Introduction
Biliary tract cancers (BTCs) include carcinomas of
the gallbladder and intra- and extra-hepatic bile ducts
(cholangiocarcinoma). Gallbladder cancer is the most common type of
BTC, and it affects females more frequently than males. Gallbladder
cancer is also considered to be the most aggressive type of BTC,
with the shortest survival (1).
Most BTCs are detected at an advanced, incurable stage, and are
typically treated with chemotherapy drugs such as 5-FU, gemcitabine
and cisplatin, often in combination. Response rates range from 20
to 40%, with a median overall survival of 8 to 14 months (1). The most notable advance in the
treatment of BTC is the result of a phase III randomized trial of
gemcitabine alone versus gemcitabine plus cisplatin; the
chemotherapy doublet improved overall survival by 3.6 months
(2). However, the median overall
survival with gemcitabine plus cisplatin remains less than 1 year
(11.7 months). In fact, there is no appropriate drug for third-line
chemotherapy following gemcitabine (plus cisplatin) and 5-FU for
BTC.
Paclitaxel is an anticancer agent (3) that acts by stabilizing polymerized
microtubules and enhancing microtubule assembly, which arrests the
cell cycle in the G0/S1 and G2/M phases and leads to cell death
(4,5). Recent studies have shown that low-dose
paclitaxel ameliorates tissue fibrosis by inhibiting TGF-β/Smad
activity (6,7). Therefore, paclitaxel may be useful for
treating BTC, a cancer that is associated with tissue fibrosis.
The present study documents the individual response
of a patient with gallbladder cancer with multiple liver metastasis
and stenosis of the bile duct who was treated with low-dose
paclitaxel as palliative chemotherapy following gemcitabine and S-1
(oral prodrug of 5-FU).
Case report
A 56-year-old woman in good health was admitted to
our hospital with right upper quadrant pain. Physical examination
was notable for right upper quadrant tenderness and hepatomegaly.
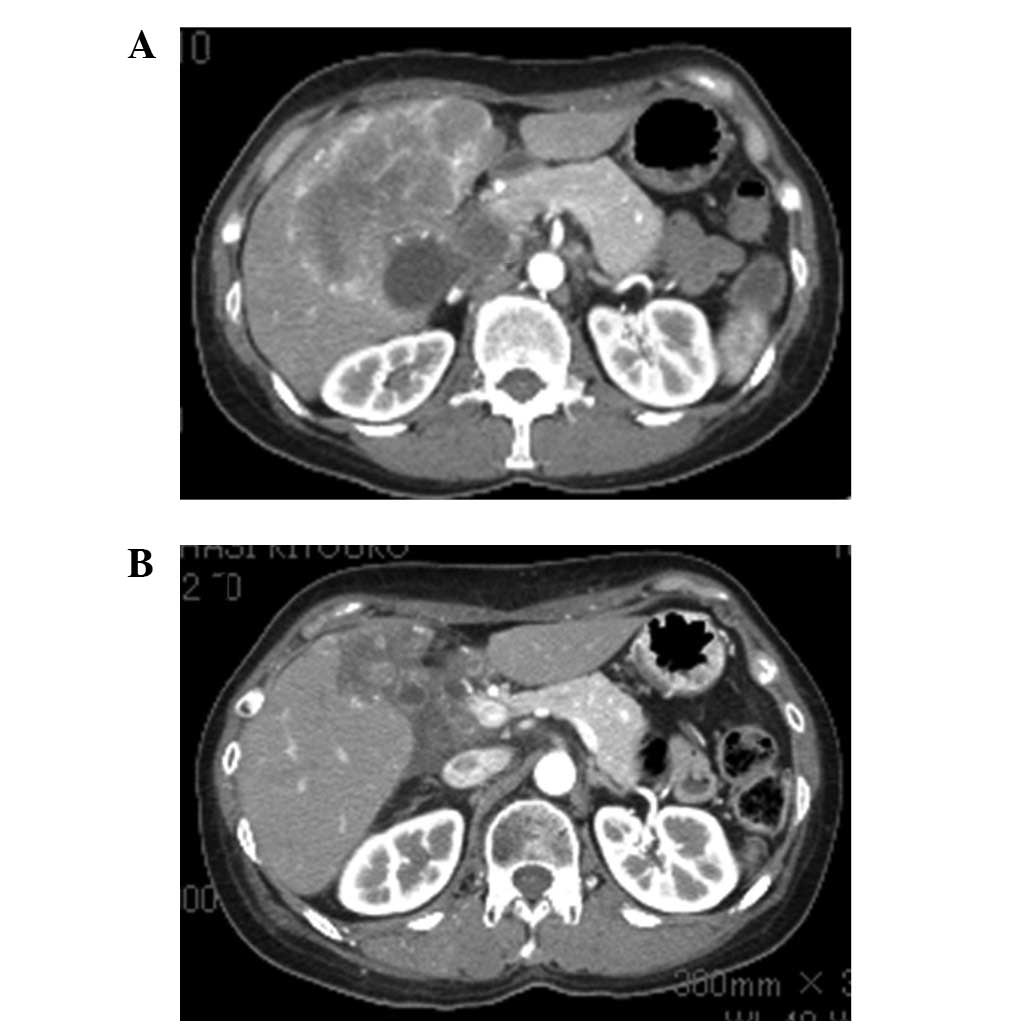
CT of the abdomen revealed multiple hepatic nodules, a thick
gallbladder wall and dilation of the intrahepatic bile duct
(Fig. 1A). CEA was 14.3 IU/ml
(upper limit of normal, 5.0 IU/ml) and CA19-9 was 58 IU/ml (upper
limit of normal, 37 IU/ml). The patient was diagnosed with
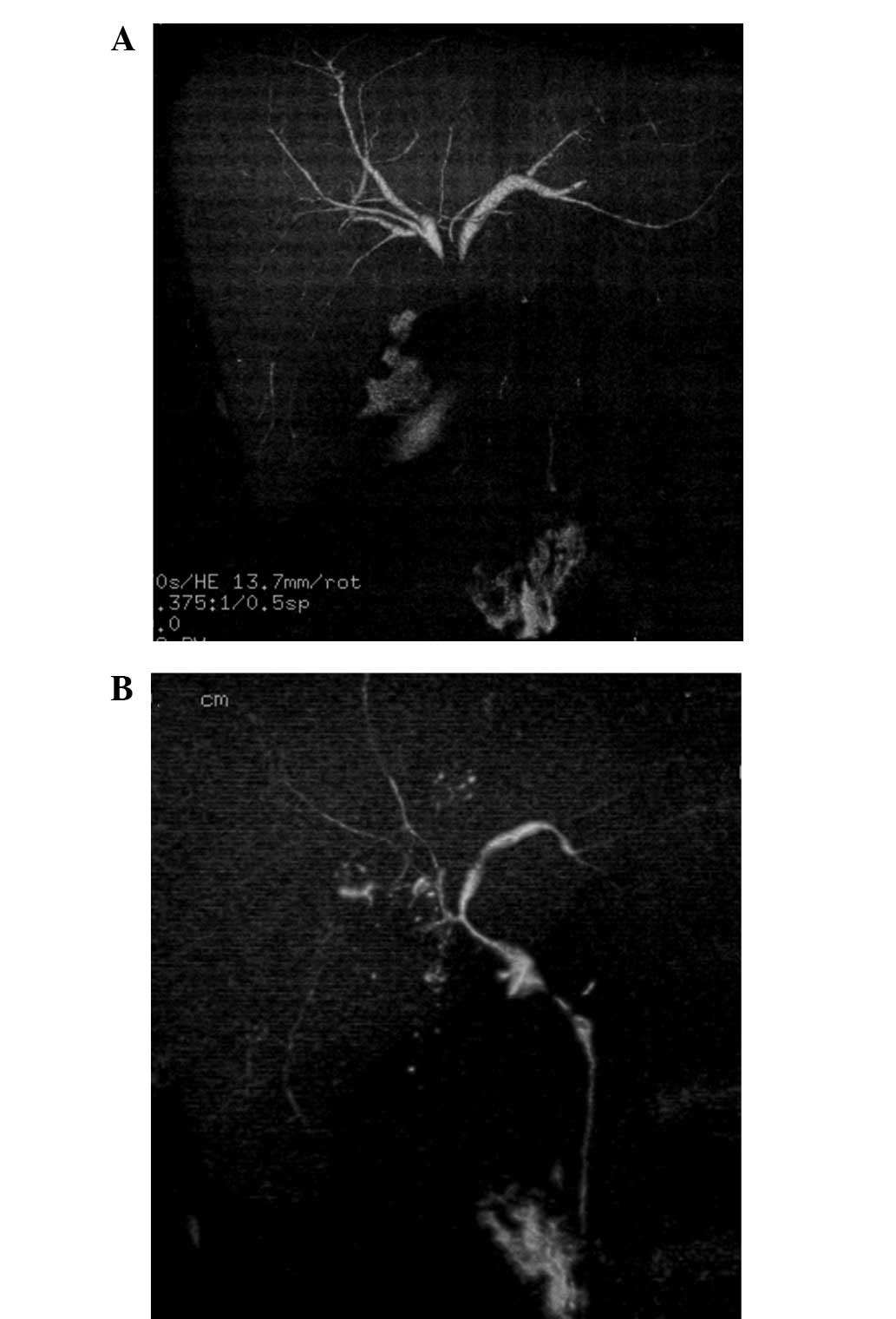
unresectable gallbladder cancer with multiple liver metastasis and
local infiltration to the liver and hilar bile duct (Fig. 2A).
The patient was treated with oral S-1 (60
mg/m2) twice daily for 14 days plus gemcitabine (800
mg/m2) administered on days 8 and 14 [every 21 days as
previously described for pancreatic carcinoma (8)]. After 4 cycles of therapy, the doses
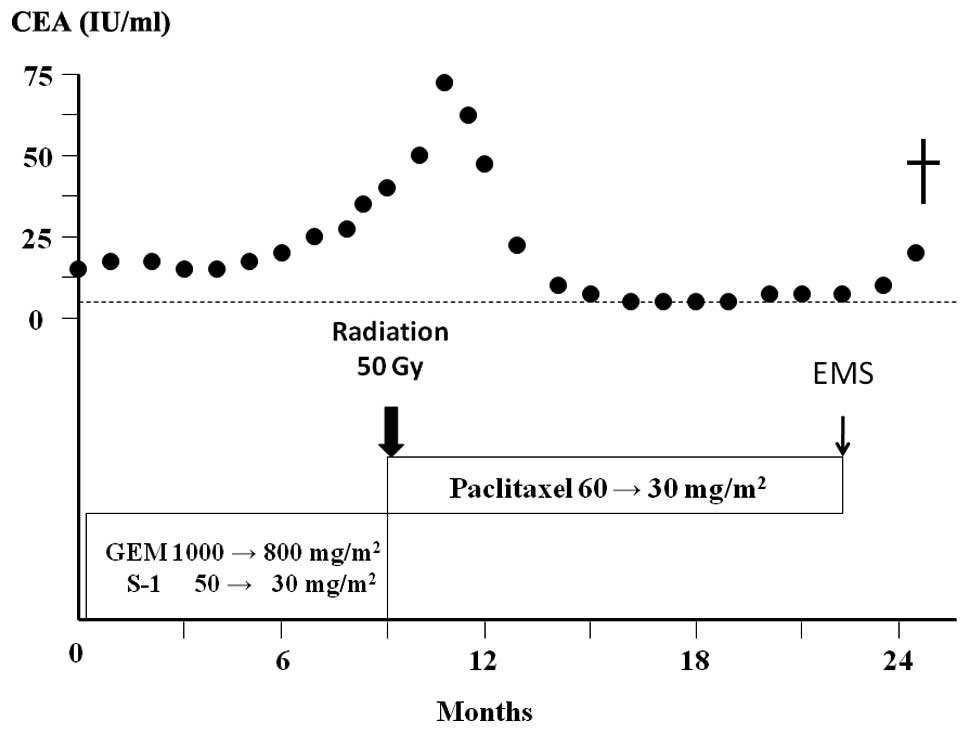
were reduced as the patient developed neutropenia. After 9 cycles
of therapy, CT showed evidence of stable disease; however, the
serum CEA level was increased (Fig.
3). Therefore, the chemotherapy regimen was changed to weekly
paclitaxel (60 mg/m2). After 12 cycles of therapy,
paclitaxel was reduced to 30 mg/m2 due to neutropenia.
The patient completed 32 cycles of paclitaxel therapy and CT showed
evidence of a partial response (Fig.
1B) and stenosis of the hilar bile duct was improved (Fig. 2B). At the beginning of paclitaxel
therapy, irradiation of the stenotic hilar bile duct was performed.
The toxicities evaluated were leucopenia (grade 2), general malaise
(grade 1) and shedding of hair; however, no serious adverse events
were observed. Therefore, the patient was treated at the outpatient
clinic, without any impairment in the quality of life (QOL).
Written informed consent was obtained from the
patient, and the treatment was undertaken with the approval of the
Local Ethics Committee of Kanazawa University Hospital.
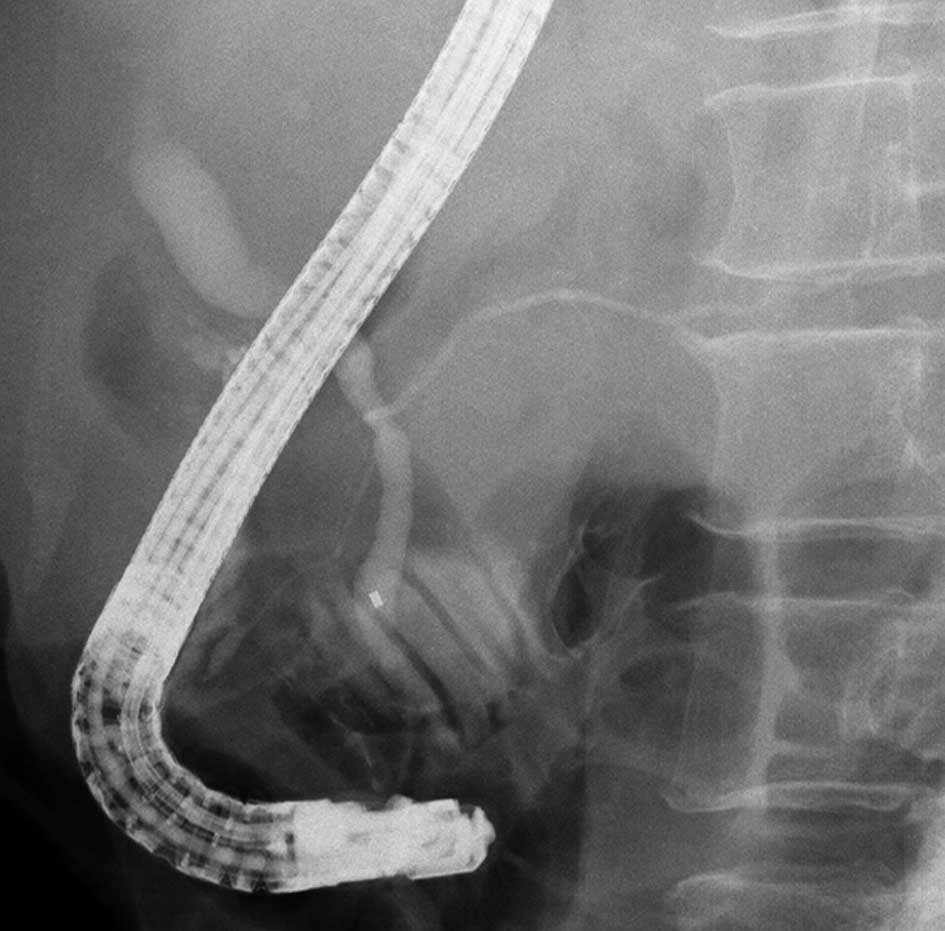
Endoscopic biliary drainage and expandable metallic
stent placement was performed for obstructive jaundice due to lymph
node metastasis 23 months after beginning treatment. The patient
was then diagnosed with pancreaticobiliary maljunction (Fig. 4). The patient succumbed to the
disease due to the progress of cachexia 25 months after the first
visit. An autopsy was performed, and the patient was diagnosed with
locally advanced, poorly differentiated adenocarcinoma of the
gallbladder with multiple liver, lung, lymph node, left ovary and
skin metastases.
Discussion
The prognosis of patients with unresectable BTC is
extremely poor. No standard chemotherapy was established for
patients with unresectable BTC until the study of cisplatin plus
gemcitabine by Valle et al (2). However, the median overall survival of
patients treated with cisplatin plus gemcitabine was only 11.7
months. 5-FU is a major drug used in the treatment of
hepatobiliary-pancreatic cancers; however, phase II studies using
combinations primarily based on 5-FU regimens have revealed little
or no benefit on survival and QOL (9,10). S-1
is an oral prodrug of 5-FU widely used in Japan (8). In a phase III trial (the GEST trial)
announced by the American Society of Clinical Oncology in 2011, S-1
produced a favorable response and was not inferior to gemcitabine
in terms of the overall survival of patients with unresectable
pancreatic cancer (11). However,
there is no appropriate drug for third-line chemotherapy following
gemcitabine (plus cisplatin) and 5-FU for BTC.
Paclitaxel is a natural substance isolated from the
western yew, Taxus brevifolia. Similar to the vinca
alkaloids, it binds to microtubules. However, while the vinca
alkaloids promote microtubule dissociation and disruption of the
mitotic spindle, paclitaxel promotes microtubule formation and
stabilization. There have been some retrospective studies and phase
I and II studies of paclitaxel and docetaxel (taxanes) for
pancreatic cancer and BTC (12–17).
The disease control rates in these studies were 33 to 57% when used
as first-line chemotherapy.
Recently, paclitaxel has been receiving attention
for its effects other than antitumor activity; for example, its use
in drug-eluting stents in coronary arteries (18). Moreover, it has been reported that
paclitaxel ameliorates fibrosis in hepatic stellate cells and renal
fibrosis through the inhibition of TGF-β/Smad activity (6,7).
Paclitaxel also interrupts TGF-β1 signaling between gallbladder
epithelial cells and myofibroblasts (19). Taghian et al reported that
paclitaxel decreases interstitial fluid pressure and improves
oxygenation in breast cancer tissues in patients treated with
neoadjuvant chemotherapy and that patients with hypoxic tumors
and/or tumors with high interstitial fluid pressure may start with
paclitaxel chemotherapy to improve their physiological status
(20). Pancreatic cancer and BTC
are well-known hypoxic tumors associated with fibrosis. Therefore,
taxanes may be effective in the treatment of pancreatic cancer and
BTC.
Certain studies have also documented taxane
chemotherapy in patients with gemcitabine-refractory pancreatic
cancer (21,22). Cancer cells experience various forms
of stress from anticancer drugs, irradiation, hypo-oxygenation,
hypo-nutrition and heat treatment, and these stresses induce
epithelial-to-mesenchymal transition (EMT), which is associated
with the invasive potential of cancer cells (23). The inhibitory effect of paclitaxel
on TGF-β/Smad activity is involved in the suppression of EMT in
epithelial cells.
In the present case, paclitaxel was more effective
than gemcitabine and S-1 for unresectable gallbladder cancer.
Furthermore, paclitaxel has fewer side-effects; therefore, it
should be suitable as a palliative chemotherapy for BTC as has been
reported for breast cancer (24).
In conclusion, palliative chemotherapy with paclitaxel after
failure of gemcitabine and 5-FU is well-tolerated and may be
effective for BTC. However, the appropriate dose of these
anticancer drugs should be determined in a future study. A phase I
study of palliative chemotherapy with weekly low-dose paclitaxel
following gemcitabine (plus cisplatin) and 5-FU is currently in
progress in patients with unresectable or recurrent BTC.
References
|
1.
|
AX XiuTS HongAF HezelDA KoobyCurrent
management of gallbladder
carcinomaOncologist15168181201010.1634/theoncologist.2009-0302
|
|
2.
|
JW ValleH WasanDH PalmerABC-02 Trial
Investigators: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancerN Engl J
Med36212731281201010.1056/NEJMoa090872120375404
|
|
3.
|
K GelmonThe toxoids: paclitaxel and
docetaxelLancet34412671272199410.1016/S0140-6736(94)90754-47967989
|
|
4.
|
KL DonaldsonGL GoolsbyPA KienerAF
WahlActivation of p34cdc2 coincident with taxol-induced
apoptosisCell Growth Differ51041105019947848905
|
|
5.
|
PB SchiffJ FantSB HorwitzPromotion of
microtubule assembly in vitro by
taxolNature277665667197910.1038/277665a0423966
|
|
6.
|
D ZhangL SunW XianF LiuLow-dose paclitaxel
ameliorates renal fibrosis in rat UUO model by inhibition of
TGF-beta/Smad activityLab
Invest90436447201010.1038/labinvest.2009.14920142807
|
|
7.
|
J ZhouDW ZhongQW WangXY MiaoXD
XuPaclitaxel ameliorates fibrosis in hepatic stellate cells via
inhibition of TGF-beta/Smad activityWorld J
Gastroenterol1633303334201010.3748/wjg.v16.i26.333020614491
|
|
8.
|
H TajimaT OhtaH KitagawaPilot study of
neoadjuvant chemotherapy with gemcitabine and oral S-1 for
resectable pancreatic cancerExp Therap Med3787792201222969969
|
|
9.
|
PA EllisA NormanA HillME O’BrienM
NicolsonT HickishD CunninghamEpirubicin, cisplatin and infusional
5-fluorouracil (5-FU) (ECF) in hepatobiliary tumorsEur J
Cancer31A15941598199510.1016/0959-8049(95)00323-B7488407
|
|
10.
|
YZ PattDV Jones JrA HoqueR LozanoA
MarkowitzI RaijmanP LynchC CharnsangavejPhase II trial of
intravenous fluorouracil and subcutaneous interferon alfa-2b for
biliary tract cancerJ Clin Oncol142311231519968708722
|
|
11.
|
T IokaM IkedaS OhkawaRandomized phase III
study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine
(GEM) in unresectable advanced pancreatic cancer (PC) in Japan and
Taiwan: GEST studyJ Clin Oncol29Supplabstr 40072011
|
|
12.
|
P PapakostasC KouroussisN
AndroulakisFirst-line chemotherapy with docetaxel for unresectable
or metastatic carcinoma of the biliary tract. A multicentre phase
II studyEuro J
Cancer3718331838200110.1016/S0959-8049(01)00214-311576836
|
|
13.
|
S OkadaY SakataS MatsunoPhase II study of
docetaxel in patients with metastatic pancreatic cancer: a Japanese
cooperative study. Cooperative Group of Docetaxel for Pancreatic
Cancer in JapanBr J
Cancer80438443199910.1038/sj.bjc.669037510408850
|
|
14.
|
DP RyanMH KulkeCS FuchsA Phase II study of
gemcitabine and docetaxel in patients with metastatic pancreatic
carcinomaCancer9497103200210.1002/cncr.1020211815964
|
|
15.
|
DV Jones JrR LozanoA HoqueA MarkowitzYZ
PattPhase II study of paclitaxel therapy for unresectable biliary
tree carcinomasJ Clin Oncol142306231019968708721
|
|
16.
|
S MaedaF MotoiT OnogawaPaclitaxel as
second-line chemotherapy in patients with gemcitabine-refractory
pancreatic cancer: a retrospective studyInt J Clin
Oncol16539545201110.1007/s10147-011-0220-821455624
|
|
17.
|
DD Von HoffRK RamanthanMJ BoradGemcitabine
plus nab-paclitaxel is an active regimen in patients with advanced
pancreatic cancer: a phase I/II trialJ Clin
Oncol2945484554201121969517
|
|
18.
|
I BaekCZ BaiJ HwangHY NamJS ParkDJ
KimPaclitaxel coating of the luminal surface of hemodialysis grafts
with effective suppression of neointimal hyperplasiaJ Vasc
Surg55806814201210.1016/j.jvs.2011.09.01222226184
|
|
19.
|
HS ChoiCE SavardJW ChoiR KuverSP
LeePaclitaxel interrupts TGF-beta1 signaling between gallbladder
epithelial cells and myofibroblastsJ Surg
Res141183191200710.1016/j.jss.2006.12.55817574589
|
|
20.
|
AG TaghianR Abi-RaadSI AssaadPaclitaxel
decreases the interstitial fluid pressure and improves oxygenation
in breast cancer in patients treated with neoadjuvant chemotherapy:
clinical implicationsJ Clin
Oncol2319511961200510.1200/JCO.2005.08.119
|
|
21.
|
T ShukuyaH YasuiN Bokupaclitaxel after
failure of gemcitabine in pancreatic cancer patients with malignant
ascites: a retrospective studyJpn J Clin
Oncol4011351138201010.1093/jjco/hyq11720656694
|
|
22.
|
S CeredaM ReniWeekly docetaxel as salvage
therapy in patients with gemcitabine-refractory metastatic
pancreatic cancerJ
Chemother20509512200810.1179/joc.2008.20.4.50918676234
|
|
23.
|
H TajimaT OhtaY ShojiExpression of
epithelial-mesenchymal transition markers in locally recurrent
hepatocellular carcinoma after radio frequency ablationExp Therap
Med1347350201010.3892/etm_00000054
|
|
24.
|
JG SchramaMM de BoerJW BaarsJH SchornagelS
RodenhuisPalliative chemotherapy after failure of high-dose
chemotherapy in breast cancer - toxicity and efficacyAnticancer
Res2327952800200312926115
|