Introduction
Mycosis fungoides (MF) is the most common type of
cutaneous T-cell lymphoma accounting for approximately 50% of all
primary cutaneous lymphoma (1). MF
is characterized clinically by an indolent course with slow
progression, over years or occasionally decades, from patches to
more infiltrated plaque and eventually tumor stages (1). MF is primarily limited to, but widely
distributed on, the skin. However, during the advanced stages of
the disease, MF has been identified in various extra-cutaneous
regions, most commonly the lymph node, spleen, liver and lung
(2). Esophageal involvement of MF
has been previously identified, however cases are extremely rare
(2–5).
MF is characterized histopathologically by
infiltrates of small to medium-sized neoplastic T lymphocytes with
cerebriform nuclei demonstrating epidermotropism. The typical
phenotype of neoplastic lymphocytes is CD3+,
CD4+ and CD8−(6). CD8+ MF has been identified,
however, it is rare (6,7).
In the present study, we report the first documented
case of CD8+ MF with esophageal involvement that was
diagnosed endoscopically antemortem.
Patient and methods
Case report
A 70-year-old Japanese male with a 15-year history
of MF presented with difficulty in swallowing. From the age of 56
years old, the patient reported observation of a gradually
enlarging erythema on his left elbow. Four years following this,
the patient was referred to Shiga University of Medical Science
Hospital due to enlargement of the erythema across his entire body,
accompanied by erosion in specific regions. Biopsy of the erythema
led to diagnosis of MF with consideration of clinical findings.
Following this, the patient recieved radiation and
psoralen ultraviolet A therapy and chemotherapy, including CEOP
(cyclophosphamide, epirubicin, vincristine and prednisolone) and
MEPP (mitoxantrone, etoposide, cisplatin and prednisolone). The
erythema underwent periods of increased severity and remission and
subsequently tumor formation was observed in specific regions.
Biopsy of lesions with tumor formation was performed.
The patient reported pharyngalgia at 70 years old.
The pharyngeal mucosa was eroded and a biopsy from the pharynx
revealed involvement of MF. MEPP therapy was added to the patient’s
therapy regimen. Two months later the patient presented with
difficulty in swallowing and positron emission tomography revealed
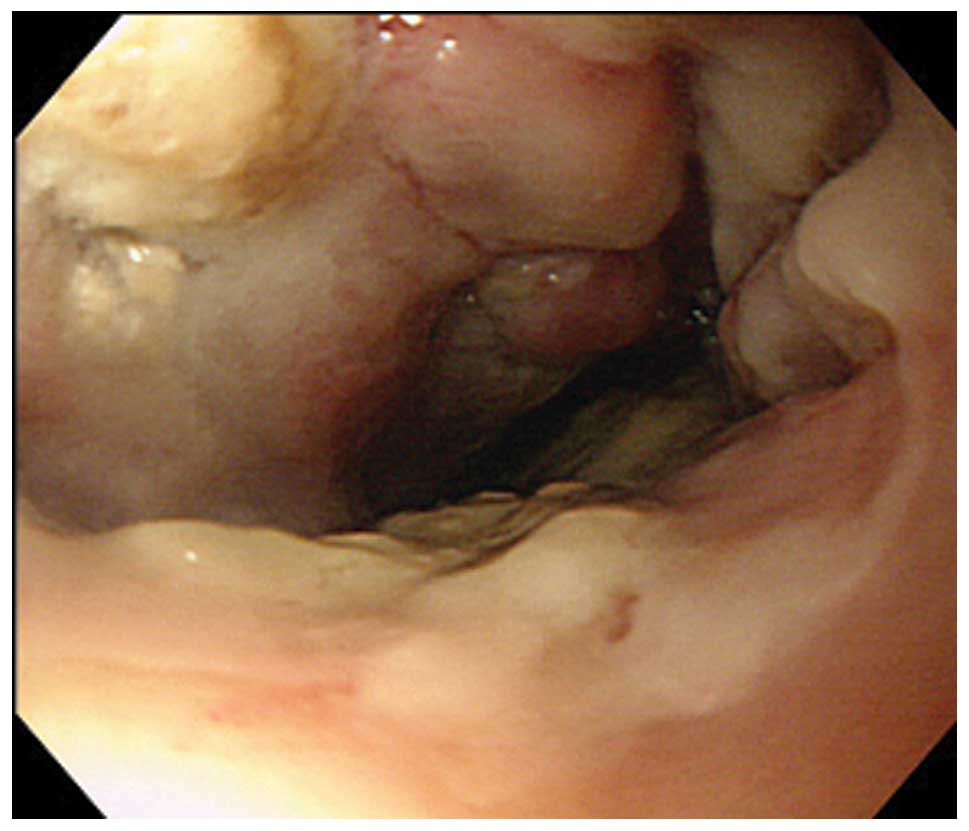
an accumulation in the esophagus and pharynx. Endoscopic
examination identified a tumorous lesion with ulceration in all
circumferences of the esophagus, 28–32 cm from the incisors
(Fig. 1). Biopsy of this lesion was
performed. Computed tomography demonstrated multiple tumorous
lesions in the right lung, that were clinically suspected to be
associated with MF. The patient received radiation and ESHAP
(etoposide, solmedrol, high-dose AraC and cisplatin) therapy prior
to succumbing to severe pneumonia and sepsis. This study was
approved by the Ethics Committee of Shiga University of Medical
Science and patient consent was obtained.
Methods
Formalin-fixed, paraffin-embedded tissue blocks of
biopsy specimens from the skin and esophagus were cut into
3-μm thick sections, deparaffinized and rehydrated. Sections
were stained with hematoxylin and eosin and used for
immunostaining. Immunostaining was performed using an autostainer
(Benchmark XT System, Ventana Medical System, Tucson, AZ, USA)
according to the manufacturer’s instructions. The following primary
antibodies were used: mouse monoclonal antibodies against βF1 (8A3,
Thermo Scientific, Waltham, MA, USA), CD3 (PS1, Novocastra
Laboratories, Ltd., Newcastle-upon-Tyne, UK), CD8 (1A5,
Novocastra), CD20 (L26, Novocastra), CD56 (CD564, Novocastra),
granzyme B (11F, Novocastra) and TIA-1 (TIA-1, GeneTex, TX, USA)
and rabbit monoclonal antibody against CD4 (SP35, Ventana Medical
System).
Results
Skin biopsy
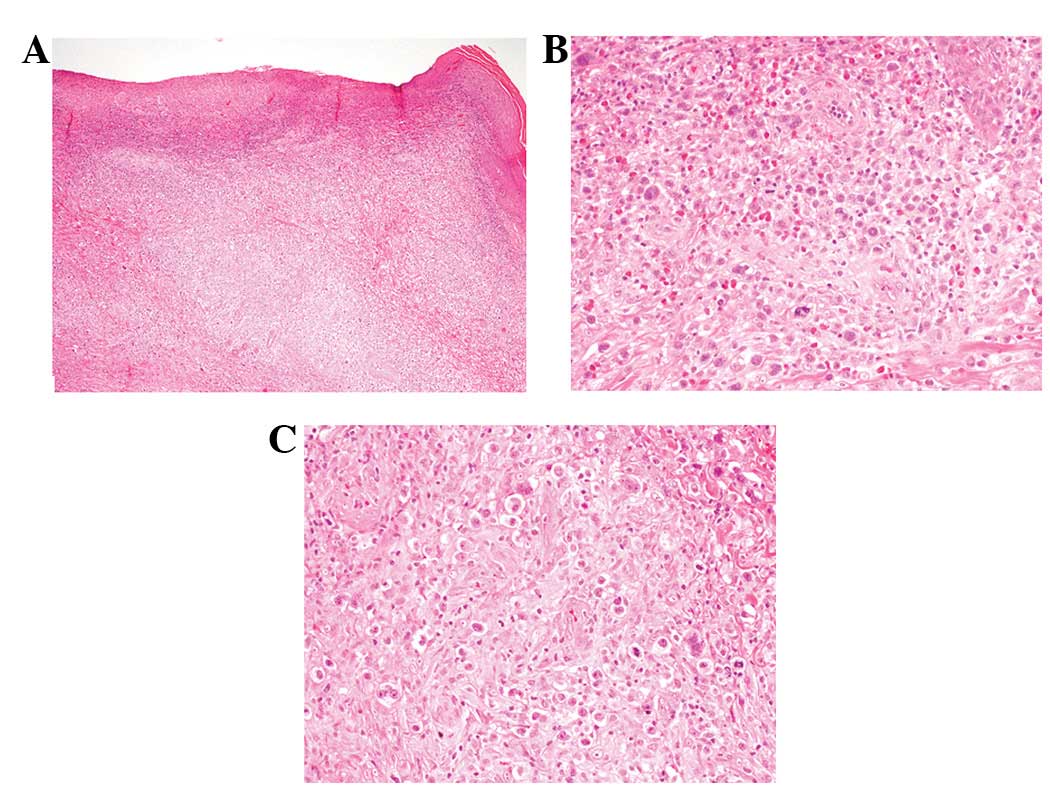
The epidermis was identified as eroded (Fig. 2A). Small to medium-sized atypical
lymphocytes had infiltrated into the entire dermis (Fig. 2A). Atypical lymphocytes had
convoluted nuclei with a small to conspicuous nucleoli (Fig. 2B). Mitotic figures were scattered.
Epidermotropism of the atypical lymphocytes was observed.
Approximately 60% of the dermal infiltrate was composed of the
above-mentioned small to medium-sized atypical lymphocytes and the
rest were large-sized lymphocytes with a rich eosinophilic
cytoplasm and large nuclei with conspicuous nucleoli.
Multinucleated atypical lymphocytes were also identified (Fig. 2C).
Immunohistochemical analyses revealed that the
atypical lymphocytes expressed βF1, CD3 and CD8 (Fig. 3A and B) and were negative for CD4
and CD56. CD30 was expressed in specific large-sized atypical
lymphocytes, but not in small to medium-sized ones (Fig. 3C). In addition, the majority of the
atypical lymphocytes were positive for granzyme B (Fig. 3D) and specific lymphocytes were
TIA-1+.
Histopathological and immunohistochemical analyses
resulted in diagnosis of MF with large cell transformation.
Esophageal biopsy
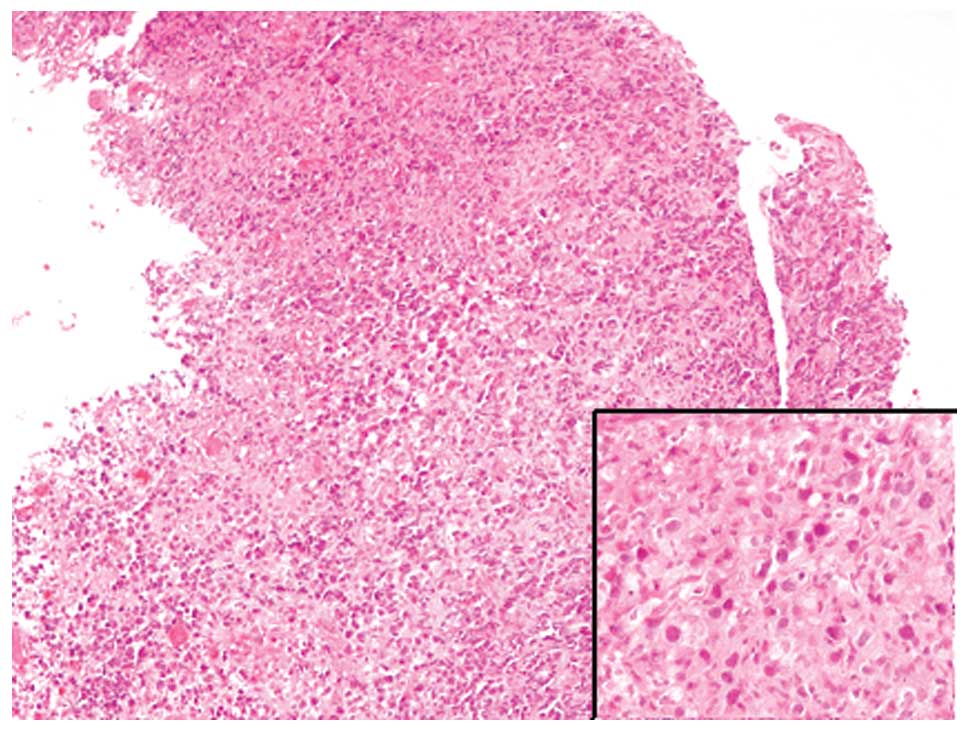
The esophageal mucosa was ulcerated. Atypical
lymphocytes with convoluted nuclei were identified within the
necrotic debris (Fig. 4). The
immunohistochemical profiles of the atypical lymphocytes of the
esophagus were identical to those of the skin specimen.
Discussion
In the present study, the first documented case of
CD8+ MF with esophageal involvement that was
endoscopically diagnosed antemortem was reported. Previously,
Rappaport and Thomas analyzed 45 autopsied cases of MF and observed
gross and/or microscopic evidence of MF in the extracutaneous
organs in 32 cases (71%). The lymph node, lung, spleen, liver,
kidney and thyroid gland were identified to be involved in MF in
24, 32, 19, 17, 14 and 13 cases, respectively. However, esophageal
involvement was rare and only 4 cases were observed, among which no
macroscopic tumor formation was revealed. In addition, laryngeal
involvement of MF was identified in only 2 cases and revealed gross
tumor formation. These analyses were performed postmortem and
esophageal involvement is considered an incidental observation at
autopsy (2). Kim et al were
the first group to report a case of MF with esophageal involvement
which was diagnosed antemortem (3).
Following this, an additional case was reported by Redleaf et
al, identifying MF involvement in the larynx, hypopharynx and
cervical esophagus by antemortem endoscopic examination (4). However, the immunohistochemical
phenotype of neoplastic T lymphocytes in the above-mentioned cases
was not available. The present report describes the first
documented case of CD8+ MF with esophageal involvement
that was endoscopically diagnosed antemortem.
It is well known that a cytotoxic phenotype, as
observed in the present case, does not reveal clinical and/or
prognostic differences in MF. Massone et al previously
classified early MF into 4 phenotypic groups: A (α/β+,
CD4+, CD8−, TIA1−), B
(α/β+, CD4−, CD8+,
TIA1+), C (α/β− CD4−,
CD8+/−, TIA1+) and D (α/β+,
CD4−, CD8−, TIA1−) (6). Survival curves did not identify
statistical differences among the groups (6), therefore, it is thought that
CD8+ cytotoxic MF should not be considered a separate
entity of MF (1). The present case
study was classified into group B according to this classification
(6). The lesions of the present
patient were limited to the skin for more than 10 years, a typical
clinical course of conventional MF. However, pharyngeal, esophageal
and lung involvement was observed during the late stage.
Large cell transformation in MF is defined by the
presence of more than 25% of large lymphoid cells in the dermal
infiltrates (1). This phenomenon is
detected in more than half of patients with tumor stage MF and is
associated with a poor prognosis (8). CD30+ and CD30−
large-sized lymphoid cells have been previously identified.
However, in the present case, approximately 40% of the lesion was
composed of large-sized atypical lymphocytes and specific cells
were revealed as CD30+. Therefore, the definition of
large cell transformation in this case was correct.
In conclusion, the present study describes the first
documented case of CD8+ MF with esophageal involvement
that was endoscopically diagnosed antemortem. MF is involved in the
visceral organs, including the esophagus. Early detection of
extracutaneous involvement of MF is important for accurate
treatment and endoscopic examination is a useful tool for detection
of MF involvement.
References
|
1
|
Ralfkiaer E, Cerroni L, Sander CA, Smoller
BR and Willemze R: Mycosis fungoides. World Health Organization
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
Swerdlow SH, Campo E, Harris NL, et al: 4th edition. IARC Press;
Lyon: pp. 296–298. 2008
|
|
2
|
Rappaport H and Thomas LB: Mycosis
fungoides: the pathology of extracutaneous involvement. Cancer.
34:1198–1229. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim OD, Cantave I and Schlesinger PK:
Esophageal involvement by cutaneous T-cell lymphoma, mycosis
fungoides type: diagnosis by endoscopic biopsy. J Clin
Gastroenterol. 12:178–182. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redleaf MI, Moran WJ and Gruber B: Mycosis
fungoides involving the cervical esophagus. Arch Otolaryngol Head
Neck Surg. 119:690–693. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dereure O and Guihou JJ: Mycosis fungoides
with predominant periorficial and mucous involvement. Ann Dermatol
Venereol. 132:877–880. 2005.PubMed/NCBI
|
|
6
|
Massone C, Crisman G, Kerl H and Cerroni
L: The prognosis of early mycosis fungoides is not influenced by
phenotype and T-cell clonality. Br J Dermatol. 159:881–886. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishida M, Hotta M, Takikita-Suzuki M,
Kojima F and Okabe H: CD8-positive granulomatous mycosis fungoides:
a case report with review of the literature. J Cutan Pathol.
37:1072–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cerroni L, Rieger E, Hödl S and Kerl H:
Clinicopathologic and immunologic features associated with
transformation of mycosis fungoides to large-cell lymphoma. Am J
Surg Pathol. 16:543–552. 1992. View Article : Google Scholar : PubMed/NCBI
|