Introduction
Cyclooxygenase (COX) enzymes are key rate-limiting
enzymes that catalyze prostaglandin (PG) synthesis from arachidonic
acid. Two isoforms of COX enzymes have been identified and
characterized. One of these forms, COX-1, is a housekeeping gene
product that performs a homeostatic role and is expressed in a
variety of tissues. The other isoform, COX-2, cannot be detected in
the majority of normal tissues, but it can be induced by
inflammatory stimuli including growth factors, cytokines and
oncogenes. These two isoforms are encoded by two separate genes and
exhibit distinct cell-specific expression, regulation and
subcellular localization; however, they share similar structural
and kinetic properties (1).
An expanding body of evidence demonstrates that
COX-2 is overexpressed in a variety of malignancies including
colorectal cancer (2,3), breast cancer (3), lung carcinoma (3) and ovarian cancer (4); while overexpression of COX-1 has been
identified in human head and neck cancer (5) as well as ovarian cancer (6). Clinical and preclinical studies have
indicated that COX-2 inhibitors are rapidly emerging as an
excellent target for prevention and/or treatment of human cancers
(7–10) due to their antiproliferative and
antiangiogenic effects and their role in enhanced immune
surveillance (8,9). Few studies have also concluded that
COX-1 inhibitors are able to reduce tumor growth by decreasing cell
proliferation and accelerating apoptosis (6,10).
Additionally, COX-2 inhibitors have demonstrated potent
life-prolonging effects in patients with esophageal and
gastroesophageal junction cancer (11) and in various animal models of cancer
(12,13).
On the basis of these findings, it was concluded
that COX inhibitors (coxibs) are well-established chemopreventative
drugs. Thus, in this study, we hypothesize that coxibs should
markedly improve survival in ovarian cancer in vivo,
possibly through inhibiting tumor growth. To examine this
possibility, we studied the potential effects of SC-560 and
celecoxib on survival time and tumor growth in an ovarian cancer
xenograft-bearing mouse model.
Materials and methods
Human ovarian tumors in nude mice
The human ovarian carcinoma cell line SKOV-3 was
used to appraise whether SC-560 and/or celecoxib were able to
prolong the survival time by inhibiting ovarian cancer growth.
SKOV-3 was purchased from China Type Culture Collection and grown
in the recommended media under standard conditions. SKOV-3 cells
were implanted subcutaneously in the dorsal skin (5×106
cells) of female athymic nude mice (BALB/cA, 40–45 days old). A
tumor was successfully formed, and after three generations, a
1.5-mm3 well-developed tumor tissue was inoculated
subcutaneously into the right axillary region of the mice.
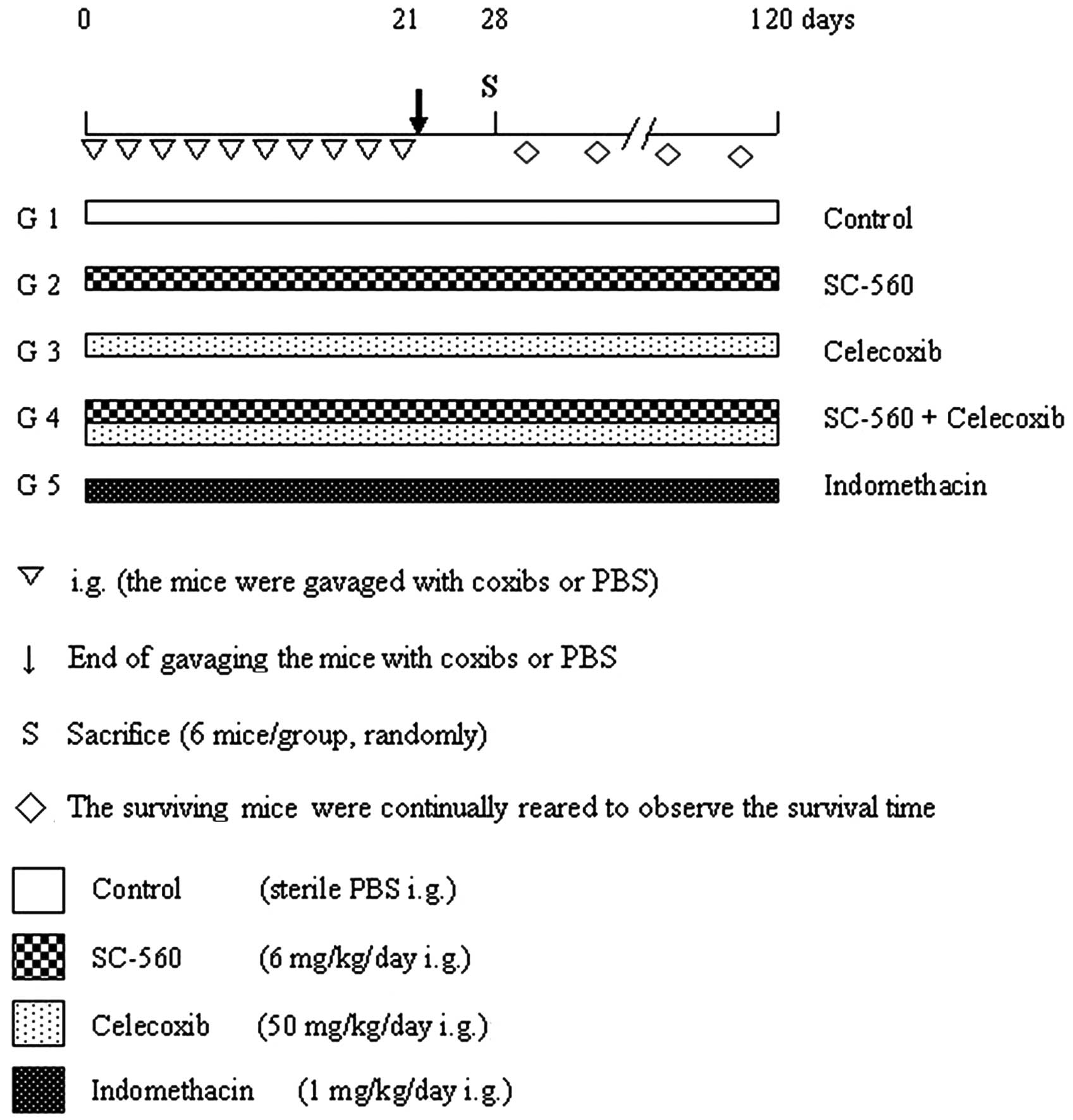
Treatment was initiated when the tumor became visible (average
volume, 118.24 mm3). Mice were randomly separated into
five groups (with 12 mice in each group) depending on their
allocated treatment: SC-560, celecoxib, SC-560/celecoxib
(combination group), indomethacin or control. The experimental
design is shown in Fig. 1. The
study was approved by the ethics committee of Nanjing Medical
University of Hangzhou Hospital, Hangzhou, China.
The COX-1-selective inhibitor (SC-560;
Sigma-Aldrich, St. Louis, MO, USA), COX-2-selective inhibitor
(celecoxib; Pfizer, New York, NY, USA), and nonselective coxib
(indomethacin; Sigma-Aldrich) were administered via gavage in a 0.5
ml suspension of 5% methylcellulose and 0.025% Tween-20 twice a day
to achieve a dose of 6 mg/kg/day SC-560, 50 mg/kg/day celecoxib and
1 mg/kg/day indomethacin. The doses were selected for their
specificity in inhibiting COX isotypes (14). The control group of mice were
treated with sterile PBS (pH 7.2), while the selected doses of
coxibs were administered to the SC-560 alone, celecoxib alone,
SC-560 in combination with celecoxib, indomethacin alone and the
control group every other day for a period of 21 days, beginning on
the day when the tumors became palpable. Mice were maintained on a
standard diet and water was made freely available.
The tumor dimensions were measured twice a week
using a linear caliper, and the tumor volume was calculated using
the equation V (mm3) = 1/2 x a x b2, where a
and b are the largest and the smallest perpendicular diameters
(15), respectively. These results
are used to calculate the relative tumor volume (RTV) using the
equation RTV=Vt/V0, where V0 is
tumor volume on the day of first administration and Vt
is the total for each measurement of tumor volume. The animals were
weighed weekly throughout the experiment. In order to observe the
effect of the coxibs on tumor growth, half of the mice in each
group were sacrificed randomly on day 28. All tumor tissue samples
were then collected and fixed in 10% phosphate-buffered formalin
solution for molecular biology or snap frozen in liquid nitrogen
and stored at −80°C for further analysis. The remaining mice were
continually reared with a basal diet to observe the survival time,
and the study was continued until all mice had been sacrificed (day
121).
Reverse transcription-polymerase chain
reaction (RT-PCR)
To investigate the expression of COX-1 and COX-2
mRNA levels in the human ovarian carcinoma cell line SKOV-3, the
coxib treatment groups and the control group were analyzed for the
expression of COX-1 and COX-2 mRNA using RT-PCR. Total RNA was
isolated from the tissue using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). Total RNA (5 μg) was
reverse transcribed using the SuperScript-II according to the
manufacturer’s protocol (Invitrogen Life Technologies). PCR for
COX-1, COX-2 and β-actin was carried out in a 50-μl reaction
mixture containing 5 μl aliquots of reverse transcribed cDNA
samples, 1X PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 2.5
units Ampi-Taq DNA polymerase and 400 nM primers. PCR was run for
40 cycles, which consisting of denaturation at 94°C for 30 sec,
annealing at 54°C for 30 sec, extension at 72°C for 45 sec and
final extension at 72°C for 5 min. A constitutively expressed
β-actin gene was used as a control with PCR conditions identical to
that for COX-1 and COX-2. The primers for COX-1 were
5′-cctcaccagtcaatccctgt-3′ (sense) and 5′-gggcagtctttgggtacaga-3′
(antisense), and those for COX-2 were 5′-tcctcccgtagcaga tgact-3′
(sense) and 5′-aagtggtaaccgctcaggtg-3′ (antisense). The primers for
β-actin were 5′-ttgctgacaggatgcagaag-3′ (sense) and
5′-acatctgctggaaggtggac-3′ (antisense).
Statistical analyses
All results were expressed as the mean ± standard
error (SE). We used the Dunnett’s test and the log-rank test based
on the joint-ranking method for evaluation of the inhibitory
activity on tumor growth and life-prolonging activity,
respectively. Additionally, to evaluate the life-prolonging effect
of the coxibs, the median survival time (MST; days) for each group
following tumor inoculation was determined from the survival times
of the mice according to the Kaplan-Meier plot using the SPSS
system 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of coxibs on tumor growth
Following tumor establishment, 12 mice in each group
were treated with SC-560, celecoxib, SC-560/celecoxib or
indomethacin. Treatment was continued for 21 days. To examine
whether the life-prolonging activities of COX-1 and COX-2 selective
inhibitors were caused by their inhibitory effects on tumor growth,
six randomly selected mice in each group were sacrificed on day 28.
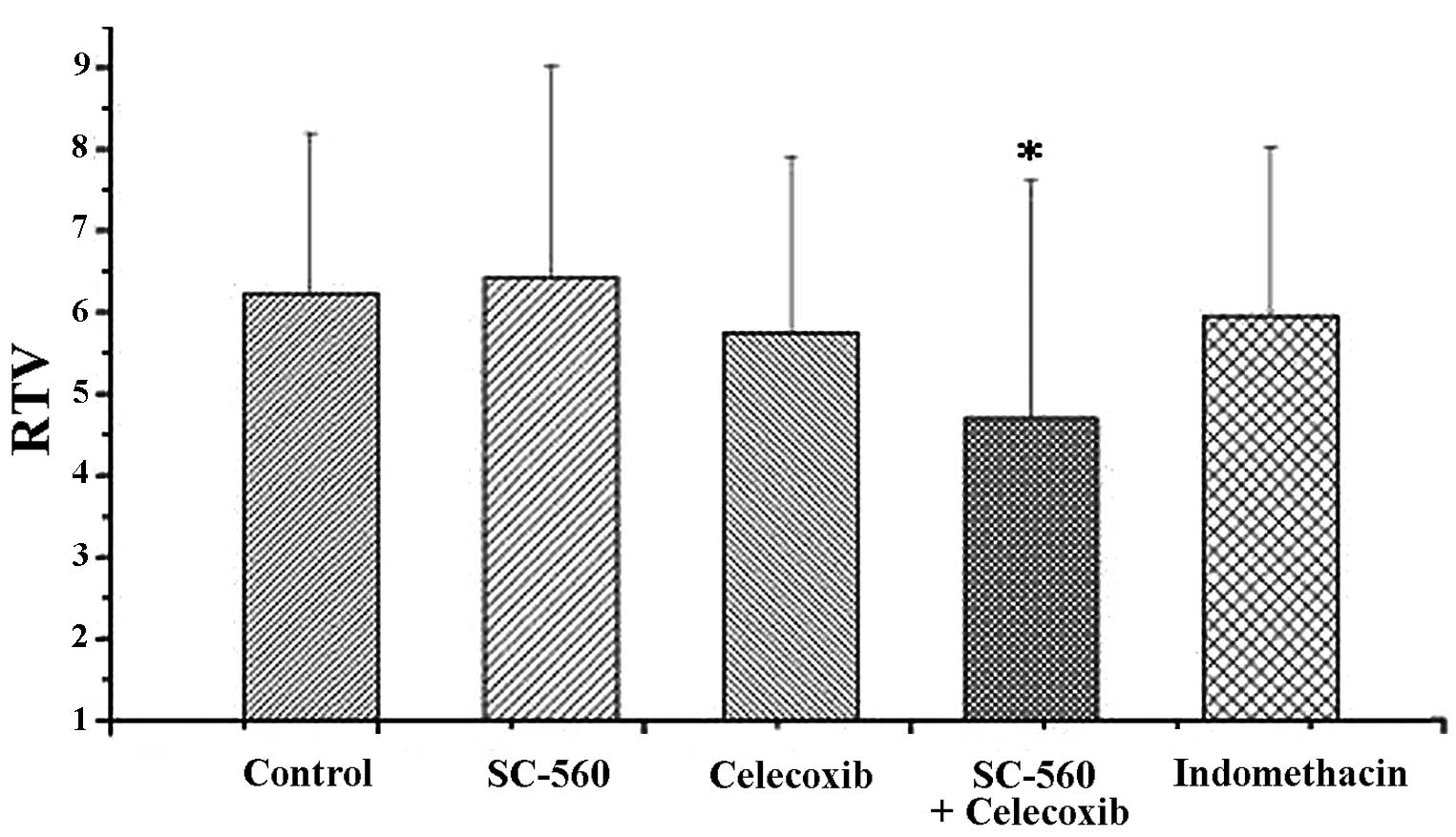
Tumor growth was evaluated by measuring the RTV, as shown in
Fig. 2. On day 21, at the end of
treatment, the RTV was 6.42±2.60 in the SC-560 group. Under similar
conditions, the RTVs were 4.69±2.93, 5.75±2.15, 5.96±2.07 and
6.23±1.97 in the SC560/celecoxib, celecoxib, indomethacin and
control groups, respectively. Combination therapy resulted in a
statistically significant inhibition of tumor size compared with
the vehicle-treated control group (P<0.05). On day 28, to assess
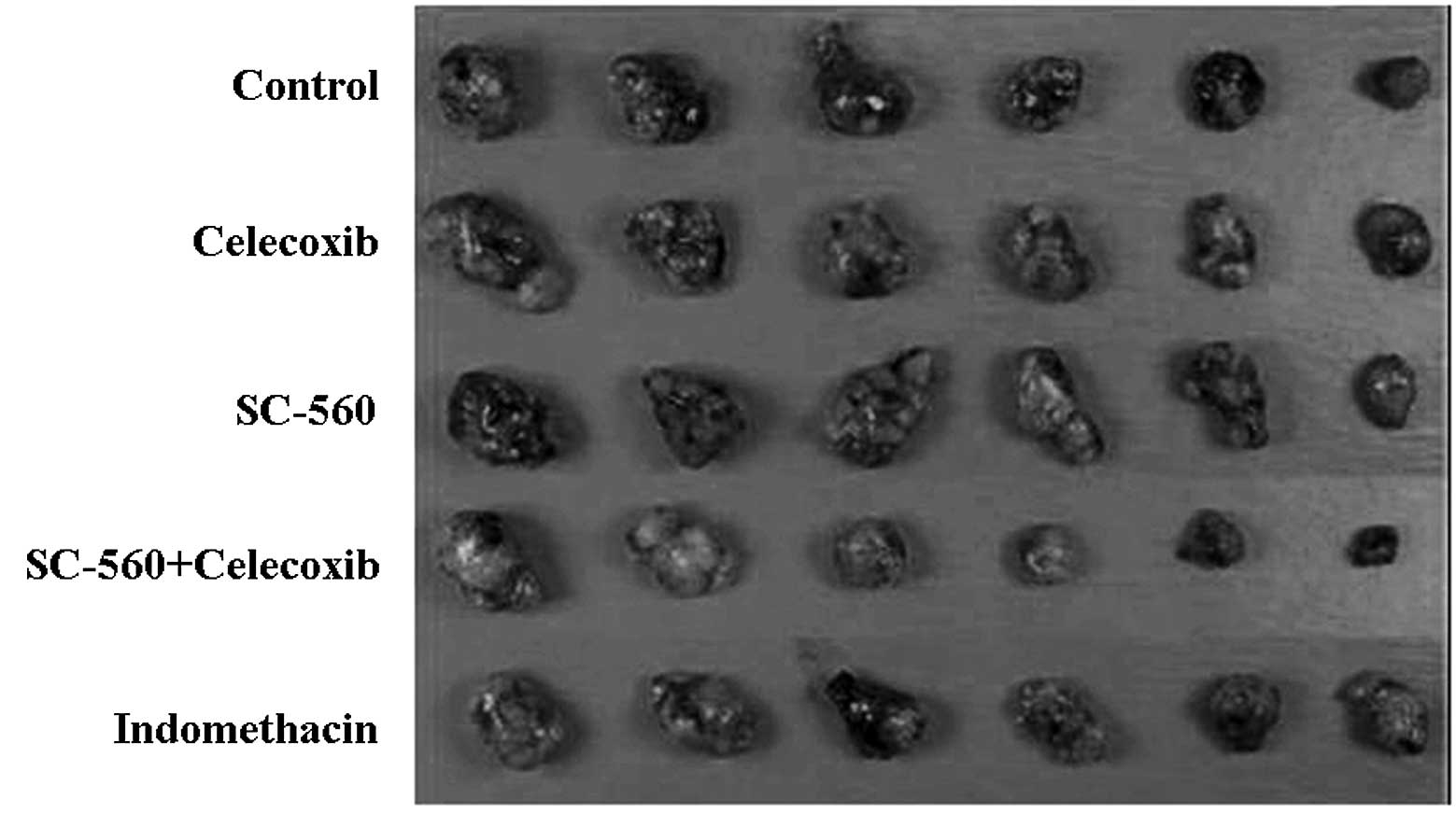
the growth-inhibitory effect of coxibs, six randomly selected mice
in each group were sacrificed, and tumor tissue samples were
collected and photographed (Fig.
3). No toxicity was observed in any of the mice, as measured by
weight gain/loss as well as gross pathological examination of the
gastrointestinal tract of the mice at necropsy.
COX mRNA expression
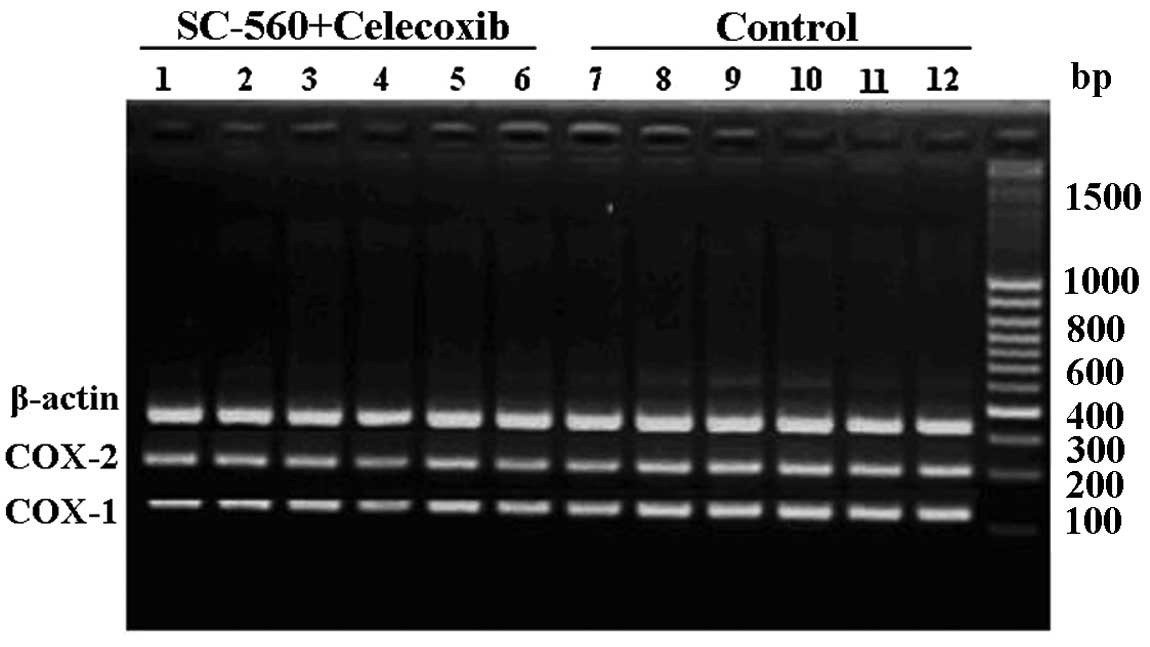
To investigate whether the coxibs regulated COX-1 or
COX-2 expression in ovarian carcinoma xenograft-bearing mice, all
collected tumor tissue samples in the combination and control
groups were analyzed for differential expression of COX-1 and COX-2
mRNA by RT-PCR analysis. The analysis revealed that following
treatment with celecoxib and SC-560, the presence of COX-1 and
COX-2 mRNA was reduced, while they were distinctly evident in
untreated tumor samples (Fig.
4).
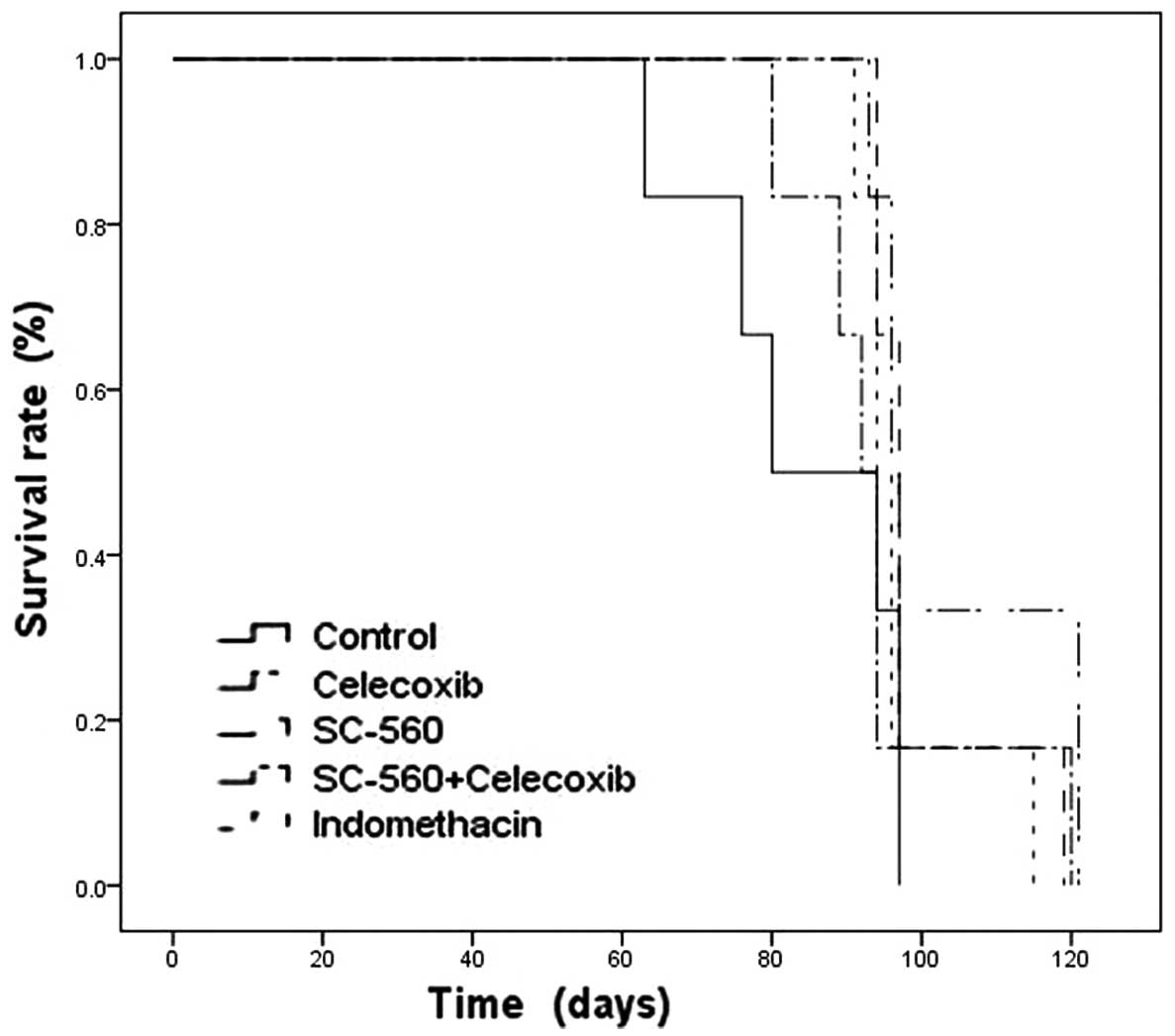
Effect of coxibs on survival
To observe the effect of coxibs in improving
survival, after day 28 the remaining six mice continued to be
reared with a standard diet and water. The mice were closely
monitored and studies were terminated when all mice were sacrificed
on day 121 due to the level of burden caused by the tumor. In the
vehicle-treated control group, the first and last mice were
sacrificed on days 63 and 97, respectively, and the mean survival
time was 84.50±5.65 days. In the coxibs-treated groups, the first
mouse was sacrificed on day 80, as in the SC560+celecoxib group,
and the last mouse was sacrificed on day 121, in the celecoxib
group. The coxibs monotherapy or combination therapy groups
resulted in a trend toward extending the survival time in
comparison to the control group (Fig.
5). Celecoxib or SC-560 treatment alone markedly prolonged the
mean survival time of xenograft-bearing mice in comparison with the
control group (P<0.05; Table I).
Particularly in the celecoxib group, the mean survival time was
extended to 104.00±5.40 days. The last mouse to be sacrificed (on
day 121) was also in this group. To evaluate the life-prolonging
effect of coxibs, the MST for each group was determined. MST is the
estimated time from diagnosis when 50% of the mice died, and is
measured using the Kaplan-Meier estimate of survival. MST for each
coxib-treated group was significantly longer in comparison with the
vehicle treated control group (Table
I).
 | Table I.Survival time in xenografted mice
with ovarian cancer. |
Table I.
Survival time in xenografted mice
with ovarian cancer.
| Group | Sample size | Survival time
(days)
|
|---|
| Mean (±SD) | MST |
|---|
| Control | 6 | 84.50±5.65 | 87 |
| Celecoxib | 6 | 104.00±5.40a | 96.5 |
| SC-560 | 6 | 99.67±3.91a | 97 |
|
SC-560+celecoxib | 6 | 94.83±5.47 | 93 |
| Indomethacin | 6 | 97.67±3.55 | 95.5 |
Discussion
The present study was designed to assess whether
coxibs are able to prolong the survival time of nude mice
transplanted with the human ovarian cancer SKOV-3 cell line. This
study revealed that the survival probability was extended following
coxib therapy. Celecoxib or SC-560 treatment alone demonstrated a
significantly prolonged mean survival time and MST in comparison
with the control group in vivo.
Recent clinical studies have provided convincing
evidence of poor survival in patients who demonstrated high COX-2
expression in stage IIB cervical adenocarcinoma and breast cancer
(16,17). Erkinheimo et al (18) suggested that elevated expression of
COX-2 is associated with reduced survival in serous ovarian
carcinomas. Another clinical study by Denkert et al
(4) using univariate and
multivariate analyses indicated that the expression of COX-2 in
patients with ovarian carcinomas is a predictor of short survival
times. Based on these findings, researchers have focused their
attention on coxibs and survival in tumors. In a phase 2 trial,
celecoxib treatment improved overall survival in patients who
suffered from COX-2-positive esophageal and gastroesophageal
junction cancer (11). In
colorectal adenocarcinoma, CS-706, a novel COX-2 selective
inhibitor, was demonstrated to have potent life-prolonging activity
in tumor-bearing mice (12). In
ovarian cancer, Xin et al (13) identified that meloxicam, categorized
as a selective COX-2 inhibitor, prolonged survival in vivo
when administered alone. However, studies concerning the
correlation of COX-1 and survival are rarely reported. In the
present study, we reveal that celecoxib and SC-560 prolong survival
in a mouse xenograft model. Our results are similar to those of
Sorenmo et al (19) which
demonstrated that dogs with prostatic carcinoma treated with coxibs
(piroxicam or carprofen) lived significantly longer than untreated
dogs.
Additionally, we attempted to find the correlation
between survival time and tumor growth. We examined the antitumor
activity of coxibs on tumor growth, and we observed that a
combination of SC-560 and celecoxib resulted in a significant
inhibition of tumor size in comparison with the control group. In
the same study (20), we identified
that combined coxib therapy produced significant potential
synergistic suppressive effects on tumor growth in comparison with
the same doses of either SC-560 or celecoxib following 14 days of
treatment. This was in accordance with the results from Kitamura
et al (21) who revealed
that combination therapy with mofezolac (a COX-1 selective
inhibitor) and nimesulide (a COX-2 selective inhibitor) has
particular potential for chemoprevention of colon carcinogenesis
compared with the effects of either coxib as monotherapy. In their
study, the number of polyps more than 2.5 mm in diameter was
markedly decreased by combined coxib treatment. We also observed
that SC-560 or celecoxib, as a single agent, had a decreasing
tendency in tumor growth, which was in accordance with other
studies demonstrating SC-560 or celecoxib suppression of tumor
growth in mouse models of ovarian cancer (6,22).
Taken together, we considered that coxibs were able to prolong
survival time by attenuating tumor growth in ovarian cancer. We
observed that suppressive effects on tumor growth were markedly
exhibited following 14 days of treatment. For this reason, on day
28, six randomly selected mice from each group were sacrificed to
observe the effect of coxibs on tumor growth, and the last six mice
were reared to record the survival time. Our novel findings suggest
that tumor growth was inhibited in each therapeutic group, which
resulted in the prolonged survival of mice. Although multiple
molecular and cellular mechanisms are involved in exerting the
antitumor effects of coxibs, the mechanisms by which coxibs extend
survival are currently unclear. Yao et al (23) suggested that selective or
nonselective coxibs improved survival in mouse models of colorectal
cancer by modulating tumor angiogenesis.
Increasing levels of COX-1 and COX-2 mRNA have been
observed in ovarian cancer (18,24).
Several studies have reported that COX-1 and COX-2 are concurrently
overexpressed and play an important role in the pathogenesis of
ovarian cancer (4,25). In the present study, RT-PCR results
revealed that COX-1 and COX-2 mRNA expression levels were increased
in untreated tumors, but were decreased in the combined treatment
tumors. In the same study, using western blotting analysis, we
identified that COX-1 and COX-2 protein levels were reduced in the
combination group cells in comparison with those in the control
group (20). The reason for the
inhibition of COX-1 and/or COX-2 mRNA and protein expression in
neoplastic tissues may be due to the inhibition of angiogenesis,
induction of apoptosis and/or antiproliferative effects (6,8,10). The
antitumor effects of coxibs have long been suggested to depend on
the inhibition of COX activity and PG synthesis. PGE2 is
produced from arachidonic acid by one of two enzymes: COX-1 or
COX-2. There is increasing evidence that PGE2
contributes to tumor progression by promoting tumor angiogenesis
and inducing tumor cell apoptosis (8,26,27).
Our same study that focused on the potential mechanisms revealed
that coxibs suppress ovarian tumor growth by influencing cell
proliferation and apoptosis (20).
These results suggest that the inhibition of COX-1 and/or COX-2 is
able to slow tumor growth, the beneficial effects of which may
prolong survival time.
In conclusion, the present study suggests that the
prolonged survival of coxib-treated animals is most likely the
result of suppressing tumor growth through multiple mechanisms,
including antiproliferative and apoptosis effects in vivo.
However, the exact mechanism of life-prolonging activity requires
further study.
References
|
1.
|
WL SmithRM GaravitoDL DeWittProstaglandin
endoperoxide H synthases (cyclooxygenases)-1 and -2J Biol
Chem2713315733160199610.1074/jbc.271.52.331578969167
|
|
2.
|
RA GuptaRN DuboisColorectal cancer
prevention and treatment by inhibition of cyclooxygenase-2Nat Rev
Cancer11121200110.1038/3509401711900248
|
|
3.
|
RA SoslowAJ DannenbergD RushBM WoernerKN
KhanJ MasferrerAT KokiCOX-2 is expressed in human pulmonary,
colonic, and mammary
tumorsCancer8926372645200010.1002/1097-0142(20001215)89:12%3C2637::AID-CNCR17%3E3.0.CO;2-B11135226
|
|
4.
|
C DenkertM KobelS PestI KochS BergerM
SchwabeA SiegertA RelesB KlosterhalfenS HauptmannExpression of
cyclooxygenase 2 is an independent prognostic factor in human
ovarian carcinomaAm J
Pathol160893903200210.1016/S0002-9440(10)64912-711891188
|
|
5.
|
BM ErovicM WoegerbauerJ PammerE SelzerMCh
GraslD ThurnherStrong evidence for up-regulation of
cyclooxygenase-1 in head and neck cancerEur J Clin
Invest386166200810.1111/j.1365-2362.2007.01896.x18173552
|
|
6.
|
T DaikokuD WangS TranguchJD MorrowS
OrsulicRN DuboisSK DeyCyclooxygenase-1 is a potential target for
prevention and treatment of ovarian epithelial cancerCancer
Res6537353744200510.1158/0008-5472.CAN-04-381415867369
|
|
7.
|
G SteinbachPM LynchRK PhillipsMH WallaceE
HawkGB GordonN WakabayashiB SaundersY ShenT FujimuraLK SuB LevinThe
effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial
adenomatous polyposisN Engl J
Med34219461952200010.1056/NEJM20000629342260310874062
|
|
8.
|
BT RagelRL JensenDL GillespieSM PrescottWT
CouldwellCelecoxib inhibits meningioma tumor growth in a mouse
xenograft modelCancer109588597200710.1002/cncr.2244117177201
|
|
9.
|
M YaoS KargmanEC LamCR KellyY ZhengE
KwongJF EvansMM WolfeInhibition of cyclooxygenase-2 by rofecoxib
attenuates the growth and metastatic potential of colorectal
carcinoma in miceCancer Res63586592200312566300
|
|
10.
|
ME UrickJR GilesPA JohnsonVEGF expression
and the effect of NSAIDs on ascites cell proliferation in the hen
model of ovarian cancerGynecol
Oncol110418424200810.1016/j.ygyno.2008.05.01818606441
|
|
11.
|
NK AltorkiP ChristosJL PortPC LeeF MirzaC
SpinelliR KeresztesD BeneckS PaulBM StilesY ZhangDS
SchrumpPreoperative taxane-based chemotherapy and celecoxib for
carcinoma of the esophagus and gastroesophageal junction: results
of a phase 2 trialJ Thorac
Oncol611211127201110.1097/JTO.0b013e31821529a921532508
|
|
12.
|
M SenzakiS IshidaA YadaM HanaiK FujiwaraS
InoueT KimuraS KurakateCS-706, a novel cyclooxygenase-2 selective
inhibitor, prolonged the survival of tumor-bearing mice when
treated alone or in combination with anti-tumor chemotherapeutic
agentsInt J Cancer12213841390200810.1002/ijc.23250
|
|
13.
|
B XinY YokoyamaT ShigetoH
MizunumaAnti-tumor effect of non-steroidal anti-inflammatory drugs
on human ovarian cancersPathol Oncol
Res13365369200710.1007/BF0294031818158574
|
|
14.
|
J ReeseX ZhaoWG MaN BrownTJ MaziaszSK
DeyComparative analysis of pharmacologic and/or genetic disruption
of cyclooxygenase-1 and cyclooxygenase-2 function in female
reproduction in miceEndocrinology14231983206200111416042
|
|
15.
|
CS WilliamsAJ WatsonH ShengR HelouJ ShaoRN
DuboisCelecoxib prevents tumor growth in vivo without toxicity to
normal gut: lack of correlation between in vitro and in vivo
modelsCancer Res6060456051200011085526
|
|
16.
|
YW JungSW KimS KimJH KimNH ChoJW KimYT
KimPrevalence and clinical relevance of cyclooxygenase-1 and -2
expression in stage IIB cervical adenocarcinomaEur J Obstet Gynecol
Reprod Biol1486266201010.1016/j.ejogrb.2009.09.01119836124
|
|
17.
|
SA GlynnRL PrueittLA RidnourBJ BoersmaTM
DorseyDA WinkJE GoodmanHG YfantisDH LeeS AmbsCOX-2 activation is
associated with Akt phosphorylation and poor survival in
ER-negative, HER2-positive breast cancerBMC
Cancer10626201010.1186/1471-2407-10-62621078168
|
|
18.
|
TL ErkinheimoH LassusP FinneBP van ReesA
LeminenO YlikorkalaC HaglundR ButzowA RistimäkiElevated
cyclooxygenase-2 expression is associated with altered expression
of p53 and SMAD4, amplification of HER-2/neu, and poor outcome in
serous ovarian carcinomaClin Cancer
Res10538545200410.1158/1078-0432.CCR-0132-0314760075
|
|
19.
|
KU SorenmoMH GoldschmidtFS ShoferC
GoldkampJ FerraconeEvaluation of cyclooxygenase-1 and
cyclooxygenase-2 expression and the effect of cyclooxygenase
inhibitors in canine prostatic carcinomaVet Comp
Oncol21323200410.1111/j.1476-5810.2004.00035.x19379307
|
|
20.
|
W LiJ WangHR JiangXL XuJ ZhangML LiuLY
ZhaiCombined effects of cyclooxygenase-1 and cyclooxygenase-2
selective inhibitors on ovarian carcinoma in vivoInt J Mol
Sci12668681201110.3390/ijms1201066821340007
|
|
21.
|
T KitamuraM ItohT NodaM MatsuuraK
WakabayashiCombined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on intestinal tumorigenesis
in adenomatous polyposis coli gene knockout miceInt J
Cancer109576580200410.1002/ijc.2001214991580
|
|
22.
|
W LiHR JiangXL XuJ WangJ ZhangML LiuLY
ZhaiCyclin D1 expression and the inhibitory effect of celecoxib on
ovarian tumor growth in vivoInt J Mol
Sci1139994013201010.3390/ijms1110399921152316
|
|
23.
|
M YaoW ZhouS SanghaA AlbertAJ ChangTC
LiuMM WolfeEffects of nonselective cyclooxygenase inhibition with
low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and
survival in a mouse model of colorectal cancerClin Cancer
Res1116181628200510.1158/1078-0432.CCR-04-169615746067
|
|
24.
|
DB HalesY ZhugeJA LagmanK AnsenbergerC
MahonA BaruaJL LuborskyJM BahrCyclooxygenases expression and
distribution in the normal ovary and their role in ovarian cancer
in the domestic hen (Gallus
domesticus)Endocrine33235244200810.1007/s12020-008-9080-z18498063
|
|
25.
|
F SpinellaL RosanoV Di CastroMR NicotraPG
NataliA BagnatoInhibition of cyclooxygenase-1 and -2 expression by
targeting the endothelin a receptor in human ovarian carcinoma
cellsClin Cancer
Res1046704679200410.1158/1078-0432.CCR-04-031515269139
|
|
26.
|
H ShengJ ShaoJD MorrowRD BeauchampRN
DuBoisModulation of apoptosis and Bcl-2 expression by prostaglandin
E2 in human colon cancer cellsCancer Res5836236619989443418
|
|
27.
|
JL MasferrerKM LeahyAT KokiBS ZweifelSL
SettleBM WoernerDA EdwardsAG FlickingerRJ MooreK
SeibertAntiangiogenic and antitumor activities of cyclooxygenase-2
inhibitorsCancer Res6013061311200010728691
|