Introduction
Mutations of the human tumour suppressor gene
adenomatous polyposis coli (APC) are frequent in both sporadic and
familial colorectal cancer (CRC) (1). Wild-type APC protein contributes to
destabilisation and degradation of β-catenin, which is a central
effector molecule in the Wnt/β-catenin signalling pathway. Loss of
APC function results in nuclear accumulation of β-catenin, which
leads to transcriptional activation, through the β-catenin/T-cell
factor complex, of target genes which may contribute to colorectal
tumourigenesis (2,3).
Insulin-like growth factors (IGFs), including IGF-1
and IGF-2, have been implicated in the development of CRC and their
effects are regulated in part by insulin-like growth factor binding
proteins (IGFBPs), which have both low and high affinity for IGFs
(4). IGFBP7, also known as IGFBP-
related protein 1 (IGFBP-rP1), is widely expressed in various
tissues, including the lung, brain, prostate and gastrointestinal
tract (5). IGFBP7 has been shown to
regulate cell proliferation, cell adhesion, differentiation and
angiogenesis in various types of cancer (6–8) and
plays a potential tumour suppressor role against colorectal
carcinogenesis (9,10). Moreover, altered expression of
IGFBP7 has been demonstrated in CRC. Down- (11) and upregulation (8,12)
patterns compared with normal tissue have been reported.
Epigenetic modifications of DNA have been postulated
to play a role in the development of multiple neoplasms in CRC
(13,14). DNA methylation of cytosine residues
in CpG dinucleotides leads to transcriptional silencing of
associated genes. Promoters with methylated CpG units, which have
their transcriptional activity lowered, may function as an
alternative mechanism of repressing tumour suppressor genes. The
aberrant methylation of gene promoter regions has been widely
studied and this epigenetic event in human malignancies may affect
the cell cycle control and differentiation (13–15).
The APC promoter methylation rate has been detected
in CRC and normal colorectal mucosa at a range of 11 to 62% in
different populations, and has been suggested to moderate the Wnt
signalling pathway (16–20). Studies have indicated that IGFBP7 is
inactivated by DNA methylation in human colon, lung and breast
cancer (21–23).
In the present study we used methylation-specific
polymerase chain reaction (MSP) to study the methylation status of
the APC and IGFBP7 genes in cancerous and paired normal tissues to
evaluate its impact on clinical factors and association with
ethnicity, represented by Swedish and Vietnamese CRC patients.
Furthermore, we also investigated the distribution of CpG islands
and the CpG dinucleotide density of each CpG island in the regions
that were the subject of our discussion of methylation status.
Materials and methods
Patients and tissue sampling
The subjects of this study were 52 CRC patients from
southeastern Sweden and 49 CRC patients from northern Vietnam.
Tissue samples were collected when the patients underwent surgical
resection for primary colorectal adenocarcinomas at the Department
of Surgery, Ryhov County Hospital (Jönköping, Sweden) and the
Department of Pathology, National Cancer Hospital (Tamhiep, Hanoi,
Vietnam). Clinicopathological characteristics of the patients were
obtained from surgical and pathological records. Tumour tissue and
adjacent normal mucosa (∼5 cm from the tumour) from each patient
were excised and immediately frozen at −80°C until analysis.
The Swedish patient group consisted of 30 males and
22 females with a mean age of 68 years (range, 29–85). The tumours
were located in the colon (n=31) and rectum (n=21) and were
classified according to the American Joint Committee on Cancer
(AJCC) classification system: stage I, n=3; stage II, n=20; stage
III, n=18; and stage IV, n=11. The Vietnamese patients comprised 28
males and 21 females with a mean age of 57 years (range, 26–87) and
were classified as stage I, n=26; stage II, n=5; stage III, n=17;
and stage IV, n=1. The tumours of the Vietnamese patients were
located in the colon (n=20) or rectum (n=29). Informed consent was
obtained from each subject and the study was approved by the ethics
committee at the Faculty of Health Sciences Linköping, Sweden and
by the guidelines of the local ethics committee in Vietnam
Cell lines
An established human colon cancer cell line, HT-29,
was purchased from the American Type Culture Collection (ATCC,
Rockville, MD, USA). The cell line was grown in the growth medium
McCoy’s 5A according to the supplier’s instructions.
DNA extraction, bisulphite modification
and MSP
DNA was isolated from tissue samples and the cell
line using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany).
Purified DNA (0.5 μg) was treated with bisulphite and purified
using the EZ DNA methylation-gold kit (Zymo Research, Irvine, CA,
USA) according to the manufacturer’s instructions.
MSP was performed as previously described (17,23,24).
The primers were synthesised commercially (TIB Molbiol, Berlin,
Germany) with sequences based on a previous study (17,23) as
follows: APC forward, 5′-TATTGCGGAGTGCGGGTC-3′ and reverse,
5′-TCGACGAACTCCCGACGA-3′ for the methylated reaction (17); APC forward,
5′-GTGTTTTATTGTGGAGTGTGGGTT-3′ and reverse,
5′-CCAATCAACAAACTCCCAACAA-3′ for the unmethylated reaction
(17); IGFBP7 forward,
5′-AGAAATTAGAGGGTGGAAGAGTCGT-3′ and reverse,
5′-CTACTAACGTCGAAAAATAAACGAA-3′ for the methylated reaction
(23); IGFBP7 forward,
5′-AGAAATTAGAGGGTGGAAGAGTTG-3′ and reverse,
5′-CTACTAACATCAAAAAATAAACAAA-3′ for the unmethylated reaction
(23).
The methylated and unmethylated MSP conditions for
APC were as follows: initial cycle at 95°C for 15 min followed by
35 cycles at 95°C for 15 sec, 60°C for 45 sec, 72°C for 30 sec and
final elongation at 72°C for 10 min. The amplified 98-bp product
for the methylated signal and the 108-bp product for the
unmethylated signal were visualised by UV-illumination on 2%
agarose gel containing Gel Red (Biotium, Inc., Hayward, CA,
USA).
The total volume of the PCR mixture was 25 μl and
contained 60 ng bisulphite-modified DNA, 0.5 μM of each primer (TIB
Molbiol), 1.5 mM MgCl2, 200 μM of each deoxynucleotide
triphosphate, 2.5 units Taq DNA polymerase and reaction
buffer [20 mM Tris-HCl (pH 8.3), 20 mM KCl, 5 mM
(NH4)2SO4 (Fermentas, Burlington,
Canada)].
The methylated and unmethylated MSP conditions for
IGFBP7 were as follows: initial cycle at 95°C for 4 min followed by
8 cycles at 95°C for 2 min, 60°C for 30 sec, 72°C for 30 sec; 32
cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec and
then a final elongation at 72°C for 5 min. The amplified 173-bp
products for both methylated and unmethylated signals were
visualised by UV-illumination on 2% agarose gel containing Gel Red
(Biotium, Inc.).
The total volume of the PCR mixture was 25 μl and
contained 60 ng bisulphite-modified DNA, 0.35 μM of each primer
(TIB Molbiol), 1.5 mM MgCl2, 200 μM of each
deoxynucleotide triphosphate, 2.5 units Taq DNA polymerase
and reaction buffer [20 mM Tris-HCl (pH 8.3), 20 mM KCl, 5 mM
(NH4)2SO4 (Fermentas)].
CpG island analysis
Using RefSeqGene of APC (GenBank: NG_008481.4) and
(GenBank: NG_031877.1) all potential transcription start sites
(TSSs) were identified. Then 3,000 bp, including 2,000 bp of
sequence extending from the 5′ upstream region to 1,000 bp
downstream of the TSS, were selected to submit to the MethPrimer
(25) and cpgplot programmes
(EMBOSS) (26) for analysis of the
CpG islands.
Promoter prediction
The 3,000-bp sequence of the IGFBP7 gene (GenBank:
NG_031877.1) was entered into the programmes of FirstEF (27) and Proscan (28) for its promoter prediction.
Statistical analysis
The Chi-square test was used to investigate the
difference in the methylation status of the groups. Statistical
analyses were performed using SPSS for Windows computer package
(Rel. 14.0, SPSS Inc., Chicago, IL, USA, 2005). P<0.05 was
considered to indicate a statistically significant result.
Results
Distribution of CpG islands in selected
region of the APC and IGFBP7 genes
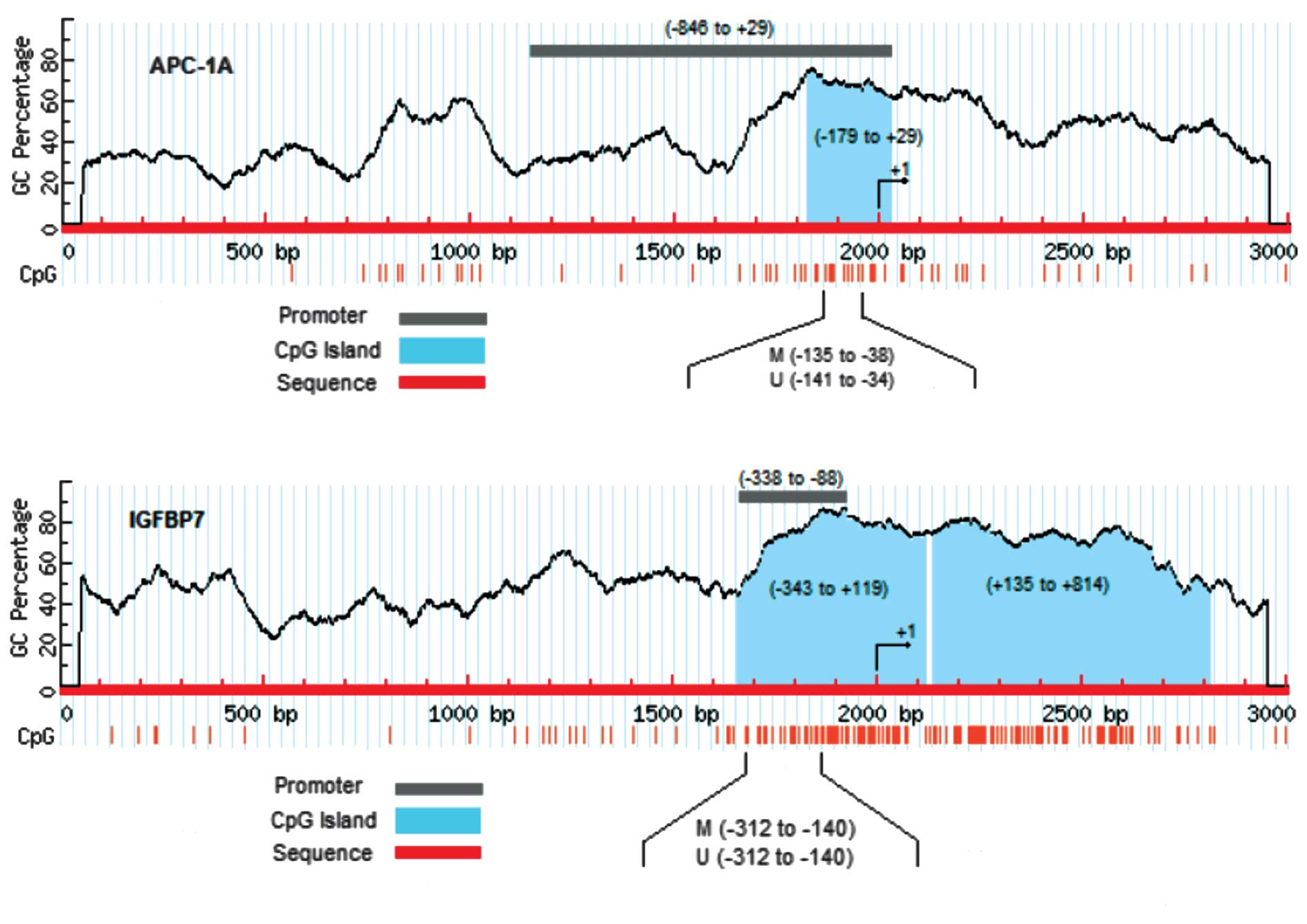
Using the MethPrimer and cpgplot programmes, 4 CpG
islands around TSS-1 (NM_001127511.1) and 1 CpG island around
TSS-2–3 (NM_001127510.1 and NM_000038.4) were determined for the
APC gene. The result of the CpG island analysis for the IGFBP7 gene
was 2 CpG islands (Fig 1).
In order to study promoter methylation, the promoter
sequences were searched in the nucleotide database. The APC
promoter sequence was found in GenBank (U02509). However, the
IGFBP7 gene promoter was not found, therefore we predicted the
promoter using two programmes (FirstEF and Proscan). The predicted
result of the IGFBP7 promoter sequence was from −338 to −88
(Proscan) and −333 to +237 (FirstEF) in the positions relative to
the TSS (+1).
In comparison of the CpG islands and promoter
sequences, the CpG island of the APC gene in position −179 to +29
is located within the 1A promoter region (accession No. U02509) and
the CpG island of the IGFBP7 gene in position −343 to +119 is
located in the predicted promoter region of the IGFBP7 gene.
Therefore, DNA sequences of these islands were used as templates
for MSP analysis (Fig. 1).
APC and IGFBP7 methylation status in CRC
tissues and paired normal tissues
An MSP assay was performed for APC in tissues from
52 Swedish CRC patients and 49 Vietnamese CRC patients and for
IGFBP7 in 51 of the tissues from the Swedish patients and 48 of the
Vietnamese patients. Representative band profiles of the MSP
reactions of the APC and IGFBP7 are illustrated in Fig. 2. Methylated (M) and unmethylated (U)
signals of the IGFBP7 and APC genes were detected in HT-29 cells
and colorectal tissue (Fig. 2).
For APC, no biallelic methylation (M/M) signals were
detected in colorectal tissue either in Swedish or Vietnamese
patients (Table I). However, the
groups exhibited a pattern that included both monoallelic
methylation (M/U) and biallelic unmethylation (U/U; Table I).
 | Table IPrevalence of APC and IGFBP7 DNA gene
methylation (M) and unmethylation (U) in CRC tissue and matched
normal tissue in Swedish and Vietnamese patients. |
Table I
Prevalence of APC and IGFBP7 DNA gene
methylation (M) and unmethylation (U) in CRC tissue and matched
normal tissue in Swedish and Vietnamese patients.
| Gene and
tissue | M/M | M/U | U/U |
|---|
| APC | | | |
| Swedish patients
(n=52) | | | |
| Cancer
tissue | 0 | 28 | 24 |
| Normal
tissue | 0 | 37 | 15 |
| Vietnamese
patients (n=49) | | | |
| Cancer
tissue | 0 | 22 | 27 |
| Normal
tissue | 0 | 29 | 20 |
| IGFBP7 | | | |
| Swedish patients
(n=51) | | | |
| Cancer
tissue | 25 | 20 | 6 |
| Normal
tissue | 22 | 29 | 0 |
| Vietnamese
patients (n=48) | | | |
| Cancer
tissue | 20 | 23 | 5 |
| Normal
tissue | 16 | 27 | 5 |
With regard to the IGFBP7 gene, we observed each
combination of methylation and unmethylation status in all tissue
samples from the two groups, with the exception of the combination
U/U in normal tissue from Swedish patients (Table I).
In the cancerous tissue from Swedish patients,
methylation in the APC and IGFBP7 genes was detected in 53.8
(28/52) and 88.2% (45/51) of the samples, respectively. In
cancerous tissue from Vietnamese patients, the methylation rate in
the APC and IGFBP7 genes was 44.9 (22/49) and 89.6% (43/48),
respectively (Table I).
The methylation rates of the APC and IGFBP7 genes in
normal tissue from Swedish patients were 71.1 (37/52) and 100%
(51/51), respectively. APC and IGFBP7 gene methylation in the
normal tissue of Vietnamese patients was present in 59.2 (29/49)
and 89.6% (43/48) of samples, respectively (Table I).
Overall, the difference in methylation frequency
between cancerous and normal tissue within and between the ethnic
groups was not statistically significant for APC or IGFBP7, with
the exception of the normal tissue from Swedish patients, which
exhibited a significantly (P<0.05) higher frequency [100%
(51/51)] of methylation of the IGFBP7 gene in comparison with the
normal tissue from Vietnamese patients [89.6% (43/48; Table I)].
When we studied the individual methylation in cancer
relative to the matched normal tissue, we found that 7 Swedish
patients had a higher degree of methylation of the IGFBP7 gene in
cancer tissue than the corresponding normal tissue. When the same
comparison was made in Vietnamese patients, we found that 17
patients showed a higher degree of methylation in cancer versus
matched normal tissue (Table II).
The difference between the ethnic groups was significant
(P<0.05). A similar comparison was made of the methylation of
the APC gene, in which we found that 7 Swedish and 8 Vietnamese
patients showed a higher degree of methylation in cancer than in
matched normal tissue, but the difference was not significant.
 | Table IIDistribution of methylation (M) and
unmethylation (U) of APC and IGFBP7 genes in CRC tissues compared
with matched normal tissue from Swedish and Vietnamese
patients. |
Table II
Distribution of methylation (M) and
unmethylation (U) of APC and IGFBP7 genes in CRC tissues compared
with matched normal tissue from Swedish and Vietnamese
patients.
| Normal tissue
|
|---|
| Gene and
tissue | M/M | M/U | U/U |
|---|
| APC | | | |
| Swedish patients
(n=52) | | | |
| Cancer
tissue | | | |
| M/M
(n=0) | 0 | 0 | 0 |
| M/U
(n=28) | 0 | 21 | 7 |
| U/U
(n=24) | 0 | 16 | 8 |
| Vietnamese
patients (n=49) | | | |
| Cancer
tissue | | | |
| M/M
(n=0) | 0 | 0 | 0 |
| M/U
(n=22) | 0 | 14 | 8 |
| U/U
(n=27) | 0 | 15 | 12 |
| IGFBP7 | | | |
| Swedish patients
(n=51) | | | |
| Cancer
tissue | | | |
| M/M
(n=25) | 18 | 7 | 0 |
| M/U
(n=20) | 2 | 18 | 0 |
| U/U
(n=6) | 2 | 4 | 0 |
| Vietnamese
patients (n=48) | | | |
| Cancer
tissue | | | |
| M/M
(n=20) | 6 | 12 | 2 |
| M/U
(n=23) | 6 | 14 | 3 |
| U/U
(n=5) | 4 | 1 | 0 |
There was no statistically significant association
between MSP findings with other clinical parameters, including
gender, age, location or stage (data not shown).
Discussion
Epigenomic instability has been postulated to play a
role in the development of multiple types of neoplasia, including
CRC (13,14). The methylation of gene promoter
regions has been widely studied and this epigenetic event affects
cell cycle control and differentiation in human malignancies.
Previous studies have reported that the aberrant hypermethylation
of promoter CpG islands is linked to gene silencing and loss of
tumour suppressor function (13–15).
The tumour suppressor gene APC is one of the key
components of the Wnt pathway (3).
The reported methylation status of the promoter of APC varies
greatly among studies of CRC in different populations (16–20).
Moreover, hypermethylation of the APC promoter has been shown to be
relatively common in other gastrointestinal neoplasms, including
those of the stomach, liver, pancreas and oesophagus (17,29).
In the Swedish and Vietnamese CRC patients,
methylation of the APC gene was detected without any significant
difference between the cancerous and normal tissues within or
between the ethnic groups. In agreement with the results of
previous studies (30,31), we also noted that the colon cancer
cell line HT-29 shows APC gene hypomethylation status.
IGFBP7 plays a potential tumour suppressor role
against colorectal carcinogenesis (9,10). The
molecular mechanism by which IGFBP7 contributes to tumour
suppression is not fully understood. Altered expression of IGFBP7
compared with normal tissue has been noted (8,11,12)
and higher tissue expression indicates favorable prognosis
(10). However, these facts
contradict the results of Adachi et al, who found IGFBP7
expression to be correlated with a poor prognosis (32). Studies have indicated that the
IGFBP7 gene is inactivated by DNA methylation in human colon, lung
and breast cancer (21–23). In agreement with previous
observations (11,33), we found that the colon cancer cell
line HT-29 shows IGFBP7 gene hypermethylation status. No
significant difference was obtained when we compared the IGFBP7
gene methylation frequency between CRC tissue from Swedish and
Vietnamese patients. However, the normal tissue from Swedish
patients exhibited a significantly higher frequency of IGFBP7 gene
methylation compared with the normal tissue of Vietnamese patients.
Moreover, a significant number of cancer tissues from Vietnamese
individuals showed higher levels of methylation versus the paired
normal tissue compared with that of the Swedish patients. It is
possible that this subset of patients had another disease
progression or that the result is due to the definition of
histologically normal tissue.
When we studied the individual methylation in cancer
compared with the matched normal tissue, we found that certain
Swedish patients had a higher degree of methylation in cancer
tissue than the corresponding normal tissue with respect to IGFBP7.
When the same comparison was made with Vietnamese patients, we
found a significantly higher number of patients with higher degree
of methylation in cancer versus matched normal tissue.
In the present study, a number of patients had a
high degree of methylation of the normal tissue. However,
epigenetic alterations are commonly observed not only in cancer
tissues but also in non-cancerous tissues, as has been noted in the
stomach (34) and colon (35). Such phenomena may be explained by
the ‘field cancerisation’ caused by carcinogen exposure (35). In our case, this may be explained by
the influence of inflammatory mediators, as inflammation has been
shown to accelerate DNA methylation in normal tissues (36).
In Vietnam, malignancies of the gastrointestinal
tract are common in the stomach and liver but are comparatively
less frequent in the colon and rectum (37). The incidence of CRC is rapidly
rising in Asian countries and is beginning to exhibit the same rate
as in Western countries (38).
However, there remains a difference in the incidence of CRC between
Vietnam and Western countries (38). As part of efforts to increase our
understanding of this difference and reflect molecular pathological
differences, we chose to consider the epigenetic aspects of CRC. To
the best of our knowledge, this is the first time that the
methylation status of the APC and IGFBP7 genes has been analysed in
Vietnamese CRC patients.
Taken together, our results suggest that
hypermethylation of the APC and IGFBP7 gene promoter region in
cancerous and normal tissue may be a prognostic factor in CRC
patients. We are aware that our finding needs to be confirmed by
extended studies before drawing a final conclusion regarding these
suggestions. Moreover, the data presented in this study are
prerequisite to a forthcoming study of CRC patients to evaluate the
influence of APC and IGFBP7 gene methylation status in cancer and
normal tissue on 5-year survival and recurrence rates.
Acknowledgements
We thank Dr Tran Van Tuan at the
Department of Cytology and Pathology, National Cancer Hospital, Tam
Hiep, Hanoi, Vietnam for providing us with tissue from Vietnamese
colorectal cancer patients. This study was supported by grants from
the Foundation of Clinical Cancer Research, Jönköping, Sweden and
grant from the project KLEPT-09-02, College of Science, Vietnam
National University, Hanoi, Vietnam.
References
|
1
|
Miyoshi Y, Nagase H, Ando H, Horii A,
Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T and Nakamura Y:
Somatic mutations of the APC gene in colorectal tumors: mutation
cluster region in the APC gene. Hum Mol Genet. 1:229–233. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneikert J and Behrens J: The canonical
Wnt signalling pathway and its APC partner in colon cancer
development. Gut. 56:417–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Durai R, Yang W, Gupta S, Seifalian AM and
Winslet MC: The role of the insulin-like growth factor system in
colorectal cancer: review of current knowledge. Int J Colorectal
Dis. 20:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Degeorges A, Wang F, Frierson HF Jr, Seth
A and Sikes RA: Distribution of IGFBP-rP1 in normal human tissues.
J Histochem Cytochem. 48:747–754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burger AM, Leyland-Jones B, Banerjee K,
Spyropoulos DD and Seth AK: Essential roles of IGFBP-3 and
IGFBP-rP1 in breast cancer. Eur J Cancer. 41:1515–1527. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato Y, Chen Z and Miyazaki K: Strong
suppression of tumor growth by insulin-like growth factor-binding
protein-related protein 1/tumor-derived cell adhesion factor/mac25.
Cancer Sci. 98:1055–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Georges RB, Adwan H, Hamdi H, Hielscher T,
Linnemann U and Berger MR: The insulin-like growth factor binding
proteins 3 and 7 are associated with colorectal cancer and liver
metastasis. Cancer Biol Ther. 12:69–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruan WJ, Lin J, Xu EP, Xu FY, Ma Y, Dengh
H, Huang Q, Lv BJ, Hu H, Cui J, Di MJ, Dong JK and Lai MD: IGFBP7
plays a potential tumor suppressor role against colorectal
carcinogenesis with its expression associated with DNA
hypomethylation of exon 1. J Zhejiang Univ Sci B. 7:929–932. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan W, Xu E, Xu F, Ma Y, Deng H, Huang Q,
Lv B, Hu H, Lin J, Cui J, Di M, Dong J and Lai M: IGFBP7 plays a
potential tumor suppressor role in colorectal carcinogenesis.
Cancer Biol Ther. 6:354–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye F, Chen Y, Knösel T, Schluns K,
Pacyna-Gengelbach M, Deutschmann N, Lai M and Petersen I: Decreased
expression of insulin-like growth factor binding protein 7 in human
colorectal carcinoma is related to DNA methylation. J Cancer Res
Clin Oncol. 133:305–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mälarstig A, Wågsäter D, Löfgren S,
Hugander A, Zar N and Dimberg J: Tumor-derived adhesion factor in
colorectal cancer. Mol Med Rep. 2:971–976. 2009.
|
|
13
|
Kondo Y and Issa JP: Epigenetic changes in
colorectal cancer. Cancer Metastasis Rev. 23:29–39. 2004.
View Article : Google Scholar
|
|
14
|
Venkatachalam R, Ligtenberg MJ,
Hoogerbrugge N, de Bruijn DR, Kuiper RP and Geurts van Kessel A:
The epigenetics of (hereditary) colorectal cancer. Cancer Genet
Cytogenet. 203:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naghibalhossaini F, Zamani M, Mokarram P,
Khalili I, Rasti M and Mostafavi-Pour Z: Epigenetic and genetic
analysis of WNT signaling pathway in sporadic colorectal cancer
patients from Iran. Mol Biol Rep. 39:6171–6178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esteller M, Sparks A, Toyota M,
Sanchez-Cespedes M, Capella G, Peinado MA, Gonzales S, Tarafa G,
Sidransky D, Meltzer SJ, Baylin SB and Herman JG: Analysis of
adenomatous polyposis coli promoter hypermethylation in human
cancer. Cancer Res. 60:4366–4371. 2000.PubMed/NCBI
|
|
18
|
Lee S, Hwang KS, Lee HJ, Kim JS and Kang
GH: Aberrant CpG island hypermethylation of multiple genes in
colorectal neoplasia. Lab Invest. 84:884–893. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee BB, Lee EJ, Jung EH, Chun HK, Chang
DK, Song SY, Park J and Kim DK: Aberrant methylation of APC, MGMT,
RASSF2A and Wif-1 genes in plasma as a biomarker for early
detection of colorectal cancer. Clin Cancer Res. 15:6185–6191.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen SP, Chiu SC, Wu CC, Lin SZ, Kang JC,
Chen YL, Lin PC, Pang CY and Harn HJ: The association of
methylation in the promoter of APC and MGMT and the prognosis of
Taiwanese CRC patients. Genet Test Mol Biomarkers. 13:67–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin J, Lai M, Huang Q, Ruan W, Ma Y and
Cui J: Reactivation of IGFBP7 by DNA demethylation inhibits human
colon cancer cell growth in vitro. Cancer Biol Ther. 7:1896–1900.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Cui T, Knösel T, Yang L, Zöller K
and Petersen I: IGFBP7 is a p53 target gene inactivated in human
lung cancer by DNA hypermethylation. Lung Cancer. 73:38–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith P, Nicholson LJ, Syed N, Payne A,
Hiller L, Garrone O, Occelli M, Gasco M and Crook T: Epigenetic
inactivation implies independent functions for insulin-like growth
factor binding protein (IGFBP)-related protein 1 and related
IGFBPL1 in inhibiting breast cancer phenotypes. Clin Cancer Res.
13:4061–4068. 2007. View Article : Google Scholar
|
|
24
|
Herman JG, Graff JR, Myöhänen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: A novel PCR assay
methylation status of CpG islands. Proc Natl Acad Sci USA.
93:9821–9826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LC and Dahiya R: MethPrimer: designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rice P, Longden I and Bleasby A: EMBOSS:
The European Molecular Biology Open Software Suite. Trends Genet.
16:276–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davuluri RV, Grosse I and Zhang MQ:
Computational identification of promoters and first exons in the
human genome. Nat Genet. 29:412–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prestridge DS: Predicting Pol II promoter
sequences using transcription factor binding sites. J Mol Biol.
249:923–932. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clément G, Bosman FT, Fontolliet C and
Benhattar J: Monoallelic methylation of the APC promoter is altered
in normal gastric mucosa associated with neoplastic lesions. Cancer
Res. 64:6867–6873. 2004.PubMed/NCBI
|
|
30
|
Sakamoto Y, Kitazawa R, Maeda S and
Kitazawa S: Methylation of CpG loci in 5′ -flanking region alters
steady-state expression of adenomatous polyposis coli gene in colon
cancer cell lines. J Cell Biol. 80:415–423. 2001.
|
|
31
|
Lind GE, Thorstensen L, Løvig T, Meling
GI, Hamelin R, Rognum TO, Esteller M and Lothe RA: A CpG island
hypermetylation profile of primary colorectal carcinomas and colon
cancer cell lines. Mol Cancer. 3:282004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adachi Y, Itoh F, Yamamoto H, Arimura Y,
Kikkawa-Okabe Y, Miyazaki K, Carbone DP and Imai K: Expression of
angiomodulin (tumor-derived adhesion factor/mac25) in invading
tumor cells correlates with poor prognosis in human colorectal
cancer. Int J Cancer. 95:216–222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin J, Lai M, Huang Q, Ma Y, Cui J and
Ruan W: Methylation patterns of IGFBP7 in colon cancer cell lines
are associated with levels of gene expression. J Pathol. 212:83–90.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakajima T, Maekita T, Oda I, Gotoda T,
Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T and Saito
D: Higher methylation levels in gastric mucosae significantly
correlate with higher risk of gastric cancer. Cancer Epidemiol
Biomarkers Prev. 15:2317–2321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen L, Kondo Y, Rosner GL, Xiao L,
Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR,
Einspahr JG, Buckmeier J, Alberts DS, Hamilton SR and Issa JPJ:
MGMT promoter methylation and field defect in sporadic colorectal
cancer. J Natl Cancer Inst. 97:1330–1338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Issa JP, Ahuja N, Toyota M, Bronner MP and
Brentnall TA: Accelerated age-related CpG island methylation in
ulcerative colitis. Cancer Res. 61:3573–3577. 2001.PubMed/NCBI
|
|
37
|
Anh PT and Duc NB: The situation with
cancer control in Vietnam. Jpn J Clin Oncol. 32(Suppl): S92–S97.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sung JJ, Lau JY, Young GP, Sano Y, Chiu
HM, Byeon JS, Yeoh KG, Goh KL, Sollano J, Rerknimitr R, Matsuda T,
Wu KC, Ng S, Leung SY, Makharia G, Chong VH, Ho KY, Brooks D,
Lieberman DA and Chan FK; Asia Pacific Working Group on Colorectal
Cancer: Asia Pacific consensus recommendations for colorectal
cancer screening. Gut. 57:1166–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|