Introduction
A successful human pregnancy requires embryonic
trophocytes to invade into the womb and adhere to the endometrium;
however, this kind of invasion is strictly regulated
temporospatially (1). The
deregulation of the invasion process may cause a series of
pregnancy-related diseases, such as hydatidiform mole and invasive
mole; however, excessive invasion of trophocytes is significantly
associated with the formation of placental choriocarcinoma
(2). In this process, one critical
step of choriocarcinoma invasion and metastasis is the degradation
of the extracellular matrix (ECM), in which the MMP-9/TIMP-1 ratio
plays a significant role (3,4).
The main treatment for choriocarcinoma is
5-fluorouracil, which has a very good therapeutic efficacy;
however, it may also cause toxic reactions, side effects and drug
resistance (5). Since conventional
treatments including surgery and chemotherapy often fail, it is
necessary to explore the metastasis mechanisms of proliferation and
invasiveness, and find a new target for drug therapy.
TGF-β belongs to a growth factor super-family which
consists of a highly evolutionary conserved group of secreted
cytokines (6). The TGF-β family
consists of TGF-β, activins, bone morphogenetic proteins (BMPs),
nodals, inhibins and anti-mullerian hormone (AMH). TGF-β1 is the
prototypic family member and is secreted as an inactive latent
precursor (7,8). It plays a significant role in the
control of growth and development, including the regulation of cell
proliferation and differentiation, the promotion of ECM formation
and suppression of the immune response (9). TGF-β1 exerts its cellular effects
through TβR I, TβR II and the Smad transcription factors, which
have pivotal roles in intracellular signaling (7,10).
Moreover, Smad4 is an essential factor in TGF-β signaling and is a
frequently mutated tumor suppressor gene found in human tumors
(11–13). Compared with other tumors, fewer
studies have explored the role of the TGF-β/Smad signaling pathway
in the initiation and development of placental choriocarcinoma.
Previous studies have revealed that TGF-β1 inhibits normal cell
proliferation, but enhances the growth of tumor cells (14). During the early stages of
tumorigenesis, TGF-β1 may be an inhibitor of tumor cells through
its suppressive functions; however, following the development of
malignance, it may promote tumor cell proliferation (15). We have conducted a series of studies
on the choriocarcinoma JEG-3 cell line (16–18), a
number of which are in agreement with these findings (15); however, the more exact mechanisms of
development of placental choriocarcinoma remain a controversial
issue.
In order to explore the potential mechanism of
placental choriocarcinoma invasion, we examined the alterations in
the invasive capacity of the JEG-3 cell line when exposed to
exogenous TGF-β1, and the protein expression levels of MMP-9 and
TIMP-1. The mRNA levels for TβR I, TβR II and Smad4 were also
detected to investigate the impacts of the TGF-β1/Smad signaling
pathway on choriocarcinoma cell proliferation and invasion. These
results may provide theoretical and laboratory evidence to improve
clinical early diagnosis for placental choriocarcinoma. They may
also give rise to novel ideas regarding a target for its early
treatment.
Materials and methods
Materials
The human placental choriocarcinoma JEG-3 cell line
was obtained from the State Key Laboratory of Reproductive Biology,
Institute of Zoology, Chinese Academy of Sciences. The study was
approved by the ethics committee of the Natural Science Foundation
of Hebei Province and the Education Department of Hebei province,
Hebei, China.
JEG-3 cells culture
JEG-3 cells were cultured in an incubator with 5%
CO2 at 37°C in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS,
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd,
Hangzhou, China), 200 mM glutamine, 100 mM sodium pyruvate, 100
μg/ml streptomycin and 100 U/ml penicillin. Cells were
subcultured with 0.25% trypsin and 0.02% EDTA when cell growth
reached 70–80%, and the density of subcultured cells was at a ratio
between 1:2 and 1:4.
Transwell invasion assay
The invasion assay for JEG-3 cells was performed on
a 24-well Transwell chamber containing an 8-μm pore size
polycarbonate membrane filter with a diameter of 6.5 mm (Corning
Inc., Acton, MA, USA). The Transwell chamber was coated with a thin
layer of 60 μl growth factor-reduced diluted Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) (19) and incubated at 37°C for 4 h. A total
of 500 μl 20% DMEM was placed on the bottom of the Transwell
chamber. After a 24 h culture in a serum-free medium, JEG-3 cells
were mixed with DMEM substrate containing 1% BSA and adjusted to a
concentration of 3x104 cells/ml. Different
concentrations of TGF-β1 [0 μg/l (control group), 5
μg/l, 50 μg/l, 100 μg/l and 200 μg/l]
were added, respectively, then the cells were plated in the upper
chambers for re-culturing at 37°C for 24 h. On removal of the
Transwell chambers, cotton swabs were utilized to rub off the upper
surface cells and then the chambers were allowed to dry naturally.
The polycarbonate membrane was fixed by 4% paraformaldehyde for 10
min, stained with hematoxylin for 5–15 min and finally set on the
slide (20). Five fields were
randomly selected and the number of cells appearing on the
undersurface of the polycarbonate membranes was counted scored
under a microscope at ×200 magnification. Cell invasion was
performed on five independent occasions.
Reverse transcription (RT)-PCR
JEG-3 cells in the exponential phase were incubated
in 6-well plates at an initial concentration of 3×104
cells/ml for 48 h and then cultured with serum-free DMEM for 24 h
to obtain cell synchronization. The cells were subsequently treated
for 48 h with a series of concentrations of TGF-β1 [0 μg/l
(control group), 5 μg/l, 10 μg/l, 25 μg/l, 50
μg/l, 100 μg/l, and 200 μg/l]. Total RNA was
isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The
final RNA concentration, integrity and purity were validated, and
meet the experiment requirements. RT-PCR was performed following
the manufacturer’s recommendations (Takara Biotechnology, Dalian,
Liaoning, China). β-actin was used as the internal control
(21). The primers used are listed
in Table I. Amplification
conditions were: 94°C for 2 min, followed by 28 or 30 cycles of
94°C for 30 sec, annealing temperature for 30 sec and 72°C for 1
min. The PCR products were confirmed by electrophoresis in 2%
agarose gel at 120V for 30 min. Gel images were taken under UV
light using the UVP image analysis system (Shanghai Jiapeng
Technology, Shanghai, China) and analyzed with Quantity One
software (Bio-Rad, Hercules, CA, USA). Each sample was repeated a
minimum of five times. The relative quantity of the specific gene
was obtained and normalized to the values of β-actin.
 | Table I.PCR primers used in reaction. |
Table I.
PCR primers used in reaction.
| Target | Primer | Sequences
(5′-3′) | Product size
(bp) | Annealing
temperature (°C) |
|---|
| TβRI | Forward | GGC CAA ATA TCC CAA
ACA GAT | 509 | 60 |
| Reverse | AAT CCA ACT CCT TTG
CCC TTA | | |
| TβRII | Forward | GAC TTG ACC TGT TGC
CTG TGT | 310 | 59 |
| Reverse | TCA GCA CAC TGT CTT
TCA TGC | | |
| Smad4 | Forward | CAG CAT CCA CCA AGT
AAT CGT | 587 | 60 |
| Reverse | CTC TCA ATG GCT TCT
GTC CTG | | |
| β-actin | Forward | AGC GGG AAA TCG TGC
GTG AC | 453 | 58 |
| Reverse | ACA TCT GCT GGA AGG
TGG AC | | |
Western blot assay
The cells were incubated in 6-well plates with an
initial concentration of 3×104 cells/ml for 48 h and
then were allowed to grow in serum-free DMEM for 24 h. Following
cell synchronization, different concentrations of TGF-β1 [0
μg/l (control group), 100 μg/l and 200 μg/l]
were added into each well and incubation continued for 48 h. The
cells were washed with PBS twice and then collected, degraded and
centrifuged at 12,000 rpm for 20 min at 4°C. The supernatants were
collected as whole cell protein extracts. The bicinchoninic acid
assay was used to determine protein quantity. Samples were mixed
with 5X sodium dodecyl sulfate (SDS) loading buffer and denatured
for 10 min at 95°C. Equal amounts of extracted proteins were
separated on 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and transferred to polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked in 5%
non-fat dried milk in tris-buffered saline Tween-20 (TBST) buffer
(10 mmol/l Tris-HCL, 100 mmol/l NaCl and 0.1% Tween-20) and then
incubated with the primary antibody at room temperature. After
washing with the TBST buffer, the membranes were incubated with the
secondary antibody, and then washed with TBST again three times, 5
minutes per wash. Immunoblots were then visualized with Super ECL
Plus luminescence fluid (Applygen Technologies Inc., Beijing,
China). β-actin was the internal control. The densities of the
bands were scanned and calculated with Quantity One software
(Bio-Rad). The relative amounts of the specific protein expression
(MMP-9 and TIMP-1) were obtained by normalization to the densities
of β-actin.
Statistical analysis
All data were expressed as the means ± standard
deviation (SD). One-way analysis of variance (ANOVA) was used to
compare differences among groups. The SNK-q test was performed to
compare the differences between each two groups. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 13.0 software (SPSS
Inc., Chicago, IL, USA).
Results
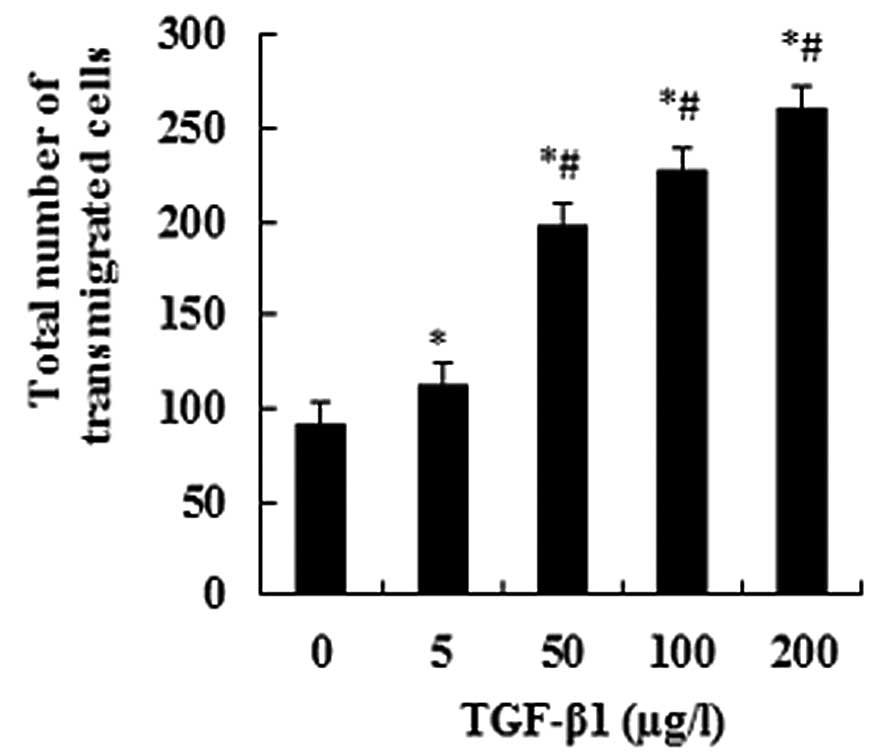
TGF-β1 promotes JEG-3 cell invasion
When JEG-3 cells were treated with TGF-β1 at a
series of concentrations (0, 5, 50, 100 and 200 μg/l), the
numbers of transmigrated cells significantly increased with an
increasing TGF-β1 concentration (P<0.05; Fig. 1). This suggests a dose-dependent
effect. The numbers of transmigrated cells displayed significant
differences between the experimental groups and the control group,
as well as between any other two groups (P<0.05; Fig. 1).
Impact of TGF-β1 on TβR I, TβR II and
Smad4 mRNA expression in the JEG-3 cell line
JEG-3 cells were separately treated by a series of
TGF-β1 concentrations (0, 5, 10, 25, 50, 100, and 200 μg/l).
It was revealed that with increasing TGF-β1 concentrations, the
mRNA expression levels for TβR I, TβR II and Smad4 gradually
reduced, suggesting a certain dose-dependence (Fig. 2A). The mRNA expression levels for
TβR I, TβR II and Smad4 showed a statistically significantly
difference (P<0.05) when compared with all other groups
(Fig. 2B, C and D).
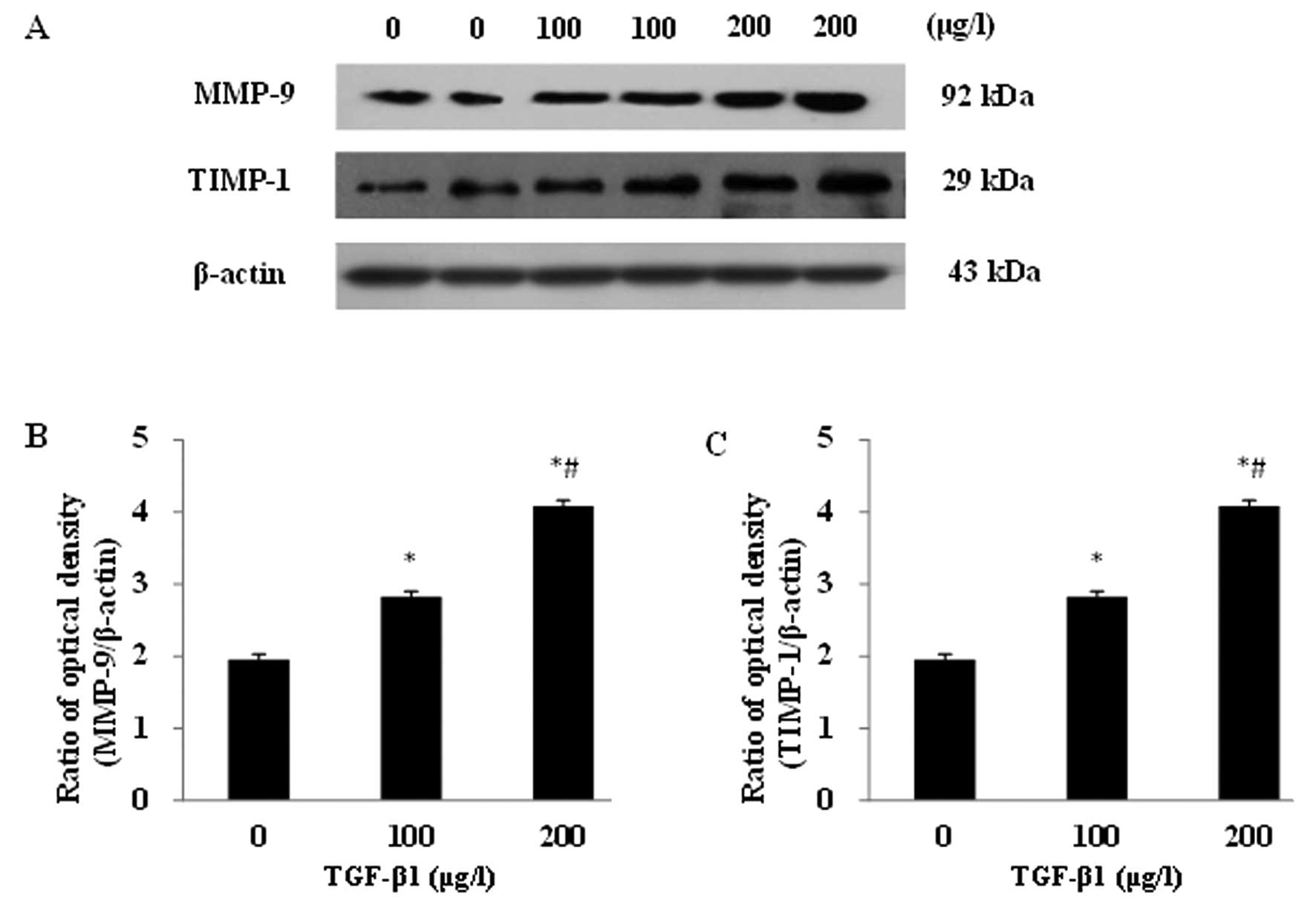
Impacts of TGF-β1 on MMP-9 and TIMP-1
protein expression in the JEG-3 cell line
After incubation with the different concentrations
of TGF-β1 (0, 100 and 200 μg/l) for 48 h, significantly
increased protein expression levels of MMP-9 and TIMP-1 were
detected in JEG-3 cells (Fig. 3A).
A dose-dependent response was observed for both proteins. The MMP-9
and TIMP-1 protein expressions displayed significant differences
between the experimental groups and the control group, as well as
between any other two groups (P<0.05; Fig. 3B and C).
Discussion
Human choriocarcinoma is the most aggressive
malignant tumor characterized by early hematogenous spread to lung
and brain tissues and can cause patients’ death. Choriocarcinoma
may occur following pregnancy and during implantation; however,
trophoblastic invasion in human pregnancy is tightly regulated.
TGF-β1 has been found to correlate closely with trophoblastic
invasion (22). The expression of
TGF-β1 can be detected in almost all types of tumors and exists at
an upregulated level in cancer cells, allowing it to inhibit normal
cell proliferation yet enhance the growth of tumor cells (23). During the early stage of
tumorigenesis, TGF-β1 may be an inhibitor of tumor cells through
the suppression of many cell functions; however, following the
development of malignance, it promotes tumor cell proliferation
(24). TGF-β1 exists within cells
as a non-active complex that must be activated in order to exert a
biological effect on its major downstream signaling molecule, Smad
(25). The signaling pathway begins
with TGF-β1 ligands, which bind to a type II receptor, and
stimulate the Smad protein to act as a transcription factor and
participate in the regulation of target gene expression. If
mutations in multiple genes occur in the TGF-β1 signaling pathway,
it can lead to TGF-β1 tolerance and loss of function in senescence
and apoptosis pathways. This may result in the induction of
malignant transformation by TGF-β1. Our previous studies (16,17)
revealed that the choriocarcinoma JEG-3 cell line responds to the
stimulation of TGF-β1 and may promote JEG-3 cell proliferation;
however, the effect of TGF-β1 on invasion and the more exact
mechanisms remain a controversial issue. In this study, we found a
significantly higher function of TGF-β1 in promoting invasion in
the JEG-3 cell line. These findings suggest that TGF-β1 may
participate in the development of choriocarcinoma. Our observations
concur with the role of TGF-β1 in other malignancies (26,27).
We also found that mRNA expression was detectable
for TβR I, TβR II and Smad4 in the choriocarcinoma JEG-3 cell line.
With increasing concentrations of exogenous TGF-β1, the expressions
of TβR I, TβR II and Smad4 were gradually reduced. This suggests
that the TGF-β1/Smad4 transduction system may participate in the
initiation process and promote the proliferation and invasion of
choriocarcinoma. This function may be correlated with the decreased
expression of TβR I, TβR II and common-mediator Smads (Smad4),
which limit the downward propagation of the TGF-β1/Smad4 signal and
subsequently cause tumor growth inhibition to reduce or even become
deficient. Certain animal experiments and clinical studies
(28–31) have also revealed that, within the
tumor, several types of mutations in the TβR II and Smad4 genes
have been identified. The loss of TβR II function may cause tumor
cells to deregulate the expression of the receptor protein,
obstruct the transmission of the TGF-β1 growth inhibiting signal
(32) and subsequently display
malignant proliferation and invasion. Moreover, TGF-β1 could also
play distinct roles in the Smad4-dependent and -independent
signaling pathways (33).
Prior to invasion or metastasis, tumor cells must
destroy the adjacent tissues and cells, degrade the basement
membrane and then infiltrate the periphery mesenchyme. TGF-β1 may
strengthen the capabilities of invasiveness and metastasis through
inducing the aberrant tumor microenvironment, participating in the
degeneration and rebuilding of the microenvironment and enhancing
the production of proteinase, such as MMPs and urokinase (34,35).
MMP-9 has the largest molecular mass of all the MMP family members
and TIMP-1 is a specific inhibitor of MMP-9. They are involved in
the degradation of the extracellular matrix (ECM) and play a role
in the invasion and metastasis of tumor cells (3,4).
Previous studies (36–38) have suggested that disruption to the
ECM during tumor progression is due to an imbalance in the MMP/TIMP
ratio. Our study revealed that a significant upregulation of MMP-9
and TIMP-1 protein expression in JEG-3 cells was observed following
treatment with TGF-β1 at concentrations of 100 and 200 μg/l.
These results were consistent with those of Yudate et al
(39). However, the ratio of
MMP-9/TIMP-1 was still higher than 1, which implied that the
balance of TIMP-1 inhibiting MMP-9 was disturbed, and resulted in
the high invasiveness of choriocarcinoma. By integrating these
results with our previous findings (17), we believe that the downregulation in
the expression of TβR I, TβR II and common mediator Smads may limit
the downward propagation of the TGF-β1/Smad4 signal, and promote
the tumor’s invasive capability. Simultaneously, the increasing
secretion of invasion-related factor MMP-9 may enhance the
degradation of the ECM and also promote the invasiveness of tumor
cells.
In summary, although some previous studies have
demonstrated the critical role of TGF-β1 in the invasiveness and
metastasis of placental choriocarcinoma, the detailed biological
mechanisms are still unclear. Our data indicate that the role of
TGF-β1 in promoting invasion and metastasis may be mediated through
TβR I, TβR II, Smads-dependent pathways and the regulation of MMP-9
and TIMP-1. Following the binding of TGF-β1 to the cell membrane
type II receptor, it activates the type I receptor and stimulates
the downstream molecules, Smad2/Smad3, to bind to Smad4 forming a
complex, which subsequently participates in regulating target gene
transcription mediated by TGF-β1 in the nucleus. Based on the
significance of TGF-β1 in tumorigenesis and metastasis, further
clarification of the mechanisms of TGF-β1 in promoting tumor
metastasis will have crucial theoretical and clinical
significance.
References
|
1.
|
leM ShihKT KuoPower of the eternal youth:
Nanog expression in the gestational choriocarcinomaAm J
Pathol173911914200810.2353/ajpath.2008.08062418755845
|
|
2.
|
DR GenestRS BerkowitzRA FisherGestational
trophoblastic diseaseWorld Health Organization Classification of
Tumours. Pathology and Genetics Tumours of the Breast and Female
Genital OrgansFA TavassoliP DevileeIARC PressLyon2502542003
|
|
3.
|
J DecockS ThirkettleL WagstaffDR
EdwardsMatrix metalloproteinases: protective roles in cancerJ Cell
Mol Med1512541265201110.1111/j.1582-4934.2011.01302.x21418514
|
|
4.
|
D BourbouliaWG Stetler-StevensonMatrix
metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesionSemin Cancer
Biol20161168201010.1016/j.semcancer.2010.05.00220470890
|
|
5.
|
NE van TrommelC LybolCM ThomasFC SweepLF
MassugerDiagnosis and treatment of gestational trophoblastic
diseaseEuropean Obstetrics & Gynaecology62832201110975558
|
|
6.
|
E PardaliP Ten DijkeTGFβ signaling and
cardiovascular diseasesInt J Biol Sci81952132012
|
|
7.
|
CH HeldinK MiyazonoP ten DijkeTGF-β
signalling from cell membrance to nucleus through SMAD
proteinsNature3904654711997
|
|
8.
|
CH HeldinA MoustakesRole of Smads in TGFβ
signalingCell Tissue Res34721362012
|
|
9.
|
M HyytiäinenC PentinenJ Keski-OjaLatent
TGF-beta binding proteins: extracellular matrix association and
roles in TGF-beta activationCrit Rev Clin Lab
Sci41233264200415307633
|
|
10.
|
P ten DijkeHM ArthurExtracellular control
of TGFβ signalling in vascular development and diseaseNat Rev Mol
Cell Biol88578692007
|
|
11.
|
B VogelsteinKW KinzlerCancer genes and the
pathways they controlNat Med10789799200410.1038/nm108715286780
|
|
12.
|
N BardeesyRA DepinhoPancreatic cancer
biology and geneticsNature Rev Cancer2897909200210.1038/nrc949
|
|
13.
|
SH LeeYS JungJY ChungNovel tumor
suppressive function of Smad4 in serum starvation-induced cell
death through PAK1-PUMA pathwayCell Death
Dis2e235201110.1038/cddis.2011.116
|
|
14.
|
WM GradyTransforming growth factor-beta,
Smads, and cancerClin Cancer
Res1131513154200510.1158/1078-0432.CCR-05-041415867206
|
|
15.
|
A MoustakasS SouchelnytskyiCH HeldinSmad
regulation in TGF-beta signal transductionJ Cell
Sci11443594369200111792802
|
|
16.
|
X LiY LiQ XuS ZhaoL ZhaoEffect of
transforming growth factor β1 on proliferation of JEG-3
choriocarcinoma cells and Smad3, 7 mRNA expressionMaternal &
Child Health Care of China25168516882010
|
|
17.
|
X LiY LiQ XuL ZhaoS ZhaoL ZhaoEffect on
TGF-β1 on MMP-9 mRNA and TIMP-1 mRNA in choriocarcinoma JEG-3
cellShandong Medical Journal4918202009
|
|
18.
|
Z ZhangQ XuC ShiY LiInterleukin-12
inhibits cell invasion in choriocarcinomaInt J Mol
Med305762201222485248
|
|
19.
|
AG BeristainH ZhuPC LeungRegulated
expression of ADAMTS-12 in human trophoblastic cells: a role for
ADAMTS-12 in epithelical cell invasion?PLoS
One6e18473201110.1371/journal.pone.001847321494557
|
|
20.
|
GL NicolsonTransfilter cell invasion
assaysCell Biology: A Laboratory HandbookJE Celis1Academic PressNew
York359362200610.1016/B978-012164730-8/50044-7
|
|
21.
|
T SuzukiPJ HigginsDR CrawfordControl
selection for RNA quantitationBiotechniques293323372000
|
|
22.
|
S RamaY SureshAJ RaoTGF beta1 induces
multiple independent signals to regulate human trophoblastic
differentiation: mechanistic insightsMol Cell
Endocrinol206123136200310.1016/S0303-7207(03)00202-8
|
|
23.
|
GJ Prud’hommePathobiology of transforming
growth factor beta in cancer, fibrosis and immunologic disease, and
therapeutic considerationsLab Invest8710771091200717724448
|
|
24.
|
A JoshiD CaoTGF-beta signaling, tumor
microenvioronment and tumor progression: the butterfly effectFront
Biosci15180194201010.2741/361420036814
|
|
25.
|
D SamantaPK DattaAlterations in the Smad
pathway in human cancersFront
Biosci1712811293201210.2741/398622201803
|
|
26.
|
M DaviesSS PrimeJW EvesonN PriceA
GanapathyA D’MelloIC PatersonTransforming growth factor-β enhances
invasion and metastasis in Ras-transfected human malignant
epidermal keratinocytesInt J Exp Pathol931481562012
|
|
27.
|
D ShangY LiuP YangY ChenY
TianTGFBI-promoted adhesion, migration and invasion of human renal
cell carcinoma depends on inactivation of von Hippel-Lindau tumor
suppressorUrology79966.e17201210.1016/j.urology.2011.12.011
|
|
28.
|
B ZhangSK HalderND KashikarYJ ChoA DattaDL
GordenPK DattaAntimetastatic role of Smad4 signaling in colorectal
cancerGastroenterology138969980201010.1053/j.gastro.2009.11.00419909744
|
|
29.
|
S BornsteinR WhiteS MalkoskiSmad4 loss in
mice causes spontaneous head and neck cancer with increased genomic
instability and inflammationJ Clin
Invest11934083419200919841536
|
|
30.
|
D RomeroM IqlesiasCP VaryM
QuintanillaFunctional blockade of Smad4 leads to a decrease in
beta-catenin levels and signaling activity in human pancreatic
carcinoma
cellsCarcinogenesis2910701076200810.1093/carcin/bgn05418310088
|
|
31.
|
B LuYN ZhouQ LiCorrelations of TGF-β RII,
Smad4 and Smad7 expression to clinicopathologic characteristics and
prognosis of gastric cancerChinese Journal of Cancer25385422009
|
|
32.
|
K HorieH YamashitaA MogiS TakenoshitaK
MiyazonoLack of transforming growth factor-beta type II receptor
expression in human retinoblastoma cellsJ Cell
Physiol175305313199810.1002/(SICI)1097-4652(199806)175:3%3C305::AID-JCP8%3E3.0.CO;2-S9572475
|
|
33.
|
X WangW SunC ZhangG JiY GeY XuY ZhaoTGF-β1
inhibits the growth and metastasis of tongue squamous carcinoma
cells through Smad4Gene4851601662011
|
|
34.
|
MG BinkerAA Binker-CosenHY GaisanoRH de
CosenLI Cosen-BinkerTGF-β1 increases invasiveness of SW1990 cells
through Rac1/ROS/NF-κB/IL-6/MMP-2Biochem Biophys Res
Commun4051401452011
|
|
35.
|
J BaoZS WuY QiQ WuF YangExpression of
TGF-beta1 and the mechanism of invasiveness and metastasis induced
by TGF-beta1 in breast cancerZhonghua Zhong Liu Za
Zhi31679682200920021864
|
|
36.
|
RD SinghN HaridasJB PatelFD ShahSN
ShuklaPM ShahPS PatelMatrixmetallo-proteinases and their
inhibitors: correlation with invasion and metastasis in oral
cancerIndia J Clin
Biochem25250259201010.1007/s12291-010-0060-821731196
|
|
37.
|
H KangSW JangJ KoHuman sLZIP induces
migration and invasion of cervical cancer cells via expression of
matrix metalloproteinase-9J Biol
Chem2864207242081201110.1074/jbc.M111.27230222009750
|
|
38.
|
K ZarrabiA DufourJ LiInhibition of matrix
metalloproteinase 14 (MMP-14)-mediated cancer cell migrationJ Biol
Chem2863316733177201110.1074/jbc.M111.25664421795678
|
|
39.
|
T YudateK IsakaY KosugiM KoshiishiK
ShiraishiM HosakaM TakayamaAnalysis of the mechanism of trophoblast
infiltrationNihon Sanka Fujinka Gakkai
Zasshi4819119819968721053
|