Introduction
The Epstein-Barr virus (EBV) belongs to the herpes
virus family and infects more than 90% of the human population
(1). It is associated with the
development of lymphoid tumors and probably also with epithelial
neoplasms. EBV colonizes B cells and can persist as a life-long
asymptomatic infection. It has been implicated as the causative
agent in a number of lymphoproliferative diseases, including
Burkitt’s lymphoma, Hodgkin’s lymphoma, plasmablastic lymphoma,
certain diffuse large B-cell lymphomas (DLBCL) and sub-types of
peripheral T-cell lymphomas, including natural killer/T-cell
lymphomas, angioimmunoblastic and lymphomatoid granulomatosis
(2–4). EBV has also been associated with
immunodeficient states such as post-transplant lymphoproliferative
disorders (LPDs) and human immunodeficiency virus (HIV)/acquired
immunodeficiency syndrome (AIDS)-associated lymphomas (5,6).
‘EBV-positive diffuse large B-cell lymphoma of the elderly’ has
been recognized as a provisional entity of DLBCL. This association
was initially described by Oyama et al in patients
predominantly older than 50; however, it has also been documented
in younger patients without immunodeficiency (7).
There is presently substantial evidence supporting
the etiological role of EBV in cancer. In vitro, the virus
transforms resting B cells into a lymphoblastoid cell line; in
vivo, EBV-infected cells do not mature to become a resting
memory cell as they are unable to exit the cell cycle. When T-cell
function that usually plays a determinant role in controlling
EBV-associated lymphoproliferative disease is blocked by
immunosuppressive agents or by HIV infection, there is an increased
risk of EBV-positive B-cell LPDs (2,6).
EBV is most frequently detected by
immunohistochemistry using antibodies to viral proteins expressed
in the cytoplasm and cellular membrane, namely latent membrane
protein (LMP) and EB nuclear antigen (EBNA) (7).
However, the standard procedure of EBV diagnosis has
been EBV-encoded RNA (EBER) detection by RNA in situ
hybridization (RNA-ISH) in tumor tissue. EBER is consistently and
highly expressed in latent infection and its detection has a high
diagnostic sensitivity for EBV-positive lymphomas (8). PCR-based techniques of genomic
isolates obtained from tumor tissue substitutes RNA-ISH with
similar sensitivity and specificity and are also useful for strain
determination in EBV types 1 or 2 (9).
Few studies have investigated EBV tumoral detection
and prognosis in DLBCL. Three published studies, from Japanese,
Peruvian and Korean populations with patient survival analyses,
demonstrated the prognostic value of EBV expression using various
methods. Generally, EBV-positive lymphomas are associated with a
worse overall survival rate (OS) and are independent of
international prognostic index (IPI) scores (7,10,11).
Another study, comparing the expression of LMP-1 with various
biological parameters in patients predominantly treated with
conventional chemotherapy without rituximab, revealed that viral
protein is the most potent prognostic factor associated with a poor
survival rate (12).
The influence of EBV positivity on the treatment
response of patients with DLBCL has also been evaluated. Studies
with nodal lymphomas associate the detection of EBV with a worse
overall response rate, and one study of extranodal lymphomas
revealed that 3/4 cases of gastric DLBCL patients had poor
responses (7,11,13).
Data concerning the influence of EBV presence in the prognosis and
response to treatment of patients with DLBCL are insufficient or
incompletely explored and have not been evaluated regarding immune
therapy with monoclonal antibodies.
However, it is possible to detect the EBV genome in
the peripheral blood of healthy seropositive individuals, although
it is usually present in extremely rare latently infected memory B
cells. By contrast, the viral load of mononuclear blood cells of
EBV-related lymphoproliferative diseases may be high (14,15).
The incidence of EBV in DLBCL varies across the world, with Asian,
Latin American and Western patients’ positivity between 5 and 15%.
In Portugal, the incidence of EBV detection among non-Hodgkin’s
lymphoma (NHL) patients or in the general population is not
known.
The detection of EBV proteins or genes is usually
performed on tumoral lymphoma tissue and this determines the
clinical prognostic impact. The search for and detection of the EBV
genome in the blood or bone marrow mononuclear cells of patients
with DLBCL and its correlation with prognosis has not been
previously reported. The presence of EBV in these cells may be
interpreted as circulating colonized tumoral cells or as colonized
physiological memory B cells.
The present study sought to investigate the presence
of the EBV genome in the blood or bone marrow mononuclear cells of
patients with DLBCL, some treated with anthracycline-based
chemotherapy alone, others treated with same type of chemotherapy
plus rituximab. The prognosis and response according to the
patient’s type of treatment was also evaluated.
Patients and methods
Case selection and patients samples
A total of 130 patients aged >18 years old and
diagnosed with DLBCL in the oncological services of the Portuguese
public health system of the cities of Porto and Braga were
included, regardless of their serological positivity for EBV.
Patients with DLBCL were selected from a pool of 350 with various
NHL histological subtypes, sequentially diagnosed and that had had
bone marrow and/or blood collected during staging procedures. The
Hospital Ethics Committees approved the study and all patients
provided written informed consent.
Histological diagnosis was obtained according to the
OMS classification (16). Samples,
including blood and/or bone marrow aspirates, were collected from
all patients. In 121 patients, both blood and bone marrow were
collected, in 4 only bone marrow was obtained and in 5 others, only
blood was collected. Bone marrow was preferentially used in this
study. Mononuclear cells from blood or bone marrow were isolated by
centrifugation on Ficoll-Hipac and obtained from the cell-enriched
fluid retained in the interface.
Registries of clinical data included demographic
variables, Ann Arbor staging system, ECOG Performance Status scale,
IPI type of chemotherapy and response to treatment.
DNA preparation
Genetic material was obtained from isolated
mononuclear cells by the following method. DNA was extracted using
a QIAamp DNA Mini kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. Purified DNA was eluted in 70
μl of buffer AE for elution and stored at −20°C.
Virus DNA detection
The identification of EBV DNA was performed by the
real-time quantitative PCR method, based on a continuous monitoring
of fluorescence by an optical system (17). The probe was labeled by two
fluorescent dyes. One served as a reporter on the 50 end (VIC dye;
Applied Biosystems, Foster City, CA, USA). The emission spectrum of
the dye was quenched by a second fluorescent dye at the 30 end
(TAMRA; Applied Biosystems). The primer and probe sequences were
selected from BALF5 gene codifying the DNA polymerase of EBV as
following: forward primer, 5′-CGGAAGCCCTCTGGACTTC-3′; reverse,
5′-CCCTGT TTATCCGATGGAATG-3′. A fluorogenic probe, 5′-TGTACA
CGCACGAGAAATGCGCC-3′, with a sequence localized between PCR primers
was obtained from Applied Biosystems.
Fluorogenic PCRs were carried out in a reaction
volume of 25 μl in a 7300 ABIPRISM System (Applied
Biosystems). Each real-time PCR reaction consisted of TaqMan
Universal Mastermix (Applied Biosystems), probe (7.5 mmol/l),
forward primer (5 mmol/l), reverse primer (5 mmol/l) and DNA
solution (2.5 μl). Thermal cycling was initiated with a
denaturation step of 50°C for 2 min and then 95°C for 10 min
followed by 50 cycles of denaturation at 95°C for 15 sec, annealing
and extension at 60°C for 1 min. Each plate consisted of patient
samples in duplicate and triplicate blanks of water as a negative
control. The calibration curve of each plate was calculated based
on a dilution series of the TaqMan Control Human Genomic DNA
Standard (Applied Biosystems) from 1 to 1x10−3 ng/ml at
10 ng/μl: 1, 0.1, 0.01, 0.001 and 0.0001 ng/ml. All data
were analyzed using the 7300 System-SDS Software (version 1.2.3)
Sequence Detection Software (Applied Biosystems).
Results
Diagnostic features
A total of 130 patients diagnosed with DLBCL were
selected for this study. The median age was 58 years and 56% were
male. In total, 127 patients were diagnosed as DLBC and 3 as
Burkitt-like NHL. Of all the cases, 33% were classified as
extra-nodal, 8% had >1 secondary extra-nodal invasion, 47% had B
symptoms, 45% had advanced stage disease (III and IV), 25.8% had an
ECOG performance status ≥2, 53% had elevated lactate dehydrogenase
(LDH) levels, 28.2% had IPI intermediate-high (IH) or high (H) and
32.7% had elevated β-2 microglobulin (B2M) levels. A minority of
patients (30%) received cyclophosphamide, doxorubicin, vincristine
and prednisolone (CHOP) or an equivalent combination (CHOP-like)
chemotherapy and 60% received the same chemotherapy combined with
rituximab (R-CHOP) or R-CHOP-like. The remaining patients were
treated with chemotherapy combinations without anthracyclines.
Certain patients were treated before 2003, the year that rituximab
was introduced in Portugal.
EBV detection
The EBV genome was detected in 28/130 patients
(21.5%) in blood and/or bone morrow samples. Bone marrow was
preferably used, but for 5 patients only blood samples were
available. In another 10, the two samples were tested and were
accordant except one case. The characteristics of the EBV-negative
and -positive patients are shown in Table I.
 | Table I.Prognostic factors for all
populations and for EVB-positive and -negative subgroups. |
Table I.
Prognostic factors for all
populations and for EVB-positive and -negative subgroups.
| Variable | Frequency | EVB-negative | EBV-positive | P-value |
|---|
| n | 130 | 102 | 28 | |
| Median age
(years) | 57.9 | 57.5 | 59.3 | |
| >60 years old, n
(%) | 65 (50) | 50 (49) | 15 (53.6) | 0.67 |
| Gender (male), n
(%) | 73 (56.2) | 58 (56.9) | 15 (53.6) | 0.76 |
| Primary
extra-nodala | 43/129 (33.3%) | 34 (33.3%) | 9/27 (33.3%) | 1 |
| Ann Arbor stage
III/IVa | 58/128 (45.3%) | 48 (47.5%) | 10 (37%) | 0.33 |
| B symptomsa | 60/127 (47.2%) | 48 (48%) | 12 (44.4%) | 0.74 |
| Performance status
≥2a | 32/124 (25.8%) | 23 (23.7%) | 9 (33.3%) | 0.31 |
| Elevated
B2Ma | 33/101 (32.7%) | 23 (29.5%) | 10 (43.5%) | 0.21 |
| Elevated
LDHa | 66/124 (53.2%) | 48 (49%) | 18 (69.2%) | 0.07 |
| IPI IH+Ha | 35/124 (28.2%) | 28 (28.6%) | 7 (26.9%) | 0.86 |
| Treatment response
≥PRa | 108/119
(90.8%) | 84 (93.2%) | 24 (88.9%) | 0.90 |
| Relapseda | 16/74 (21.6%) | 15 (26.3%) | 1 (5.9%) | 0.07 |
| 4-year PFSb, % | 71.6 | 70.2 | 82.9 | 0.69 |
| 5-year
survivalb, % | 65.2 | 64.3 | 76.7 | 0.91 |
Compared with EBV-negative patients, EBV-positive
patients had a similar mean age (57.5 versus 59.3 years), gender,
nodal or extra-nodal presentation, Ann Arbour staging, IPI risk
factors, serum LDH and B2M level, response to treatment,
progression-free survival and OS. Response rate [complete response
(CR) + partial response (PR)] was 93.2 and 88.9% for EBV-negative
and -positive patients, respectively (not significant).
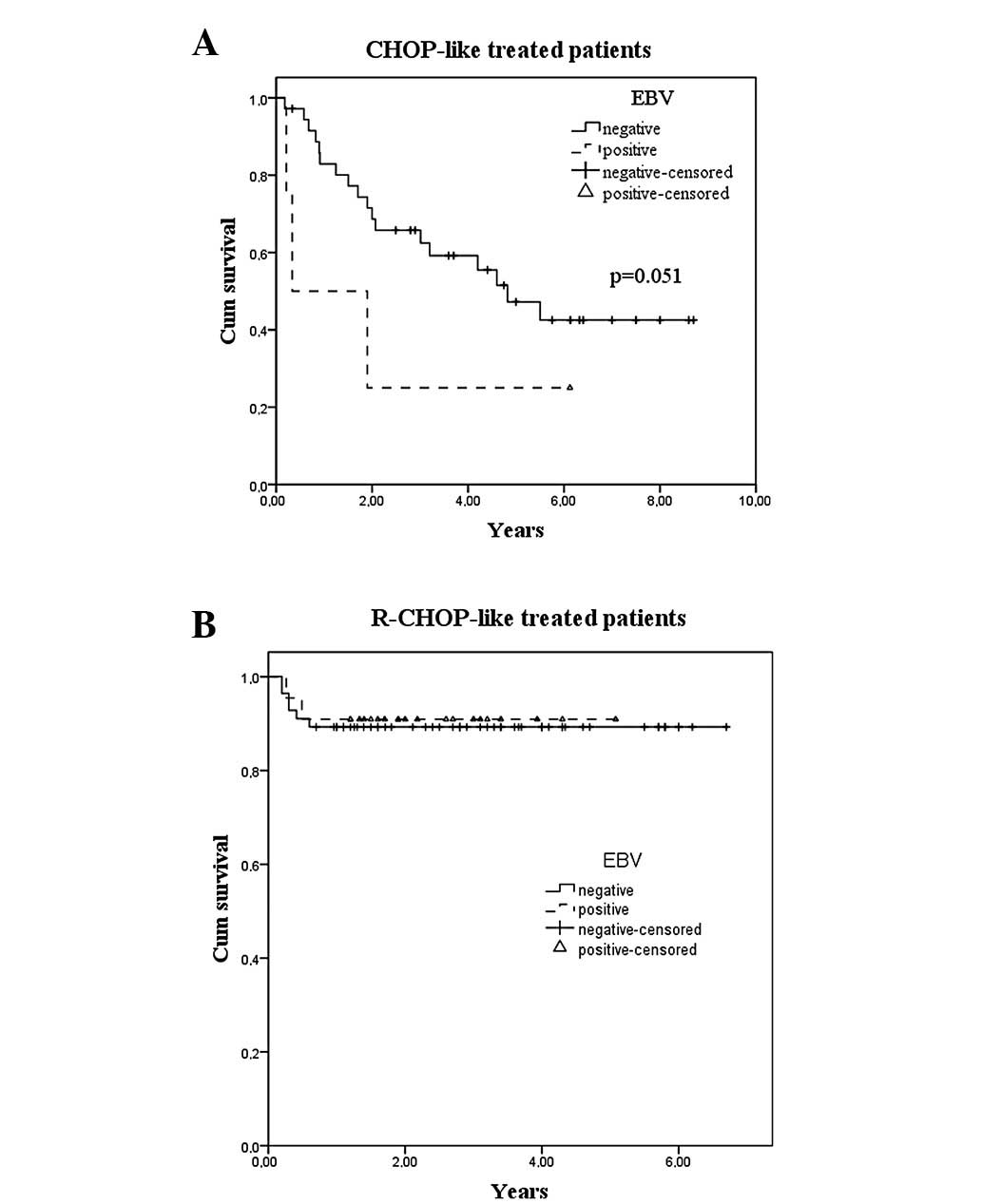
The global survival rate was 65% at 5 years. There
was a significant difference at 5 years of survival in the CHOP/
CHOP-like and R-CHOP/R-CHOP-like groups, with 47.7 and 88.6%,
respectively (P=0.001). Globally there was no difference between
EBV-positive and -negative cases, but when a Breslow test was used
to compare EBV-positive patients treated with CHOP-like or
R-CHOP-like chemotherapy, a trend (P=0.051) favoring R-CHOP-like
was attained (Fig. 1).
Clinical variables, treatment response rate and
survival was not significantly different in EB-positive and
-negative groups (Table I).
Fig. 1 shows the survival curves by
EBV status and type of chemotherapy. Univariate analysis revealed
that age, Ann Arbor stage, B symptoms, ECOG system performance
status and LDH and serum albumin levels anticipated a prognostic
factor value, independent of the type of treatment, chemotherapy
with or without rituximab and EBV status (Table I). When considering IPI in these
series, a good discrimination in prognosis of the groups was
observed but there was an asymmetrical distribution of the
population, with 12% of patients having a score of H. Fig. 2 shows the survival curves for the
IPI groups.
Four cases of DLBCL were diagnosed in patients with
AIDS. AIDS diagnosis occurred between 3.5 years and 4 months before
the diagnosis of DLBCL and 3 of them were EBV positive Of these
patients, 2 succumbed; one from an infectious complication during
treatment and the other had no response to 3 different lines of
immune-chemotherapy and succumbed to the disease within 6
months.
Discussion
There is a limited number of studies investigating
EBV tumoral detection and prognosis in DLBCL. To the best of our
knowledge, none of these explore the virus presence in mononuclear
cells of the blood or bone marrow while also evaluating the
prognostic endpoints (7,10,11).
The current study evaluated the correlation between the EBV genome
expression in mononuclear cells of the bone marrow or peripheral
blood in patients with DLBCL with response to treatment and
survival.
Noteworthy data have emerged from a Japanese study
(7) that evaluated the presence of
EBER in tumor tissue, comparing a group of EBV-positive with a
group of EBV-negative elderly DLBCL patients. A shorter survival
was observed in EBV-positive patients with a median OS of 24 months
versus OS not reached in EBV-negative patients, which was a
prognostic factor independent of IPI scores. Similar results have
been reported in a Peruvian population (10) treated with chemotherapy without
rituximab with an OS of 7 and 47 months for EBV-positive and
-negative DLBCL patients, respectively. In this study, EBER tumoral
expression and IPI scores were independent prognostic factors in a
multivariate analysis. A study of Korean patients (11) treated with chemotherapy with only a
small percentage combined with rituximab also demonstrated that EBV
expression was associated with a worsened OS. This association is
maintained in sub-groups of high IPI scores and phenotypical
non-Germinal Center DLBCL patients. The differences between
patients treated with a chemotherapy combination containing
rituximab and those treated with a chemotherapy not containing
rituximab were not analyzed, likely due to a small number of
patients receiving this monoclonal antibody.
In a different approach, Paydas et al
(12) evaluated the prognostic
significance of biological factors correlated with angiogenic,
anti-apoptotic, inflammatory and viral process in 88 DLBCL patients
and determined that the expression of LMP-1 is the most important
factor to determine the survival rate. Contrasting with those
studies, the results of the current study using bone marrow and
blood, but not tumoral tissue, revealed no significant difference
in survival between EBV-positive and -negative patients.
Cases of EBV-positive aggressive DLBCL have been
described among Caucasian populations but a survival analysis has
not been reported (18,19). We observed a case with an especially
aggressive course in a patient co-infected with EBV and HIV.
The influence of EBV positivity in treatment
response on patients with DLBCL has been evaluated by a limited
number of studies. Oyama et al compared 62 EBV-positive
Japanese patients treated with anthracycline-containing regimens
without rituximab with 104 EBV-negative patients and observed
overall response rates of 80 and 99%, respectively (P<0.0001)
with CR rates of 66 and 91% (7).
Park et al reported an overall response rate but not a
complete remission rate of 25 EBV-positive and 207 EBV-negative
patients; the authors identified a significant difference of 72 and
92%, respectively (11). In a
gastric DLBCL study, 4 EBV-positive patients had a poor response to
chemotherapy and radiotherapy with 2 of those presenting with
progressive disease, one partial and one complete remission
(13).
The search for EBV presence in mononuclear cells in
bone morrow and/or blood and the evaluation of its value as a
prognostic tool has not been systematically performed. In a
Brazilian study of children with Burkitt’s lymphoma comparing
different methods of EBV detection, only 9 cases were
simultaneousely tested in tumor mass samples and bone marrow
(9). The authors searched clonal
lymphoma cells by immunoglobulin H gene rearrangement. They found
that only EBV-tumor cell-positive cases with bone marrow clonal
infiltrating cells were also EBV-positive. However, risk factors
were not evaluated in this study.
The present study was conducted on blood and bone
marrow searching for the presence of EBV in the mononuclear cells
of these tissues and aimed to evaluate the prognostic relevance of
the virus in patients with DLBCL. With a sensitive technology such
as PCR it was possible to detect EBV-positive memory cells,
non-tumoral lymphocytes and true malignant lymphocytes. However,
the frequency of colonized cells in peripheral blood is very rare
in healthy seropositive individuals, with an count under 1/50,000
in the majority of studies (14,15).
These findings were the basis of the present study.
When comparing the results of the present study
using bone marrow/blood samples with studies performed elsewhere
that used tumor tissue cells, the frequency of detection is not
substantially different, with 21.5 and 5–15%, respectively. This
frequency is higher than that published for the western population,
but it included 4 cases of HIV-positive patients. This result also
indicates that searching in bone marrow or blood mononuclear cells
may be a reliable method of EBV detection. However, it cannot
distinguish tumor B cells from the rare EBV-colonized memory B
cells, either in circulation or in bone marrow. Therefore, we plan
to search for EBV on tissue cells and to compare the results
between bone marrow and blood in the same patient.
In contrast to the results of others, the negative
prognostic impact usually observed in DLBCL has not been verified
in the current study. This may be explained by the introduction of
rituximab in therapeutic armamentarium to B-cell NHL. The majority
of patients received R-CHOP and some were administered
dose-densified R-CHOP-14, which had not occurred in the majority of
the patients in previous studies. In fact, the prognostic influence
of EBV presence in the rituximab era remains unknown. The addition
of rituximab to CHOP chemotherapy may overcome the prognostis of
Bcl2 expression on DLBCL in patients treated with CHOP and an
identical influence may be possible on EBV expression (20). Favoring this hypothesis is the
efficacy of rituximab in post-transplant lymphoproliferative
disease, a syndrome typically associated with EBV and
immunodepression (21). The current
study also supports the favorable influence of rituximab, given
that when CHOP-like versus R-CHOP-like patients were analyzed, the
trend of a decreased survival rate in EBV-positive patients was
only observed in the first group, whose treatment did not include
rituximab.
In conclusion, this study demonstrated the presence
of a high percentage of EBV in mononuclear cells from bone marrow
aspirates or the peripheral blood of patients with DLBCL. Detection
of the virus genome was not associated with any clinical variable,
response rate or survival rate, regardless of treatment, but the
majority of patients in this study received chemotherapy and
rituximab. Moreover, we provide evidence to support the use of
rituximab in addition to conventional chemotherapy to improve the
clinical outcomes associated with EBV detection in DLBCL.
References
|
1.
|
JI CohenEpstein-Barr virus infectionN Engl
J Med343481492200010.1056/NEJM20000817343070710944566
|
|
2.
|
DA Thorley-LawsonA GrossPersistence of the
Epstein-Barr virus and the origins of associated lymphomasN Engl J
Med35013281337200410.1056/NEJMra03201515044644
|
|
3.
|
G KellyA BellA RickinsonEpstein-Barr
virus-associated Burkitt lymphomagensis selects for downregulation
of the nuclear antigen EBNA2Nat
Med810981104200210.1038/nm75812219084
|
|
4.
|
PM BanksRA WarnkeMature T-cell and NK-cell
neoplasmsWHO Classification of Tumors: Pathology and Genetics of
Tumours of Hematopoetic and Lymphoid TissuesES JaffeNL HarrisH
SteinJW VardimanIARC PressLyon1892302001
|
|
5.
|
K KuzushimaH KimuraY HoshinoLongitudinal
dynamics of Epstein-Barr virus-specific cytotoxic T lymphocytes
during posttransplant lymphoproliferative disorderJ Infect
Dis182937940200010.1086/315791
|
|
6.
|
SJ Hamilton-DutoitM RaphaelJ AudouinIn
situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in
acquired immunodeficiency syndrome-related lymphomas: correlation
with tumor morphology and primary siteBlood826196241993
|
|
7.
|
T OyamaK YamamotoN AsanoAge-related
EBV-associated B-cell lymphoproliferative disorders constitute a
distinct clinicopathologic group: a study of 96 patientsClin Cancer
Res1351245132200710.1158/1078-0432.CCR-06-282317785567
|
|
8.
|
RF AmbinderRB MannDetection and
characterization of Epstein-Barr virus in clinical specimensAm J
Pathol14523925219948053485
|
|
9.
|
R HassanLR WhiteCG StefanoffEpstein-Barr
(EBV) detection and typing by PCR: a contribution to diagnostic
screening of EBV-positive Burkitt’s lymphomaDiagn
Pathol11723200616893464
|
|
10.
|
D MoralesB BeltranFH De
MendozaEpstein-Barr virus as a prognostic factor in de novo nodal
diffuse large B-cell lymphomaLeuk
Lymphoma516672201010.3109/1042819090330801519860616
|
|
11.
|
S ParkJ LeeYH KoThe impact of Epstein-Barr
virus status on clinical outcome in diffuse large B-cell
lymphomaBlood110972978200710.1182/blood-2007-01-06776917400912
|
|
12.
|
S PaydasM ErginG SeydaogluPrognostic
significance of angiogenic/lymphangiogenic, anti-apoptotic,
inflammatory and viral factors in 88 cases with diffuse large B
cell lymphoma and review of the literatureLeuk
Res3341174126200910.1016/j.leukres.2009.02.01519286254
|
|
13.
|
T YoshinoS NakamuraY MatsunoEpstein-Barr
virus involvement is a preditive factor for the resistance to
chemoradiotherapy of gastric diffuse large B-cell lymphomaCancer
Sci97163166200610.1111/j.1349-7006.2006.00155.x16441428
|
|
14.
|
RJ TierneyN StevenLS YoungAB
RickinsonEpstein-Barr virus latency in blood mononuclear cells:
Analysis of viral gene transcription during primary infection and
in the carrier stateJ Virol68737473851994
|
|
15.
|
LL DeckerLD KlamanDA
Thorley-LawsonDetection of the latent form of Epstein-Barr virus
DNA in peripheral blood of healthy individualsJ
Virol703286329919968627812
|
|
16.
|
SH SwerdlowE CampoNE HarrisWHO
Classification of Tumours of Haematopoietic and Lymphoid Tissue4th
editionIARC PressLyon2008
|
|
17.
|
CA HeidJ StevensKJ LivakPM WilliamsReal
time quantitative PCRGenome
Res6986994199610.1101/gr.6.10.9868908518
|
|
18.
|
SE GibsonED HsiEpstein-Barr virus-positive
B-cell lymphoma of elderly at a United States tertiary medical
center: an uncommon aggressive lymphoma with a nongerminal center
B-cell phenotypeHum
Pathol40653661200910.1016/j.humpath.2008.10.007
|
|
19.
|
S HoellerA TzankovSA PileriEpstein-Barr
virus-positive diffuse large B cell lymphoma in elderly patients is
rare in Western populationsHum
Pathol41352357201010.1016/j.humpath.2009.07.02419913281
|
|
20.
|
N MounierJ BriereC GisselbrechtRituximab
plus CHOP (R-CHOP) overcomes bcl-2-associated resistance to
chemotherapy in elderly patients with diffuse large B-cell lymphoma
(DLBCL)Blood10142794284200310.1182/blood-2002-11-344212576316
|
|
21.
|
N MilpiedB VasseurN ParquetHumanized
anti-CD20 monoclonal antibody (Rituximab) in post transplant
B-lymphoproliferative disorder: A retrospective analysis on 32
patientsAnn
Oncol11113116200010.1093/annonc/11.suppl_1.S11310707791
|