Introduction
Synchronous primary endometrial and ovarian cancers
coexist in approximately 10% of all females with ovarian cancer and
in 5% of all females with endometrial cancer. These cancers are
uncommon and often misdiagnosed as FIGO stage III of endometrial
cancer or FIGO stage II of ovarian cancer (1). Several molecular analyses, including
immunohistochemistry, DNA flow cytometry and gene mutation
analysis, have been adapted in order to elucidate more definitely
the dilemma of diagnosis; however, to date there is no consensus on
the most appropriate method (2,3).
Previous studies have suggested that patients diagnosed with
synchronous primary cancers have a better overall prognosis than
those classified as single organ disease with metastasis. A
Gynecologic Oncology Group (GOG) study reported that 74 patients
with simultaneously detected endometrial and ovarian cancer limited
to the pelvis without distinction between independent primary sites
and metastasis had a 5-year survival rate of 86% and a 10-year
survival rate of 80% (4).
In the present study, 43 patients with synchronous
primary endometrial and ovarian cancers diagnosed and treated at
the Obstetrics and Gynecology Hospital of Fudan University
(Shanghai, China) between 1999 and 2009 were retrospectively
reviewed.
Patients and methods
Patient characteristics
Between 1999 and 2009, the medical records and
pathological reports in the Obstetrics and Gynecology Hospital of
Fudan University (Shanghai, China) database of 43 patients were
retrospectively reviewed. The pathological specimens were reviewed
and diagnosed by two gynecological pathologists according to the
criteria described by Young and Scully (5): i) histological dissimilarity of the
tumors; ii) no or only superficial myometrial invasion of
endometrial tumor; iii) no vascular space invasion of endometrial
tumor; iv) atypical endometrial hyperplasia additionally present;
v) absence of other evidence of spread of endometrial tumor; vi)
ovarian unilateral tumor (80–90% of cases); vii) ovarian tumor
located in parenchyma; viii) no vascular space invasion, surface
implants or predominant hilarlocation in ovary; ix) absence of
other evidence of spread of ovarian tumor; x) ovarian endometriosis
present; xi) different ploidy of DNA indices, if aneuploid, of the
tumors; and xii) dissimilar molecular genetic or karyotypic
abnormalities in the tumors.
All the patients were followed up for at least 12
months. The clinicopathological data included age at diagnosis,
presenting symptoms, body mass index (BMI), parity, personal and
family history, assisted examination, treatment, histology, grade
and recurrence. Patients were considered lost to follow-up when the
duration of follow-up was <12 months following surgery (if no
recurrence had occurred within that period).
The study was approved by the ethics committee of
Fudan University, Shanghai, China. Written informed patient consent
was obtained from the patient.
Statistical analysis
The data were analyzed using SPSS 13.0 software.
Recurrence-free interval rates were determined using the
Kaplan-Meier method. Recurrence-free intervals were calculated even
for patients who had relapse. The log-rank test was used to test
differences in survival within variables. P<0.05 was considered
to indicate a statistically significant result.
Results
Age and BMI
The median age of the 43 patients at the time of
diagnosis was 51 years (range, 29–71). Two (4.7%) patients were
aged <40 years and 18 (41.9%) patients were aged ≤50 years. A
total of 17 patients were menopausal (39.5%), 8 patients were
nulliparous (18.6%), the median BMI was 23.44 kg/m2
(range, 15.79–36.33), 7 (16.3%) of the 43 patients were obese (BMI
>28). One patient received hormone replacement treatment prior
to admission to hospital. Data shown in Table I.
 | Table ICharacteristics of the patients with
synchronous primary endometrial and ovarian cancers. |
Table I
Characteristics of the patients with
synchronous primary endometrial and ovarian cancers.
| Characteristic | Number | % |
|---|
| Symptoms (n=43) | | |
| Abnormal uterine
bleeding | 28 | 65.12 |
| Abdominal pain | 17 | 39.53 |
| Abdominal mass | 11 | 25.58 |
| Vaginal
discharge | 1 | 2.3 |
| CA125 (n=26) | | |
| Normal | 6 | 23.08 |
| <500 U/ml | 12 | 46.15 |
| >500 U/ml | 8 | 30.77 |
| Ultrasound
examination (n=40) | | |
| Abdominal mass | 34 | 85.0 |
| Uterine
enlargement | 17 | 42.5 |
| Endometrial
thickening | 9 | 22.5 |
| Mass in uterine
cavity | 17 | 42.5 |
| Pelvic hydrops | 13 | 32.5 |
| D and C (n=25) | | |
| Endometrial
carcinoma | 23 | 92.0 |
| Complex
hyperplasia | 2 | 8.0 |
Chief complaint and symptoms
The most common presenting symptoms were abnormal
uterine bleeding (AUB; n=28; 65.12%), abdominal pain and abdominal
fullness (n=17; 39.53%) and abdominal mass (n=11; 25.58%); only one
case exhibited vaginal discharge (n=1; 2.3%). Data shown in
Table I.
Laboratory findings
The CA125 level was assessed in 26 patients, 20 of
whom had an elevated CA-125 level (76.92%), the median CA125 was
161.7 U/ml. Eight patients had >500 U/ml (30.77%) Data shown in
Table I..
Assisted examination
Ultrasonography is the most common radiographic test
to evaluate adnexal masses and endometrial thickening. A total of
40 patients underwent pelvic ultrasound examination, the results of
which revealed that 34 patients had an abdominal mass (85%), 17
patients had uterine enlargement (42.5%), 9 patients had
endometrial thickening (22.5%), 17 patients were found to have a
mass occupation in the uterine cavity (42.5%) and 13 patients had
pelvic hydrops (32.5%). Data shown in Table I.
Pathological specimens from 25 patients were
obtained by diagnostic curettage, and of these 23 patients had
endometrial carcinoma and 2 had endometrial complex hyperplasia.
One of the patient’s ascites smear was positive prior to
surgery.
Pathological findings
Clinical stage
Most patients were diagnosed as early stage (stage I
and II) in both uterus and the ovary. According to FIGO stage, 27
cases were endometrial cancer stage I (62.8%), 9 cases were
endometrial cancer stage II (20.9%) and 7 cases were endometrial
cancer stage III (16.3%). Of the ovarian cancers, 27 cases were
stage I (62.8%), 4 cases were stage II (9.3%) and 12 cases were
stage III (27.9%). Data shown in Table
II.
 | Table IIHistological characteristics of 43
patients. |
Table II
Histological characteristics of 43
patients.
| Endometrial cancer
| Ovarian cancer
|
|---|
| FIGO stage | Number | % | Number | % |
|---|
| I | 27 | 62.8 | 27 | 62.8 |
| II | 9 | 20.9 | 4 | 9.3 |
| III | 7 | 16.3 | 12 | 27.9 |
| IV | 0 | 0 | 0 | 0 |
Pathological type. Histological
characteristics of the 43 cases are shown in Table III. Endometrioid cancer (n=26,
60.47%) was the main pathological type in uterine carcinoma and the
other pathological types included serous adenocarcinoma, clear cell
carcinoma, adenosquamous and acanthoadenocarcinoma. Ovarian cancer
mostly occurred unilaterally (n=32; 74.4%), however 11 cases
(25.58%) had bilateral involvement of the ovary. The majority of
pathological types were endometrioid adenocarcinoma and serous
adenocarcinoma. In the present study, we noted specific
pathological types of endometrial and ovarian cancers, such as
endometrioid polypus canceration in the uterus with endometrioid
carcinoma in the left ovary and carcinosarcoma in the right side.
None of the 43 patients had vascular invasion or lymph node
metastasis.
 | Table IIIDifferent pathological characteristics
in synchronous primary endometrial and ovarian cancers. |
Table III
Different pathological characteristics
in synchronous primary endometrial and ovarian cancers.
| Ovarian cancer
|
|---|
| Endometrial
cancer | Endometrioid | Serous
adenocarcinoma | Clear cell | Adenosquamous | Other |
|---|
| Endometrioid | 12 | 1 | 1 | 2 | 9 |
| Serous
adenocarcinoma | 0 | 7 | 1 | 0 | 0 |
| Clear cell | 0 | 0 | 0 | 0 | 1 |
| Adenosquamous | 0 | 0 | 0 | 0 | 1 |
| Other | 2 | 0 | 1 | 0 | 2 |
Treatment
Surgical treatment
All patients initially underwent surgery in our
hospital. Of the 43 patients, 6 had hysterectomy and bilateral
salpingo-oophorectomy and the remaining 37 patients had total
hysterectomy, bilateral salpingo-oophoretomy, omentectomy with
appendectomy and pelvic lymph node dissection.
Adjuvant therapy
A total of 38 patients received platin-based
adjuvant chemotherapy, 3 patients received both adjuvant
chemotherapy and radiotherapy and 4 patients received neoadjuvant
chemotherapy. Data shown in Table
IV.
 | Table IVAdjuvant therapy of the synchronous
primary endometrial and ovarian cancers (n=43). |
Table IV
Adjuvant therapy of the synchronous
primary endometrial and ovarian cancers (n=43).
| Adjuvant therapy | Number | % |
|---|
| Neoadjuvant
chemotherapy | 4 | 9.3 |
| Chemotherapy | 38 | 88.4 |
| Chemotherapy and
radiotherapy | 3 | 6.98 |
Recurrence and prognosis
Nine patients had recurrence (20.93%). The median
time to recurrence was 10 months (range, 5–30). The five-year
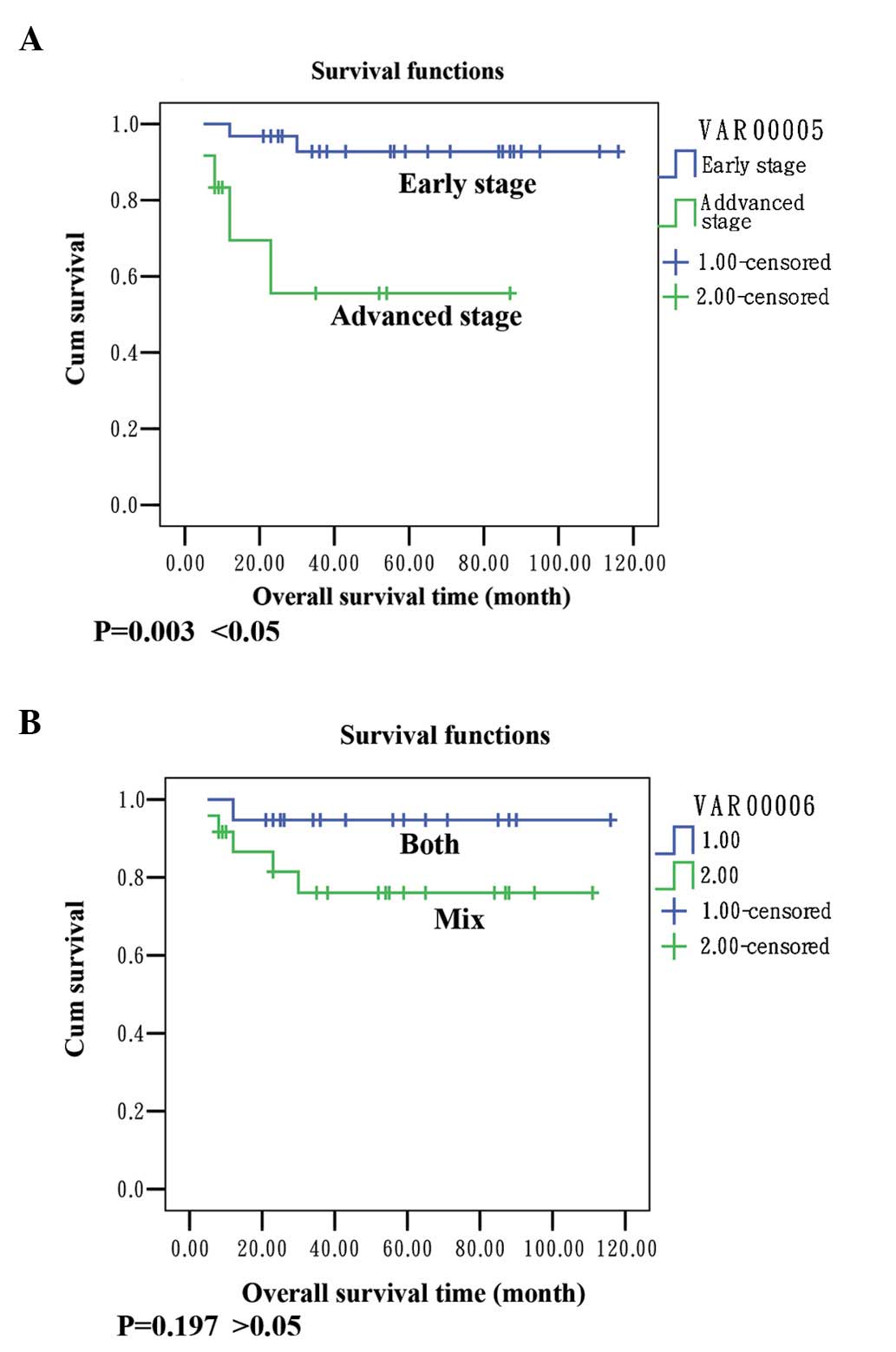
survival rate of the patients was 86.05%. Data shown in Fig. 1.
Discussion
Synchronous primary endometrial and ovarian cancers
coexist in approximately 10% of all females with ovarian cancer and
in 5% of all females with endometrial cancer. These cancers are
uncommon and often misdiagnosed as FIGO stage III of endometrial
cancer or FIGO stage II of ovarian cancer (1). In our study, the incidence was 3.08%
in patients with endometrial cancer and 3.18% in patients with
ovarian cancer. Our study was conducted in a single institution
rather than multicenter analysis, the study only included cases
with confirmed diagnosis of synchronous tumors and other
conditions, such as primary endometrial cancer with ovarian
metastasis and primary ovarian cancer with endometrial metastasis,
were excluded.
Synchronous primary endometrial and ovarian cancers
are unlike endometrial or ovarian cancer alone (6). Pathologists have listed histological
criteria to evaluate these tumors. Molecular profiling in
synchronous endometrioid and ovarian cancers may aid in determining
a differential diagnosis. Halperin et al reported that 62.5%
of synchronous primary endometrial and ovarian cancers can be
classified by detection of ER and PR content and that 31.3% of
synchronous primary endometrial and ovarian cancers can be
identified by detecting Bcl-2 (6).
The median age of patients with synchronous primary
endometrial and ovarian cancers was 50 years, and the median age of
those with endometrial cancer or ovarian cancer was 60 years, so
the incidence age of primary carcinoma was lower. Previous studies
have also reported a younger median age in patients with
synchronous primary endometrial and ovarian cancers (7). In a prospective series of 74 patients
with simultaneously detected endometrial and ovarian cancers, the
GOG reported a median age of 49 years. Synchronous primary
endometrial and ovarian cancer patients are younger than those who
develop endometrial or ovarian cancer alone. In our study, the
median age of the patients at the time of diagnosis was 51 years
(range, 29–71).
Obesity is a well-known risk factor for the
development of endometrial cancer. In the study by Soliman et
al, 36% of patients (17/47) in the endometrioid/endometrioid
group, 30% of patients (3/10) in the endometrioid/serous group and
40% of patients (2/5) in the endometrioid/clear cell group were
obese (BMI >30) (7). Nishimura
et al reported that the mean BMI of Japanese females with
synchronous primary endometrial and ovarian cancers was 22.6±3.4
kg/m2 (range, 16–31) (8), but the authors did not report the
obesity rates. In the present study, the median BMI was 23.44
kg/m2 (range, 15.79–36.33), only 7 (16.3%) of the 43
patients were obese (BMI >28). Asian ethnicity may be correlated
with a lower obesity rate.
The clinical symptom and sign of synchronous primary
endometrial and ovarian cancers are similar to the independent
endometrial and ovarian cancers. AUB and abdominal mass were the
main symptoms. In accordance with the results described in the
literature, the common presenting symptoms in the present study
were AUB (65.12%), abdominal mass (25.58%), abdominal pain and
abdominal fullness (39.53%).
Surgical treatment is the main treatment for
endometrial and ovarian cancers. Synchronous primary endometrial
and ovarian cancers are often misdiagnosed as FIGO stage III of
endometrial cancer or FIGO stage II of ovarian cancer in early
years, hence patients are often over-treated. Although cancer of
both uterine body and that of the ovary occurs simultanenously,
pathological changes are mainly of early stage. The scope of
general surgery included hysterectomy, bilateral
salpingo-oophorectomy, omentectomy and appendectomy. Pelvic lymph
node dissection depended on the pathological findings (9,10). The
treatment of patients with ovarian cancer is generally based on
chemotherapy with paclitaxel plus cisplatin (TP) regimen or
cisplatin + doxorubicin + cyclophosphamide (PAC) regimen and the
length of treatment ranges from 2 to 10 cycles, generally 3 to 6
cycles; however, patients with stage I or II grade 1 ovarian cancer
do not require chemotherapy. In 1982, Eifel proposed that patients
who have the following risk factors of endometrial cancer should
receive adjuvant radiotherapy: i) pathological type of papillary
serous adenocarcinoma or adenosquamous; ii) tumor differentiation
as G2, G3 grade; iii) deep myometrial invasion.
However, the adjuvant treatment for these patients
is controversial. In our study, surgical treatment was used for all
patients; 6 patients underwent hysterectomy and bilateral
salpingo-oophorectomy and the remaining 37 patients had total
hysterectomy, bilateral salpingo-oophoretomy, omentectomy,
appendectomy and pelvic lymph node dissection. The majority of
patients received postoperative platinum-based chemotherapy. A
total of 38 patients received adjuvant chemotherapy, 3 patients
received both adjuvant chemotherapy and radiotherapy and 4 patients
received neoadjuvant chemotherapy. It is difficult to evaluate the
prognosis of these patients without consideration of the impact of
adjuvant chemotherapy.
Our results showed that the mean survival in the
group with early stage (I and II) disease was 109 months and the
survival rate was 93.5%; the mean survival was 54 months in the
group with advanced stage (III/IV) disease and the survival rate
was 63.7%. There was a statistically significant difference between
the groups (P=0.003 <0.05; Fig.
1A). The survival rate in the group of endometrioid carcinoma
in the endometrium and ovaries was 94.1%, the survival rate in the
group with mixed pathological type was 80.8% and the result was not
significant (P= 0.197 >0.05; Fig.
1B). Ayhan et al concluded that the stage of ovarian
cancer and grade of endometrial cancer are important prognostic
factors (4). Our results indicated
that the stage had a greater influence on the survival than the
histology. We suggest that the advanced stage has a detrimental
influence on survival and was a poor prognostic predicator in
synchronous primary endometrial and ovarian cancers.
References
|
1
|
Zaino R, Whitney C, Brady MF, et al:
Simultaneously detected endometrial and ovarian carcinomas - a
prospective clinicopathologic study of 74 cases: a gynecologic
oncology group study. Gynecol Oncol. 83:355–362. 2001. View Article : Google Scholar
|
|
2
|
Kaneki E, Oda Y, Ohishi Y, et al: Frequent
microsatellite instability in synchronous ovarian and endometrial
adenocarcinoma and its usefulness for differential diagnosis. Hum
Pathol. 35:1484–1493. 2004. View Article : Google Scholar
|
|
3
|
Ricci R, Komminoth P, Bannwart F, et al:
PTEN as a molecular marker to distinguish metastatic from primary
synchronous endometrioid carcinomas of the ovary and uterus. Diagn
Mol Pathol. 12:71–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ayhan A, Guvenal T, Coskun F, et al:
Survival and prognostic factors in patients with synchronous
ovarian and endometrial cancers and endometrial cancer mestastatic
to the ovaries. Eur J Gynaecol Oncol. 24:171–174. 2003.PubMed/NCBI
|
|
5
|
Young RH and Scully RE: Metastasic tumors
of the ovary. Blaustein’s Gynecological Pathology of the Female
Genital Tract. Kurman RJ: 6th edition. Springer; New York: pp.
987–990. 2002
|
|
6
|
Halperin R, Zehavi S, Hadas E, et al:
Simultaneous carcinoma of the endometrium and ovary vs endometrial
carcinoma with ovarian metastases: a clinical and
immunohistochemical determination. Int J Gynecol Cancer. 13:32–37.
2003. View Article : Google Scholar
|
|
7
|
Soliman PT, Slomovitz BM, Broaddus RR, et
al: Synchronous primary cancers of the endometrium and ovary, a
single institution review of 84 cases. Gynecol Oncol. 94:456–462.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura N, Hachisuga T, Yokoyama M, et
al: Clinicopathologic analysis of the prognostic factors in women
with coexistence of endometrioid adenocarcinoma in the endometrium
and ovary. J Obstet Gynaecol Res. 31:120–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiang YC, Chen CA, Huang CY, et al:
Synchronous primary cancers of the endometrium and ovary. Int J
Gynecol Cancer. 18:159–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Signorelli M, Fruscio R, Lissoni AA, et
al: Synchronous early-stage endometrial and ovarian cancer. Int J
Gynaecol Obstet. 102:34–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eifel PJ, Ross J, Hendrickson M, Cox RS,
Kempson R and Martinez A: Adenocarcinoma of the endometrium.
Analysis of 256 cases with disease limited to the uterine corpus:
treatment comparisons. Cancer. 52:1026–1031. 1983. View Article : Google Scholar : PubMed/NCBI
|