Introduction
Endometrial carcinoma is a malignant epithelial
carcinoma of the endometrium, which accounts for approximately 80%
of adenocarcinomas. The growing incidence of endometrial carcinoma
among younger generations worldwide jeopardizes the health of young
females (1).
Cyclooxygenase (COX) is composed of two isoenzymes,
namely COX-1 and COX-2. COX is a rate-limiting enzyme that
catalyzes the transformation of arachidonic acid to prostaglandin
H2 (PGH2). PGH2 is mainly
expressed in normal tissues. However, the expression of COX-2 is
induced by many exterior and interior blood vessel activators,
including cytokines, growth factors and carcinogenic-promoting
agents during inflammation, tissue damage and tumorigenesis.
Studies have shown that the genesis and development
of endometrial carcinoma are closely related to COX-2. The
expression of COX-2 has been reported to be increased in
endometrial cancer tissue and this was associated with the
inhibition of apoptosis and the promotion of angiogenesis (2). These results provided strong evidence
for the potential clinical use of a selective COX-2 inhibitor in
the treatment of endometrial carcinoma. JTE-522, a selective COX-2
inhibitor, has been shown to inhibit the proliferation of RL952
cells and induce their apoptosis (3). Wood et al investigated the
effects of rofecoxib on endometrial carcinoma in vivo
(4). Rofecoxib significantly
inhibited the proliferation of endometrial carcinoma, but not its
apoptosis. Celecoxib, a relatively new NSAID, selectively inhibits
COX-2 but not COX-1, which minimizes undesired effects, including
platelet functional disturbance, renal impairment and
gastrointestinal reactions, caused by the long-term use of
non-selective NSAIDs. The relatively low incidence of side effects
with selective COX-2 inhibitors does not affect their widespread
application in clinical practice.
Wei et al found that celecoxib effectively
inhibited proliferation by inducing apoptosis (5). However, studies concerning the effects
of celecoxib, in vivo and in vitro, on human
endometrial carcinoma cells (HEC-1B) in mice are not available.
Munir et al identified that the high expression level of
COX-2 in HEC-1B cells was further increased by prostaglandin
E2 (PGE2) (6). A cancer-bearing model involving
hairless mice was successfully established by Gong et al by
the subcutaneous inoculation of HEC1-B cells (7). To investigate the effect of celecoxib
on the proliferation, invasion and apoptosis of HEC-1B cells, in
vitro cell culturing and in vivo cancer-bearing models
were used. Further information was obtained about the effect of
celecoxib on the biological behavior of human endometrial
adenocarcinoma. The findings may provide a new technique for
controlling the development of endometrial carcinoma (8).
Materials and methods
Cell culture
The HEC-1B cell line (Shanghai Cell Bank, Chinese
Academy of Sciences from American Type Culture Collection,
Manassas, VA, USA) was routinely cultivated in a RPMI-1640 (Gibco,
Carlsbad, CA, USA) nutrient medium with 10% FBS (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd., Hangzhou, China) in an
incubator ventilated with 5% CO2 at 37°C. The cells in
the exponential growth phase were used for the assay.
Cytostasis rate
The HEC-1B cells were inoculated into 96-well plates
(1×104/well) and were routinely cultivated in an
incubator ventilated with 5% CO2 at 37°C. After 24 h,
200 μl 1.0, 3.0, 9.0 or 27.0 μmol/l celecoxib (batch
number: 0408064, Pfizer, New York, NY, USA) solution was added. No
drug was used in the control and blank groups for zero adjustment.
A total of 3 parallel wells were used in each group. After 24, 48
and 72 h of cultivation, 20 μl MTT solution (5 mg/ml) was
added to each well. The cultivation was terminated after 4 h. The
supernate in each well was discarded and 150 μl DMSO was
added (Sigma, St. Louis, MO, USA). After agitating the solution for
10 min, the absorbance (A490) was determined using an ELISA at 490
nm. The cytostasis rate was calculated using the following formula:
Cytostasis rate = [(Acontrol group−Atest
group)/Acontrol group] × 100. The assay was
conducted three times.
Cell cycle and apoptosis
The HEC-1B cells in the logarithmic phase were
inoculated into culture flasks with an area of 25 cm2,
routinely cultivated in an incubator for 24 h and ventilated with
5% CO2 at 37°C with saturated humidity. When the cells
were well-grown and adherent, they were cultivated in serum-free
RPMI-1640 for 12 h. The culture fluid was drained from the test
group, and 200 μl celecoxib was added with the following
concentrations: 1.0, 3.0, 9.0 and 27.0 μmol/l. No drug was
added to the control group. Three parallel samples were prepared
for each group. After 24 h, ∼2×106 cells were obtained
from the single cell suspension from each culture flask. After
centrifuging and fixation, the cells were stained with propidium
iodide and were kept in the dark for 30 min. Cell cycle and
apoptosis assays were then performed in the Shanghai Cell Bank,
Chinese Academy of Sciences. All assays were performed three
times.
Establishment of an invasion cabin and
determination of invasiveness in vitro
The bottom of the Transwell cabin was coated with a
diluted solution (1:8) of 50 mg/l Matrigel (ID: 356243, Corning,
Inc., Corning, NY, USA), air dried at 4°C and sterilized by
vitalight lamp for 2 h. The supernatant was discarded, and 50
μl serum-free medium containing 10 g/l BSA (Corning Inc.)
was added. The samples were cultivated in an incubator at 37°C for
30 min. The Transwell cabin (Corning) was placed in a 24-pore plate
and 400 μl conditioned medium (supernate of the HEC-1B cell
culture) and the complete culture fluid were added in a 1:1 ratio.
A tumor cell suspension (∼200 μl) comprising RPMI-1640
culture fluid with 10 g/l BSA, 1% FBS and 2×105 cells/ml
was added to the cabin. After 24 h, 500 μl celecoxib at
concentrations of 1.0, 3.0, 9.0 and 27.0 μmol/l was added to
the test groups. The control group contained no celecoxib. The
Transwell cabin was taken out and washed with PBS after 24 h. The
cells above the microporous membrane were removed using a cotton
swab, fixed with 95% alcohol and stained with Giemsa. Three
parallel pores were formed in each group. The cell count was
determined using an inverted microscope in 5 fields of vision at a
magnification of x100. The average was used to evaluate the
invasiveness.
COX-2 expression
The HEC-1B cells were plated in a 6-well plate
(5×105 cells/well) and were routinely cultivated in an
incubator ventilated with 5% CO2 at 37°C. After 24 h,
the original culture fluid was discarded. The cells in the test
groups were treated with 1 ml celecoxib solution at concentrations
of 1.0, 3.0, 9.0 and 27.0 μmol/l, whereas the control group
was left untreated (n=3). According to the pre-cooling procedure, a
lysis buffer was added and the following procedures were performed:
cell quassation using an Ultrasonic Cell Disruptor, centrifuging at
10,000 rpm for 10 min, SDS-PAGE, electrotransfer, sealing, addition
of antibody, imaging and analysis of the results.
Animals and breeding
A total of 35 4-week-old BALB/c (nu/nu) nude mice
from Shanghai Laboratory Animal Co. Ltd. (Shanghai, China) weighing
18–22 g were raised in SPF conditions at the Shanghai Institutes
for Biological Sciences. The experiment was approved by the Ethics
Committee for Animal Experiments of the Chinese Academy of
Sciences.
The HEC-1B human endometrial adenocarcinoma cell
line was routinely cultivated in RPMI media supplemented with 10%
FBS in an incubator ventilated with 5% CO2 at 37°C.
Cells in the logarithmic phase were harvested and lysed with 0.25%
trypase-EDTA. The suspension was collected and, after centrifuging,
the supernate was discarded. The cells were washed twice with a
serum-free medium and centrifuged. The suspension was diluted with
PBS to a concentration of 2.5×107 cells/ml
(5×106 cells in 0.2 ml). The suspension (∼0.2 ml) was
subcutaneously injected into the groin of each mouse using a 1-ml
syringe sterilized with 75% alcohol. Mice with a subcutaneous
xenografted tumor >5 mm in diameter were chosen as the
cancer-bearing model. The cancer-bearing nude mice were randomly
divided into groups A, B and C (n=10). Celecoxib was dissolved in
normal saline to provide two solutions of different concentrations,
10 and 5 mg/ml, and ∼0.4 ml celecoxib solution was administered
through a gastric tube to each mouse. For groups A and B, the
solution concentrations were 10 mg/ml (4 mg/day) and 5 mg/ml (2
mg/day), respectively. Group C was treated with normal saline. The
volume of the tumor was determined every 3 days to obtain the tumor
growth curve.
Tumor inhibition rate (IR)
After two weeks, the xenografted tumor was excised
following the sacrifice of the mice by cervical luxation. The long
diameter (a, mm) and short diameter (b, mm) of the tumor were
determined. The volume of the tumor (V, mm3) and IR were
calculated according to the following formulae: V = (a ×
b2)/2; IR = [(Vmean control group−Vmean
test group)/(Vmean control group)] × 100. The
tumor tissues were fixed with 4% paraformaldehyde and preserved in
liquid nitrogen.
COX-2 levels and microvessel density
(MVD)
The COX-2 levels and MVD were determined using the
immunohistochemical method. As described in the manufacturer’s
instructions, modifications were performed after fixing the cells
in 10% methanol. The cells were then prepared as 5-μm thick
paraffin sections, dewaxed and hydrated. COX-2 and CD34 were
diluted to 1:100 and 1:50, respectively. A positive response was
characterized by a brownish yellow color under a light microscope.
The standard score for immunohistochemical staining was similar to
that obtained by the Rahman method. The vessels stained brown by
anti-factor VIII antibodies were counted at a magnification of
x200. The average vessel number in the three fields of vision was
used as the MVD.
Statistical analysis
The experimental data are expressed as the means ±
standard deviation. SAS6.12 package was used to proces the
experimental data using the t-test, analysis of variance and
correlation analysis. P <0.05 was considered to indicate a
statistically significant result.
Results
Cell proliferation
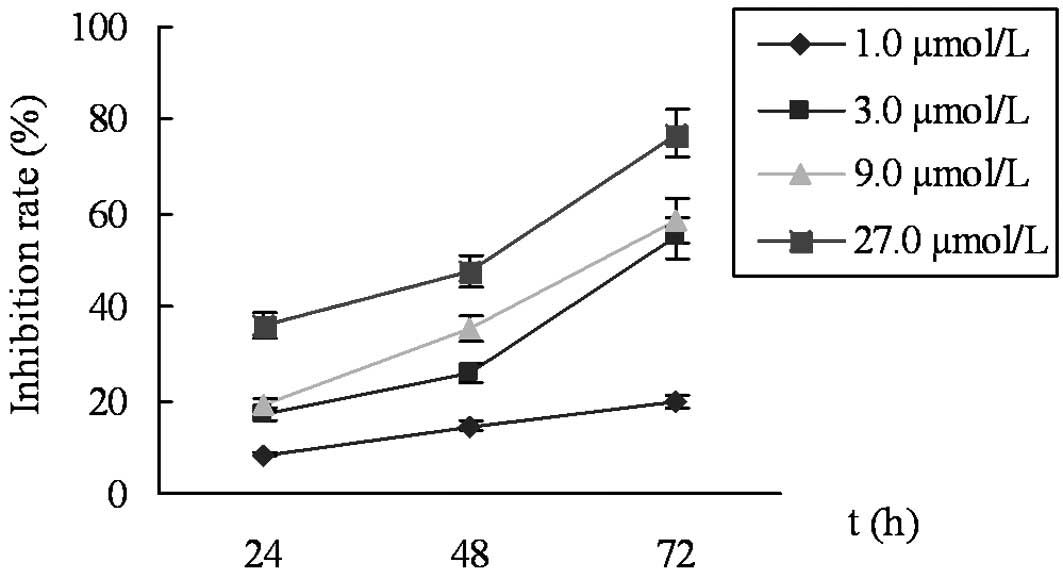
The results from the MTT assay revealed that cell
proliferation was significantly inhibited by celecoxib in a
time-dependent and concentration-dependent manner. The IR was 8.5
and 36.0% for 1.0 and 27.0 μmol/l celecoxib, respectively,
at 24 h, and the IR values of 27.0 μmol/l celecoxib were
36.0 and 77.2% at 24 and 72 h, respectively (Fig. 1). The cells exposed to celecoxib for
24 h were used in further experiments to avoid cell damage due to
prolonged exposure to the drug.
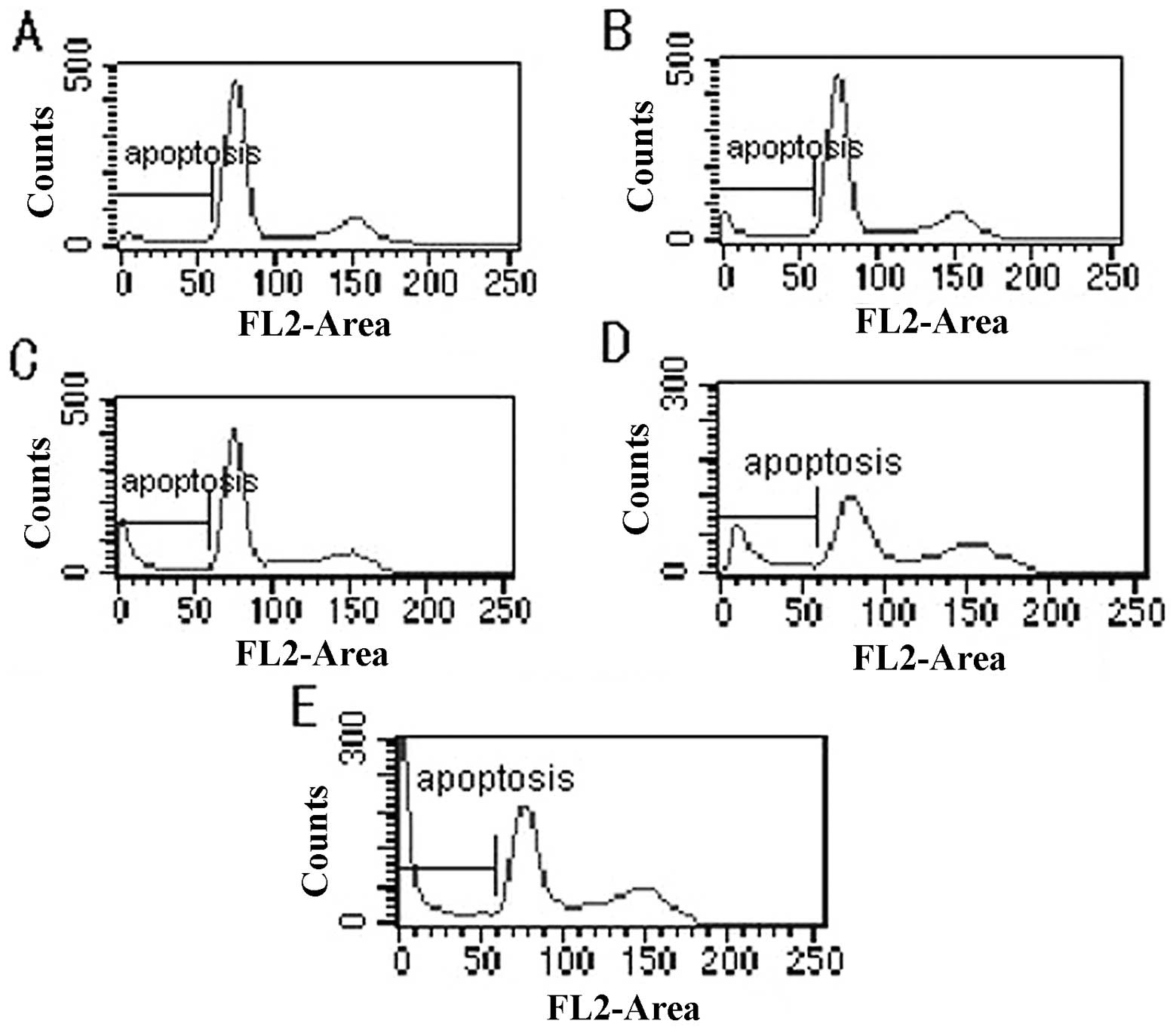
Cell cycle and apoptosis
After treating the cells with celecoxib for 24 h,
the results from flow cytometry revealed that the cell cycle was
characterized by an increase in the proportion of cells in the
G0/G1 phase, blockage of the G0/G1 phase, decreases in the
proportion of cells in the S and G2/M phases, and increases in the
apoptosis peak and apoptosis rate. Statistical significance was
observed between the test group and control group (P<0.05)
(Fig. 2; Table I).
 | Table I.Changes in cell cycle and apoptosis
in HEC-1B cells following treatment with celecoxib for 24 h
detected by flow cytometry. |
Table I.
Changes in cell cycle and apoptosis
in HEC-1B cells following treatment with celecoxib for 24 h
detected by flow cytometry.
| Cell cycle
distribution (%)
| |
|---|
| Celecoxib
(μmol/l) | G0/G1 phase | S phase | G2/M phase | Apoptosis rate
(%) |
|---|
| 0 | 40.97±3.92 | 32.36±2.63 | 26.67±2.47 | 2.78±0.20 |
| 1.0 | 45.32±4.06b | 28.83±1.45a | 25.85±1.94a | 7.21±0.80a |
| 3.0 | 64.67±3.83b | 20.07±2.35a | 15.26±3.41b | 14.50±1.34a |
| 9.0 | 69.53±5.06b | 16.49±1.47a | 13.98±2.85b | 19.28±1.56a |
| 27.0 | 76.10±2.87a | 11.23±2.01b | 12.67±1.54a | 33.80±1.83a |
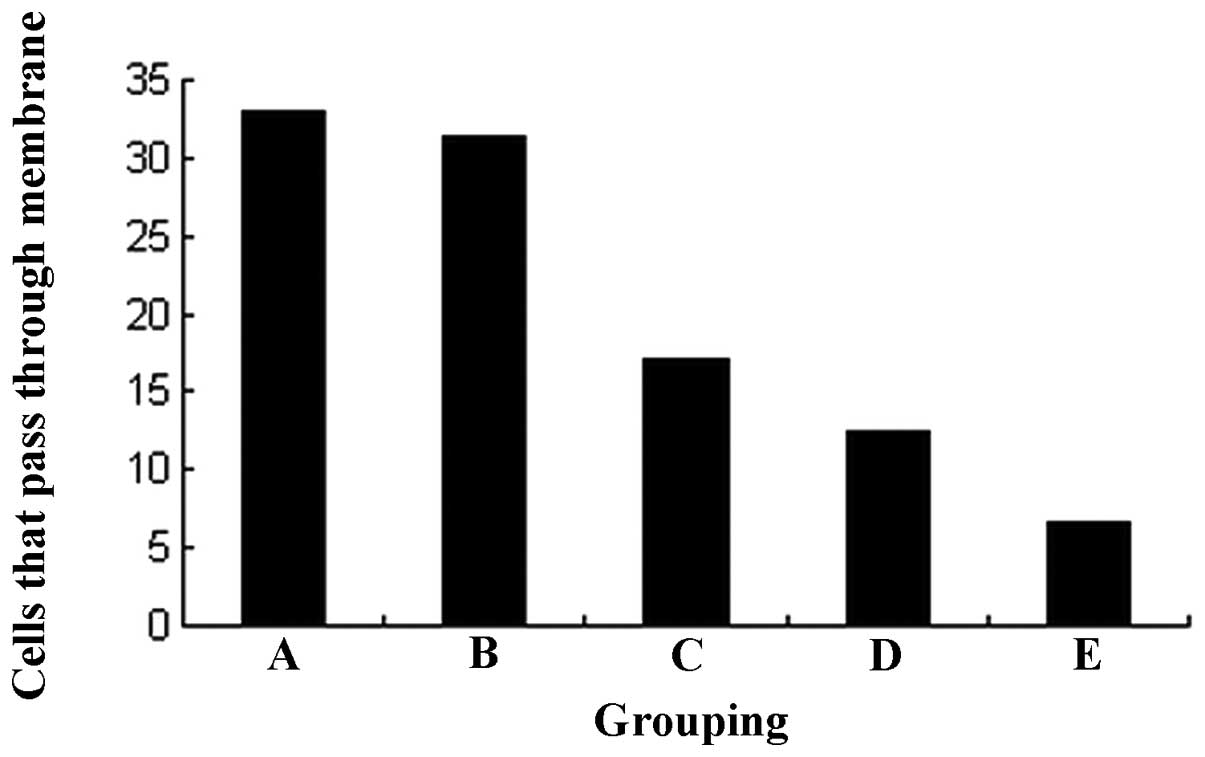
Changes in invasiveness
The results of the Transwell invasive test indicated
that the HEC-1B cells were able to permeate through the
polycarbonate membrane coated with Matrigel. However, the
invasiveness was greatly decreased by celecoxib in a
concentration-dependent manner. No statistical significance was
observed between the 1.0 μmol/l test group and the control
group (31.4±2.2 vs. 32.9±2.1, P>0.05). However, compared with
the control group, the number of cells that permeated through the
membrane was greatly decreased following treatment with 3.0, 9.0
and 27.0 μmol/l celecoxib (17.0±2.6, 12.5±2.1 and 6.7±1.2,
respectively, P<0.01; Fig.
3).
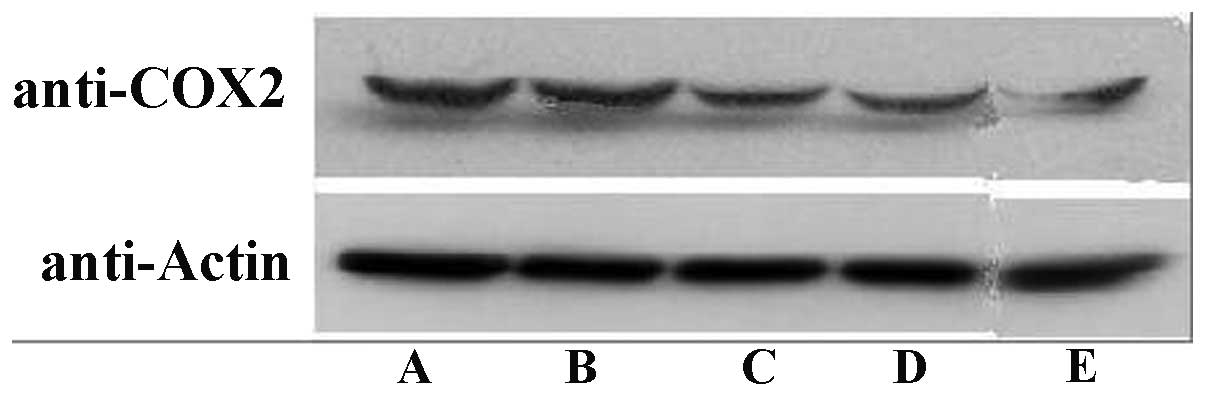
COX-2 expression
The results from the western blot analysis revealed
that celecoxib greatly attenuated the COX-2 expression in a
concentration-dependent manner. However, no significant difference
was observed between the 1.0 μmol/l celecoxib test group and
the control group. The inhibition of COX-2 expression followed a
concentration-dependent pathway (P<0.05; Fig. 4).
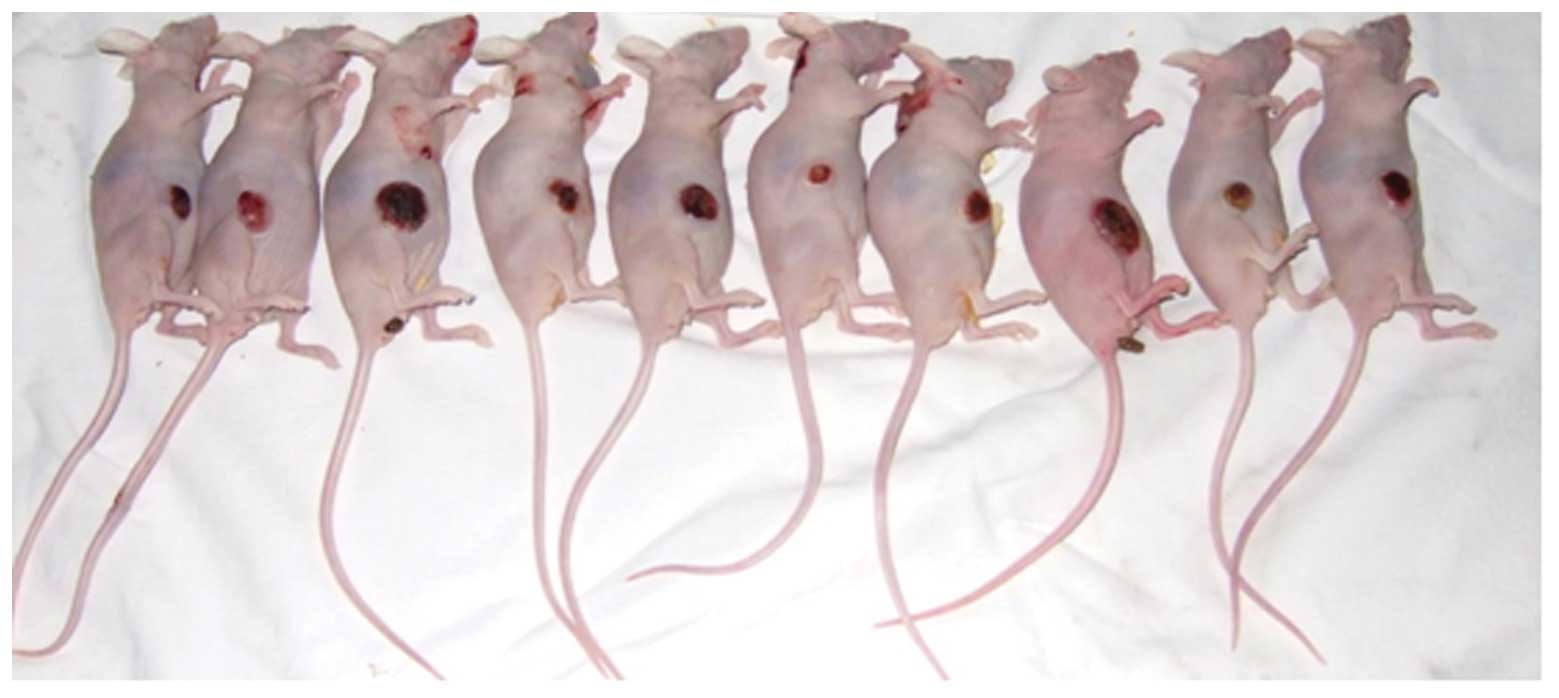
Endometrial adenocarcinoma model
Fourteen days after the subcutaneous inoculation,
all 35 nude mice with an observed tumor growth survived with a
balanced diet. According to the standards of cancer-bearing models,
30 mice were chosen for further experiments. The mice were
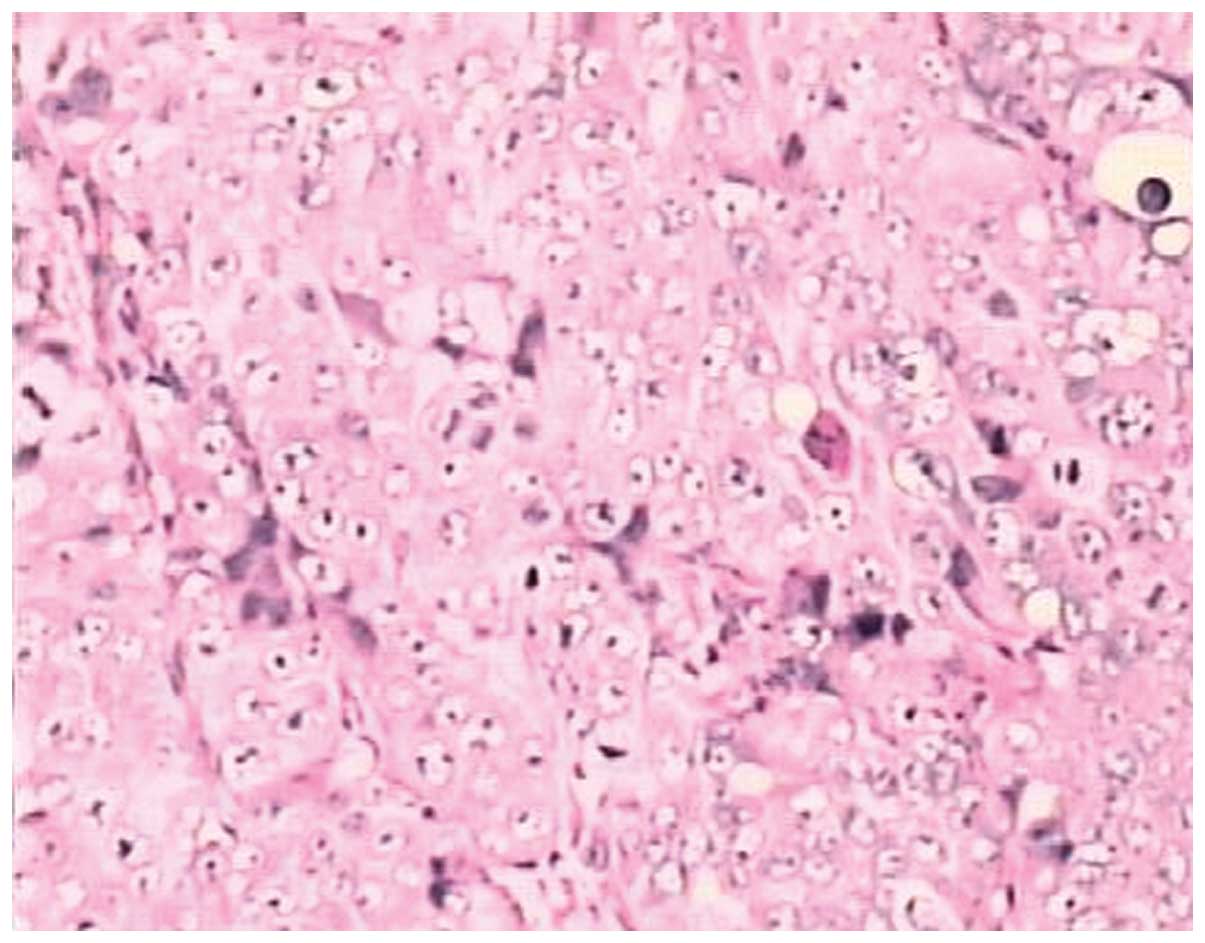
sacrificed and their tumor tissues were excised for pathological
examination using H&E staining. The morphologies of the model
and human endometrial adenocarcinoma tissues were identical. Thus,
the validity of the nude mice model of human endometrial
adenocarcinoma was confirmed (Figs.
5 and 6).
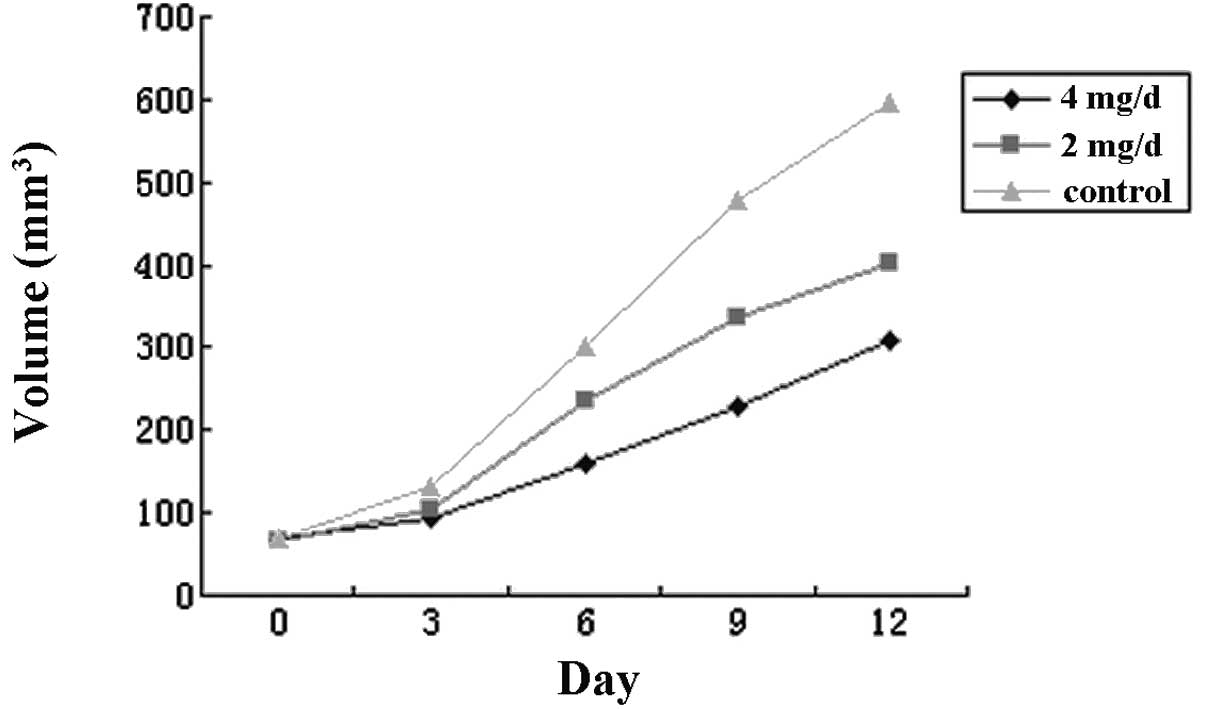
Xenograft growth in nude mice
The results indicated that the growth of the
xenograft was significantly decreased by celecoxib in a
concentration-dependent manner. The IRs were 32.4 and 48.6% for the
groups treated with 2 and 4 mg/day celecoxib, respectively.
Statistical significance was observed in the difference in the
weight and volume of the xenograft between the test and the control
groups and between the test groups treated with different doses
(P<0.05, P<0.01, respectively). The results are shown in
Fig. 7 and Table II.
 | Table II.Alteration in transplantation volume
and weight following celecoxib treatment (mean ± s). |
Table II.
Alteration in transplantation volume
and weight following celecoxib treatment (mean ± s).
| Group | Volume
(mm3) | Weight (g) | IR (%) |
|---|
| C, Control | 596.5±77.3 | 0.607±0.058 | 0 |
| B, 2 mg/day | 403.2±53.8a | 0.41±0.062b | 32.4 |
| A, 4 mg/day | 306.7±43.6a | 0.33±0.046b | 48.6 |
COX-2 expression and MVD in the
xenografts
The results from the immunochemical assay indicated
that COX-2 was mainly expressed in the cytoplasm of the tumor cells
in the form of buffy granules. Celecoxib dose-dependently
attenuated the expression of COX-2 and reduced the MVD. Statistical
significance was observed in the COX-2 expression level and MVD
between the test group and the control group (P<0.05 and
P<0.01, respectively). The expression level of COX-2 was
positively correlated with MVD (r=0.921, P<0.01) (Table III).
 | Table III.Effect of celecoxib on COX-2
expression and MVD in nude mice transplantation tumor. |
Table III.
Effect of celecoxib on COX-2
expression and MVD in nude mice transplantation tumor.
| Group | n | COX-2 | MVD |
|---|
| C, Control | 10 | 9.3±1.6 | 24.6±3.7 |
| B, 2 mg/day | 10 | 5.6±1.3a | 13.5±2.6b |
| A, 4 mg/day | 10 | 3.5±1.1a | 7.8±2.1b |
Discussion
Endometrial cancer, which is one of the three
malignant tumors of the female genital tract, is a malignant
epithelial tumor of the endometrium. COX is the key enzyme that
converts arachidonic acid to prostaglandins (PGs). COX isozymes 1
and 2 are the rate-limiting enzymes in the biosynthesis of PGs.
COX-1 and COX-2 convert arachidonic acid to PGH2.
PGH2 is then converted to various PGs by specific
synthases. COX-2 is induced by a variety of stimulants such as
cytokines, growth factors, oncogens and tumors, whereas COX-1 is a
non-inducible isozyme (9). Although
COX-2 selective inhibitors suppress many COX-2 expressing tumor
cells, the mechanism remains unclear at present. Liu et al
revealed that celecoxib suppresses the growth of liver cancer cells
and facilitates apoptosis (10).
During the investigation of the effect of celecoxib
on the human endometrial carcinoma cell line HEC-1A, Hasegawa et
al (11) demonstrated that
celecoxib effectively suppressed the proliferation of HEC-1A cells
and increased the proportion of cells in the G0/G1 phase (6). COX-2 is expressed in HEC-1B cells, and
its expression is gradually attenuated by different concentrations
of celecoxib solution (3.0-27.0 μmol/l). The results from
the MTT assay and flow cytometry indicated that the proliferation
of HEC-1B cells was markedly inhibited by celecoxib in a time- and
concentration-dependent manner. After treating the cells with
celecoxib for 24 h, the HEC-1B cells were characterized by an
increase in the proportion in the G0/G1 phase, blockage in the
G0/G1 phase, decreases in the proportions of cells in the S and
G2/M phases, and elevated apoptosis peak and apoptosis rate. These
results were consistent with those of Wei et al (5). Based on these results, celecoxib may
induce apoptosis and inhibit proliferation by downregulating the
expression of COX-2 in the HEC-1B cells.
Tumor invasion and metastasis are complicated
processes involving many steps, which begin with the exfoliation of
tumor cells and adhesion to the extracellular matrix and ultimately
lead to degradation of the cell matrix. The tumor attaches itself
to an organ to form a new metastasis following the proliferation of
malignant cells. Numerous cytokines and proteins are involved in
the process of tumor invasion and metastasis. In the Transwell
cabin, the Matrigel coating on the polycarbonate microporous film
contains laminin, fibronectin, and collagen IV extracted from the
Esh sarcomas of rats which are similar components to those of the
basement membrane. The Transwell test effectively imitates the
invasion progress in vitro. In this test, invasiveness is
characterized as the ability of the cells to pass through
8-μm pore films. The results from the Transwell invasive
test indicated that HEC-1B cells were capable of passing through
the Matrigel-coated polycarbonate membrane, and this invasiveness
was greatly decreased by celecoxib in a concentration-dependent
manner. No statistical significance was observed between the
control group and the test group treated with 1.0 μmol/l
celecoxib (P>0.05). However, the number of cells that passed
through the membrane greatly decreased when they were treated with
3.0, 9.0 or 27.0 μmol/l celecoxib (P<0.05). The present
study indicated that celecoxib attenuated the invasiveness of
HEC-1B cells by downregulating the expression of COX-2 (12).
The model involving nude mice with endometrial
adenocarcinoma xenografts was successfully established. The
validity of the model was confirmed by the observation that the
pathological morphology of the model and that of the human
endometrial adenocarcinoma tissue were identical. Pathological
karyokinesis was observed in the endometrial adenocarcinoma tissue
with a densely stained nucleus using a light microscope. An
immunohistochemical assay was used to evaluate the expression of
COX-2 and the MVD. The results indicated that the expression level
of COX-2 and the MVD were attenuated by celecoxib in a
concentration-dependent manner. Statistical significance was
observed in the COX-2 expression level and MVD between the test
group and the control group (P<0.05 and P<0.01 respectively).
The expression level of COX-2 positively correlated with MVD
(r=0.921, P<0.01).
The results from in vivo experiments
indicated that celecoxib inhibited the growth of the tumor
xenografts. This inhibition may be related to the inhibition of
COX-2 by celecoxib and a consequent inhibition of vasculogenesis,
which is the prerequisite of tumorigenesis and metastasis. Thus,
the inhibition of vasculogenesis is a significant indicator of the
inhibition of tumor progression and metastasis. COX-2 promotes
tumorigenesis through vasculogenesis induction and is catalyzed by
PGs. The overexpression of COX-2 in the tumor cells may lead to the
increased expression of PGE2, which is common in the
early stages of tumorigenesis. The reduction of PGE2
levels due to COX-2 inhibition results in a lowered cAMP level and
this promotes the apoptosis of endothelocytes and impedes capillary
genesis by inhibiting anti-apoptotic key enzymes, including Akt
(13). COX-2 is expressed in
numerous tissues, including human lung, breast, colon, prostate and
nascent blood vessel endothelium. COX-2 selective inhibitors such
as celecoxib inhibited vasculogenesis in the cornea, but COX-1
inhibitors did not (14). The
results indicated that COX-2 selective inhibitors suppress the
growth of tumor cells by inhibiting nascent capillary growth
through a number of biochemical mechanisms. The concentrations of
these inhibitors affect the progression of cancer cells, and may be
related to pathways involving Akt and JNK inhibition rather than
the inhibition of COX-2 alone.
In summary, the present study demonstrates that the
genesis, progression and invasion of endometrial adenocarcinoma is
closely correlated to the overexpression of COX-2 through upstream
regulation. The COX-2 selective inhibitor celecoxib inhibited the
progression of endometrial adenocarcinoma. This study provides
evidence for the potential of gene-targeted therapy and
drug-assisted therapy in the treatment of endometrial
adenocarcinoma. However, further studies are required to determine
whether COX-2 selective inhibitors may be used as effective means
for treating endometrial adenocarcinoma in clinical practice.
References
|
1.
|
P UharcekPrognostic factors in endometrial
carcinomaJ Obstet Gynaecol
Res34776783200810.1111/j.1447-0756.2008.00796.x
|
|
2.
|
S OhnoY OhnoN SuzukiG SomaM
InoueCyclooxygenase-2 expression correlates with apoptosis and
angiogenesis in endometrial cancer tissueAnticancer
Res2737653770200717970040
|
|
3.
|
HL LiHW ZhangDD ChenL ZhongXD RenR
St-TuJTE-522, a selective COX-2 inhibitor, inhibits cell
proliferation and induces apoptosis in RL95-2 cellsActa Pharmacol
Sin23631637200212100758
|
|
4.
|
NJ WoodNA QuintonS BurdallE SheridanSR
DuffyExploring the potential chemopreventative effect of aspirin
and rofecoxib on hereditary nonpolyposis colorectal cancer-like
endometrial cancer cells in vitro through mechanisms involving
apoptosis, the cell cycle, and mismatch repair gene expressionInt J
Gynecol Cancer17447454200710.1111/j.1525-1438.2007.00867.x
|
|
5.
|
SC WeiYS LinPN TsaoJJ Wu-TsaiCH WuJM
WongComparison of the anti-proliferation and apoptosis-induction
activities of sulindac, celecoxib, curcumin, and nifedipine in
mismatch repair-deficient cell linesJ Formos Med
Assoc103599606200415340658
|
|
6.
|
I MunirK FukunagaH KanasakiExpression of
cyclooxygenase 2 by prostaglandin E(2) in human endometrial
adenocarcinoma cell line HEC-1BBiol
Reprod63933941200010.1095/biolreprod63.3.93310952941
|
|
7.
|
Y GongLC MurphyLJ MurphyHormonal
regulation of proliferation and transforming growth factors gene
expression in human endometrial adenocarcinoma xenograftsJ Steroid
Biochem Mol Biol501319199410.1016/0960-0760(94)90167-88049128
|
|
8.
|
R EitanCC SaenzES VenkatramanPilot study
prospectively evaluating the use of the measurement of preoperative
sonographic endometrial thickness in postmenopausal patients with
endometrial
cancerMenopause122730200510.1097/00042192-200512010-00007
|
|
9.
|
K SubbaramaiahD ZakimBB WekslerAJ
DannenbergInhibition of cyclooxygenase: a novel approach to cancer
preventionProc Soc Exp Biol
Med216201210199710.3181/00379727-216-441709349689
|
|
10.
|
NB LiuT PengC PanYY YaoB ShenJ
LengOverexpression of cyclooxygenase-2 in human HepG2, Bel-7402 and
SMMC-7721 hepatoma cell lines and mechanism of cyclooxygenase-2
selective inhibitor celecoxib-induced cell growth inhibition and
apoptosisWorld J Gastroenterol1162816287200516419156
|
|
11.
|
K HasegawaY OhashiK IshikawaExpression of
cyclooxygenase-2 in uterine endometrial cancer and anti-tumor
effects of a selective COX-2 inhibitorInt J
Oncol2614191428200515809736
|
|
12.
|
Y CaoSM PrescottMany actions of
cyclooxygenase-2 in cellular dynamics and in cancerJ Cell
Physiol190279286200210.1002/jcp.1006811857443
|
|
13.
|
E FosslienMolecular pathology of
cycloxygenase-2 in neoplagiaJ Ann Clin Lab Sci303212000
|
|
14.
|
JL MasferrerKM LeahyAT KokiAntiangiogenic
and antitumor activities of cyclooxygenase-2 inhibitorsCancer
Res6013061311200010728691
|