is an enzyme which
belongs to the Cip/Kip family of Cdk inhibitor proteins (35). It binds to and prevents the
activation of cyclin E-Cdk2 and cyclin D-Cdk4 complexes, and thus
controls the cell cycle progression at G1 (36). It is often referred to as a cell
cycle inhibitor protein since its major function is to stop or slow
down the cell division cycle (35–38).
In the current study, we found that 8-Cl-cAMP induced the
upregulation of the Cdk inhibitor p27. Therefore, we assessed the
significance of p27. Fig. 4 shows
that p27 knockdown inhibited the 8-Cl-cAMP-induced decreases in
cell viability, cell cycle arrest and cell death. These results
indicate that the 8-Cl-cAMP-induced apoptosis in MM cells is
p27-dependent, and p27 may be the target of 8-Cl-cAMP.
The proto-oncogene c-myc is involved in the control
of cell proliferation, apoptosis and differentiation, and its
aberrant expression is frequently observed in human cancer
(39). It has been previously
reported that small-molecule inhibitors of c-myc suppress
proliferation and induce apoptosis of promyelocytic leukemia cells
via cell cycle arrest (39,40). Although our data show that 8-Cl-cAMP
induced the downregulation of c-myc, the mechanism for this action
is not well defined. We also hypothesize that c-myc is significant
in the mediation of 8-Cl-cAMP-induced cytotoxicity.
In addition, we identified that the pro-apoptotic
effect of 8-Cl-cAMP was accompanied by a progressive increase of
p38 MAPK phosphorylation. The p38 MAPK is a key regulator of cell
survival (41,42) and has been implicated in the
pro-apoptotic effect of 8-Cl-cAMP in HL60 and HeLa cells. In
conclusion, our data indicate that 8-Cl-cAMP has a potent
antiproliferative effect on MM cell lines through multiple
pathways.
Acknowledgements
This study was supported by the
Scientific Research Program of the Health Bureau of Shanghai (Grant
No. 2010101) and the Shanghai Committee of Science and Technology,
China (Grant No. 12ZR1416800). The authors are grateful to all
members of the Hematology Department of Shanghai Ninth People’s
Hospital for their continuous support and encouragement.
References
|
1.
|
M HallekPL BergsagelKC AndersonMultiple
myeloma: increasing evidence for a multistep transformation
processBlood9132119989414264
|
|
2.
|
X FengJ YanY WangJR ZierathM
NordenskjöldJI HenterThe proteasome inhibitor bortezomib disrupts
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
expression and natural killer (NK) cell killing of TRAIL
receptor-positive multiple myeloma cellsMol
Immunol4723882396201010.1016/j.molimm.2010.05.003
|
|
3.
|
LE PerezN ParquetM MeadsC AnasettiW
DaltonBortezomib restores stroma-mediated APO2L/TRAIL apoptosis
resistance in multiple myelomaEur J
Haematol84212222201010.1111/j.1600-0609.2009.01381.x19922463
|
|
4.
|
T IguchiT Yachide-NoguchiY HashimotoS
NakazatoM SagawaY IkedaM KisakiNovel tubulin-polymerization
inhibitor derived from thalidomide directly induces apoptosis in
human multiple myeloma cells: possible anti-myeloma mechanism of
thalidomideInt J Mol Med211631682008
|
|
5.
|
JA ZonderJ CrowleyMA HusseinV BolejackDF
Moore SrBF WhittenbergerLenalidomide and high-dose dexamethasone
compared with dexamethasone as initial therapy for multiple
myeloma: a randomized Southwest Oncology Group trial
(S0232)Blood11658385841201010.1182/blood-2010-08-30348720876454
|
|
6.
|
M OffidaniP LeoniL CorvattaC PolloniS
GentiliAM LiberatiOutcome and toxicity in the modern era of new
drugs for multiple myeloma: a reappraisal for comparison with
future investigational trialsClin Lymphoma Myeloma
Leuk10353360201010.3816/CLML.2010.n.06821030348
|
|
7.
|
D DingliSV RajkumarHow best to use new
therapies in multiple myelomaBlood
Rev2491100201010.1016/j.blre.2010.03.00120359801
|
|
8.
|
M PetterssonH Jernberg-WiklundLG LarssonC
SundströmI GivolY TsujimotoK NilssonExpression of the bcl-2 gene in
human multiple myeloma cell lines and normal plasma
cellsBlood7949550219921730093
|
|
9.
|
SD PeetersS HovengaS RosatiE
VellengaBcl-xl expression in multiple myelomaMed
Oncol22183190200510.1385/MO:22:2:18315965282
|
|
10.
|
T CaravitaA SiniscalchiA TendasL CupelliM
AlesA PerrottiSafety and efficacy of a combination therapy with
Revlimid, Adriamycin and dexamethasone (RAD) in relapsed/refractory
multiple myeloma (MM): a single-centre experienceAnn
Hematol90115116201110.1007/s00277-010-0967-4
|
|
11.
|
S LucchiD CalebiroT de FilippisES GrassiMO
BorghiL Persani8-Chloro-cyclic AMP and protein kinase A I-selective
cyclic AMP analogs inhibit cancer cell growth through different
mechanismsPLoS
One6e20785201110.1371/journal.pone.002078521695205
|
|
12.
|
N DumazR MaraisIntegrating signals between
cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the
anniversary prize of the Gesellschaft für Biochemie und
Molekularbiologie Lecture delivered on 5 July 2003 at the Special
FEBS Meeting in BrusselsFEBS J27234913504200516008550
|
|
13.
|
YS Cho-ChungRole of cyclic AMP receptor
proteins in growth, differentiation, and suppression of malignancy:
new approaches to therapyCancer Res507093710019902224844
|
|
14.
|
DJ PropperMP SaundersAJ SalisburyL LongKJ
O’ByrneJP BraybrookePhase I study of the novel cyclic AMP (cAMP)
analogue 8-chloro-cAMP in patients with cancer: toxicity, hormonal,
and immunological effectsClin Cancer Res516821689199910430069
|
|
15.
|
JH HanYH AhnKY ChoiSH HongInvolvement of
AMP-activated protein kinase and p38 mitogen-activated protein
kinase in 8-Cl-cAMP-induced growth inhibitionJ Cell
Physiol218104112200910.1002/jcp.2157318756496
|
|
16.
|
V BajicZ StanimirovicJ StevanovicB
Spremo-PotparevicL ZivkovicZ MilicevicCytogenetic effects of
8-Cl-cAMP on human and animal chromosomesJ
BUON147177200919373950
|
|
17.
|
V VucićA NićiforovićM AdzićMB RadojcićS
RuzdijićThe combination of gamma ionizing radiation and 8-Cl-cAMP
induces synergistic cell growth inhibition and induction of
apoptosis in human prostate cancer cellsInvest New
Drugs26309317200818060599
|
|
18.
|
M PesicK DrabekC EslerS RuzdijicV
PejanovicZ PietrzkowskiInhibition of cell growth and proliferation
in human glioma cells and normal human astrocytes induced by
8-Cl-cAMP and tiazofurinNucleosides Nucleotides Nucleic
Acids19963975200010.1080/1525777000803303610893715
|
|
19.
|
Y LiX QuJ QuY ZhangJ LiuY TengArsenic
trioxide induces apoptosis and G2/M phase arrest by inducing Cbl to
inhibit PI3K/Akt signaling and thereby regulate p53
activationCancer
Lett284208215200910.1016/j.canlet.2009.04.03519457607
|
|
20.
|
DT VisticaP SkehanD ScudieroA MonksA
PittmanMR BoydTetrazolium-based assays for cellular viability: a
critical examination of selected parameters affecting formazan
productionCancer Res512515252019912021931
|
|
21.
|
YH AhnJM JungSH Hong8-Chloro-cyclic
AMP-induced growth inhibition and apoptosis is mediated by p38
mitogen-activated protein kinase activation in HL60 cellsCancer
Res6548964901200510.1158/0008-5472.CAN-04-312215930311
|
|
22.
|
TW RallEW SutherlandFormation of a cyclic
adenine ribonucleotide by tissue particlesJ Biol
Chem23210651076195813549487
|
|
23.
|
RW ButcherEW SutherlandAdenosine
3′,5′-phosphate in biological materials. I. Purification and
properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of
this enzyme to characterize adenosine 3′,5′-phosphate in human
urineJ Biol Chem237124412501962
|
|
24.
|
GA RobisonRW ButcherI OyeHE MorganEW
SutherlandThe effect of epinephrine on adenosine 3′,5′-phosphate
levels in the isolated perfused rat heartMol
Pharmacol11681771965
|
|
25.
|
CW AbellTM MonahanThe role of adenosine
3′,5′-cyclic monophosphate in the regulation of mammalian cell
divisionJ Cell Biol595495581973
|
|
26.
|
IH PastanGS JohnsonWB AndersonRole of
cyclic nucleotides in growth controlAnnu Rev
Biochem44491522197510.1146/annurev.bi.44.070175.002423166606
|
|
27.
|
DL FriedmanRole of cyclic nucleotides in
cell growth and differentiationPhysiol Rev566527081976185633
|
|
28.
|
DJ FranksJP MacManusJF WhitfieldThe effect
of prostaglandins on cyclic AMP production and cell proliferation
in thymic lymphocytesBiochem Biophys Res
Commun4411771183197110.1016/S0006-291X(71)80210-34334273
|
|
29.
|
JP MacManusJF WhitfieldT
YoudaleStimulation by epinephrine of adenyl cyclase activity,
cyclic AMP formation, DNA synthesis and cell proliferation in
populations of rat thymic lymphocytesJ Cell
Physiol77103116197110.1002/jcp.10407701124322941
|
|
30.
|
P RalphR HymanR EpsteinI NakoinzM
CohnIndependence of theta and TL surface antigens and killing by
thymidine, cortisol, phytohemagglutinin, and cyclic AMP in a murine
lymphomaBiochem Biophys Res
Commun5510851091197310.1016/S0006-291X(73)80006-34358928
|
|
31.
|
P CoffinoHR BourneGM TomkinsMechanism of
lymphoma cell death induced by cyclic AMPAm J
Pathol811992041975170834
|
|
32.
|
K PonzettiM KingA GatesMS AnwerCR
WebsterCyclic AMP-guanine exchange factor activation inhibits
JNK-dependent lipopolysaccharide-induced apoptosis in rat
hepatocytesHepat Med20101112010
|
|
33.
|
D MoujalledR WestonH AndertonR NinnisP
GoelA ColeyCyclic-AMP-dependent protein kinase A regulates
apoptosis by stabilizing the BH3-only protein BimEMBO
Rep127783201110.1038/embor.2010.19021151042
|
|
34.
|
G TortoraF CiardielloS PepeP TagliaferriA
RuggieroC BiancoPhase I clinical study with 8-chloro-cAMP and
evaluation of immunological effects in cancer patientsClin Cancer
Res137738419959815994
|
|
35.
|
S OginoT KawasakiA OgawaGJ KirknerM LodaCS
FuchsCytoplasmic localization of p27 (cyclin-dependent kinase
inhibitor 1B/KIP1) in colorectal cancer: inverse correlations with
nuclear p27 loss, microsatellite instability, and CpG island
methylator phenotypeHum
Pathol38585592200710.1016/j.humpath.2006.09.014
|
|
36.
|
S IgrejaHS ChahalSA AkkerM GueorguievV
PopovicS DamjanovicAssessment of p27 (cyclin-dependent kinase
inhibitor 1B) and aryl hydrocarbon receptor-interacting protein
(AIP) genes in multiple endocrine neoplasia (MEN1) syndrome
patients without any detectable MEN1 gene mutationsClin Endocrinol
(Oxford)70259264200910.1111/j.1365-2265.2008.03379.x
|
|
37.
|
B WangF NiL LiZ WeiX ZhuJ WangAnalysis of
cyclin-dependent kinase inhibitor 1B mutation in Han Chinese women
with premature ovarian failureReprod Biomed
Online21212214201010.1016/j.rbmo.2010.04.02520615757
|
|
38.
|
Q TongW ZhangS JinS LiZ ChenThe
relationship between p27(kip1) expression and the change of
radiosensitivity of esophageal carcinoma cellsScand J
Gastroenterol46173176201110.3109/00365521.2010.52272120923380
|
|
39.
|
MJ HuangYC ChengCR LiuS LinHE LiuA
small-molecule c-Myc inhibitor, 10058-F4, induces cell-cycle
arrest, apoptosis, and myeloid differentiation of human acute
myeloid leukemiaExp
Hematol3414801489200610.1016/j.exphem.2006.06.01917046567
|
|
40.
|
KC JeongKO AhnCH YangSmall-molecule
inhibitors of c-Myc transcriptional factor suppress proliferation
and induce apoptosis of promyelocytic leukemia cell via cell cycle
arrestMol Biosyst615031509201010.1039/c002534h20485733
|
|
41.
|
K OnoJ HanThe p38 signal transduction
pathway: activation and functionCell
Signal12113200010.1016/S0898-6568(99)00071-6
|
|
42.
|
T WadaJM PenningerMitogen-activated
protein kinases in apoptosis
regulationOncogene2328382849200410.1038/sj.onc.120755615077147
|