Introduction
Bladder cancer is the fifth most common malignancy
in western countries. Incidence increased at a rate of almost 0.8%
per year between 1975 and 1987 and has since leveled off, with an
estimated 68,810 new cases in 2008 (1). Despite recent multidisciplinary
advances in its treatment, bladder cancer continues to carry
unacceptably high rates of mortality and morbidity, with a 10-year
survival rate of 40–50% (2). More
than 70% of bladder cancers present as moderate- to
well-differentiated non-muscle-invasive papillary cancer and are
treated with endoscopic transurethral resection. However, 50% of
patients suffer intravesical recurrence within 2 years, and 5–25%
progress to muscle-invasive cancer following repeated recurrence
(3). Local recurrence and distant
metastasis of tumor cells are not a simple process, but a highly
organized phenomenon composed of a series of molecular events. To
date, several molecules including chemokines have been identified
to play pivotal roles in cancer progression (3). Variable biological behavior in bladder
cancer may also be related to differences in expression of
chemokines and its receptors (4).
Chemokines are a superfamily of small, secreted
proteins (8–10 kDa) and another class of biomarkers that primarily
direct the migration of various leukocyte types through
interactions with a group of seven-transmembrane-stretch G
protein-coupled receptors resulting in angiogenesis, collagen
production, B-cell lymphopoiesis and bone marrow myelopoiesis
(5). To date, over 50 chemokines
and 20 chemokine receptors have been identified, and are grouped
into four categories (C, CC, CXC and CX3C) according to the
location of the main cysteine residues near the N terminal of these
proteins (6). CXCL16 is one of the
two known transmembrane chemokines; it is found not only in immune
cells, but also expressed constitutively in fibroblasts,
keratinocytes and cancer cells of different origins (7–9). In a
previous study, CXCL16 was identified as a ligand for CXCR6, which
is expressed by peripheral blood leukocytes (10). Eisenhardt et al(4) showed that the chemokine receptor was a
noteworthy candidate for the future investigation of metastasis of
bladder cancer in vivo. However, there is no study
concerning the clinical significance of CXCL16/CXCR6 in bladder
cancer.
To investigate whether the CXCL16/CXCR6
ligand-receptor system is involved in the progression of human
bladder cancer, we performed comparative analyses of
immunohistochemical (IHC) staining for CXCL16/CXCR6 and
quantitative real-time reverse transcription polymerase chain
reaction (RT-PCR) in bladder cancer tissues and benign bladder
tissues and evaluated the correlation between CXCL16/CXCR6
expression and the clinicopathological findings with reference to
characteristics of bladder cancer.
Materials and methods
Tissue collection
Bladder cancer and benign tissues were obtained from
160 patients. Of these, 155 patients with bladder cancer underwent
radical cystoprostatectomy and 5 patients with benign bladder
diseases (control group) underwent partial cystectomy or bladder
biopsy. Benign bladder lesions included 2 cases with inflammation,
1 with bladder rupture and 2 with pathologically normal bladder.
This study was conducted with the approval of Pusan National
University clinical trial (PNUH IRB-2011203). Written informed
patient consent was obtained from the patients.
The bladder cancer patients were initially evaluated
at 1 month after surgery, then every 3 months for 2 years, every 6
months for the next 3 years and every year thereafter until disease
progression or mortality. Overall survival (OS) data that reflected
overall mortalities were obtained from the Korean National
Statistics Registry Database.
IHC staining
We evaluated IHC staining with 155 paraffin blocks
and frozen tissues in patients with bladder cancer or benign
bladder diseases. The tissues were fixed routinely with formalin
and embedded in paraffin. Antigen retrieval was carried out and
0.3% H2O2 in phosphate-buffered saline (PBS)
was used to block endogenous peroxidase activity in the sections.
Following treatment with protein-blocking solution containing 10%
bovine serum albumin (BioShop, Burlington, ON, Canada) in PBS to
block non-specific binding, the sections were incubated for 1 h at
room with rabbit anti-human CXCR6 (1 mg/ml, GeneTex Corporation,
Zeeland, MI, USA) or rabbit anti-human CXCL16 (0.3 μg/ml,
PeproTech, Rocky Hill, NJ, USA) antibodies. Ensuing incubations
were carried out with Cy3-coupled secondary antibodies (Molecular
Probes, Eugene, OR, USA). The tissue samples were observed at ×100
magnification using a fluorescent microscopy (Axiovert 200, Carl
Zeiss, Göttingen, Germany) and images were obtained with AxioVs40
software (version 4.7.2, Carl Zeiss). The immunostaining intensity
was scored as no staining (score 0), light red (score 1) and deep
red (score 2) at ×100 magnification. The percentage of positive
cells was calculated as <5% (score 0), 5–25% (score 1), 25–50%
(score 2), 50–75% (score 3) and >75% (score 4) at ×100
magnification. We defined the IHC staining score as the sum of the
intensity and percentage scores.
Total RNA extraction
We evaluated 13 frozen tissues to extract mRNA (8
patients with bladder cancer and 5 with benign bladder diseases).
To confirm the expression of mRNA transcripts of CXCR6 and its
ligand CXCL16, quantitative real-time RT-PCR was performed. Total
RNA was extracted using the QIAzol™ lysis reagent (Qiagen,
Valencia, CA, USA).
Reverse transcription of RNA
The prepared RNA was reverse transcribed into
complementary DNA (cDNA) in a volume of 20 μl containing 5X
RT reaction buffer plus 0.005 M DTT, 1 mM of each dNTP, 20 units
RNase inhibitor, 50 μM oligo(dT) primer, 2 μg total
RNA and 200 units of DiaStar™ RTase (SolGent, Daejeon, South
Korea). The mixture was incubated at 50°C for 50 min and then at
70°C for 10 min.
Quantitative real-time RT-PCR
CXCR6 and its ligand CXCL16 mRNA were quantified
using commercial LightCycler FastStart DNA Master SYBR-Green I
(Roche, Basel, Switzerland) using the LightCycler instrument (Roche
Molecular Diagnostics, Pleasanton, CA, USA) for real-time PCR and
all subsequent quantification steps, according to the
manufacturer’s instructions.
The PCR primer pairs used for cDNA amplification
were as follows: 5′ CTGACTCAGCCAGGCAATGG-3′ (sense) and
5′-TGAGTGGACTGCAAGGTGGA-3′ (antisense) for human CXCL16;
5′-ATGGCAATGTCTTTAATCTCGACAA-3′ (sense) and
5′-TGAAAGCTGGTCATGGCATAGTATT-3′ (antisense) for human CXCR6; and
5′-GGGGAGCCAAAA GGGTCATCATCT-3′ (sense) and 5′-GAGGGGCCATCC
ACAGTCTTCT-3′ (antisense) for human GAPDH. A typical
20-μl one-tube PCR assay contained 1 μl cDNA (sample)
or serially diluted standard cDNA. PCR amplifications were
performed in separate tubes for 50 cycles (10 sec at 95°C; 5 sec at
57°C; and 15 sec at 72°C) using PCR master mixtures specific for
CXCL16, CXCR6 or the GAPDH housekeeping gene. The
GAPDH reaction product served as a control for PCR and as a
reference for relative quantification of CXCL16 and CXCR6 mRNA.
The number of PCR cycles required to reach the
fluorescence threshold was the cycle threshold (Ct). The Ct value
for each sample was proportional to the log of the initial amount
of input cDNA. By plotting the Ct value of an unknown sample on the
standard curve, the amount of target sequence in the sample could
be calculated. To normalize the CXCL16 and CXCR6 mRNA expression
for sample-to-sample differences in RNA input, RNA quality and
reverse transcriptase efficiency, we amplified the GAPDH
housekeeping gene. From the standard curve, we derived the
calculated amounts of CXCL16, CXCR6 and GAPDH.
Statistical analyses
Statistical analyses were conducted using the SPSS
software (version 15.0 for Windows; SPSS, Chicago, IL, USA).
Statistical differences in CXCL16 and CXCR6 protein expression
between bladder cancer and benign bladder tissue were evaluated
using Pearson’s Chi-square test. The Pearson coefficient was used
to assess the statistical significance of differences between
molecular and clinicopathological parameters. Univariate survival
analysis was performed according to Kaplan-Meier, and differences
in the survival curves were assessed with the log-rank test.
Multivariate analysis was performed on all of the parameters that
were found to be significant on univariate analysis using the Cox
regression model with P<0.05 considered to indicate a
statistically significant result.
Results
mRNA level of CXCL16 and CXCR6 in bladder
cancer and control group
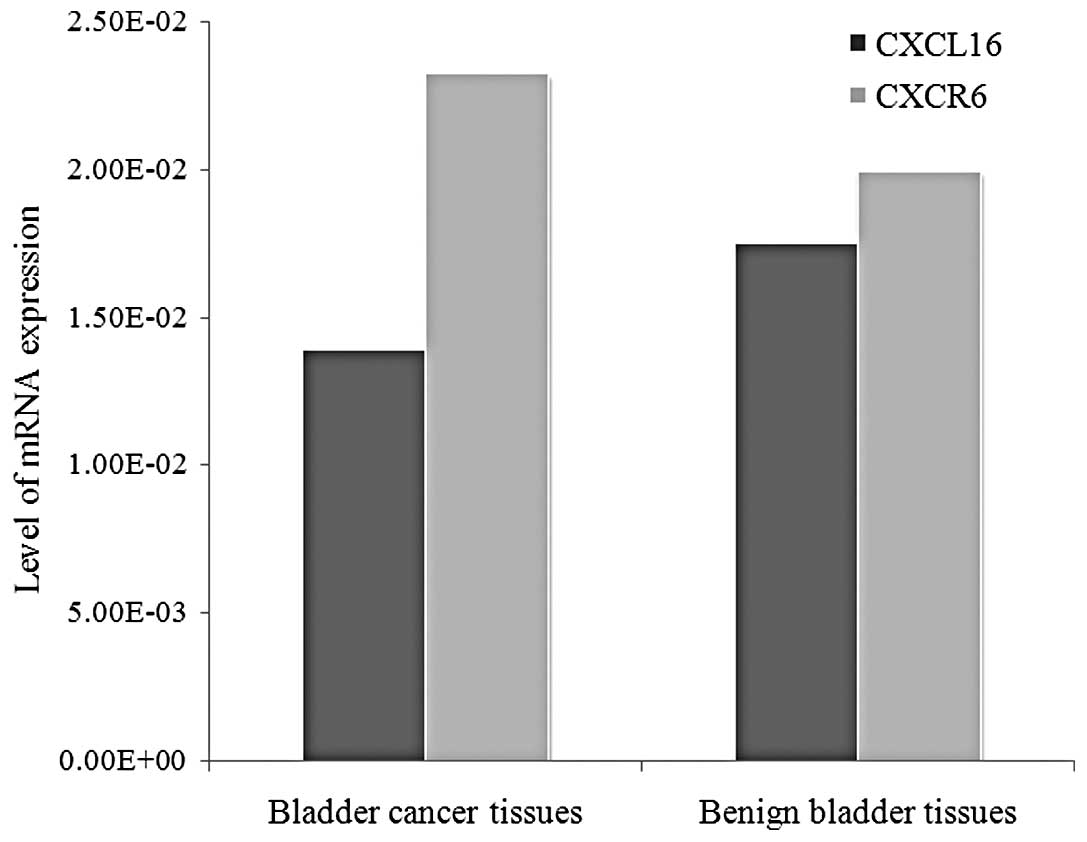
The mean mRNA levels of CXCL16 in patients with
bladder cancer and in the control group were 1.39×10−2
and 1.75×10−2, respectively. The mean mRNA levels of
CXCR6 in the cancer and control groups were 2.32×10−2
and 1.99×10−2, respectively. The expression of mRNA
levels of CXCR6 was higher in the bladder cancer group than in the
control group (P=0.033), whereas CXCL16 mRNA expression in bladder
cancer did not show statistical difference when compared with the
control group (Fig. 1).
IHC staining scores in bladder cancer and
benign bladder tissues
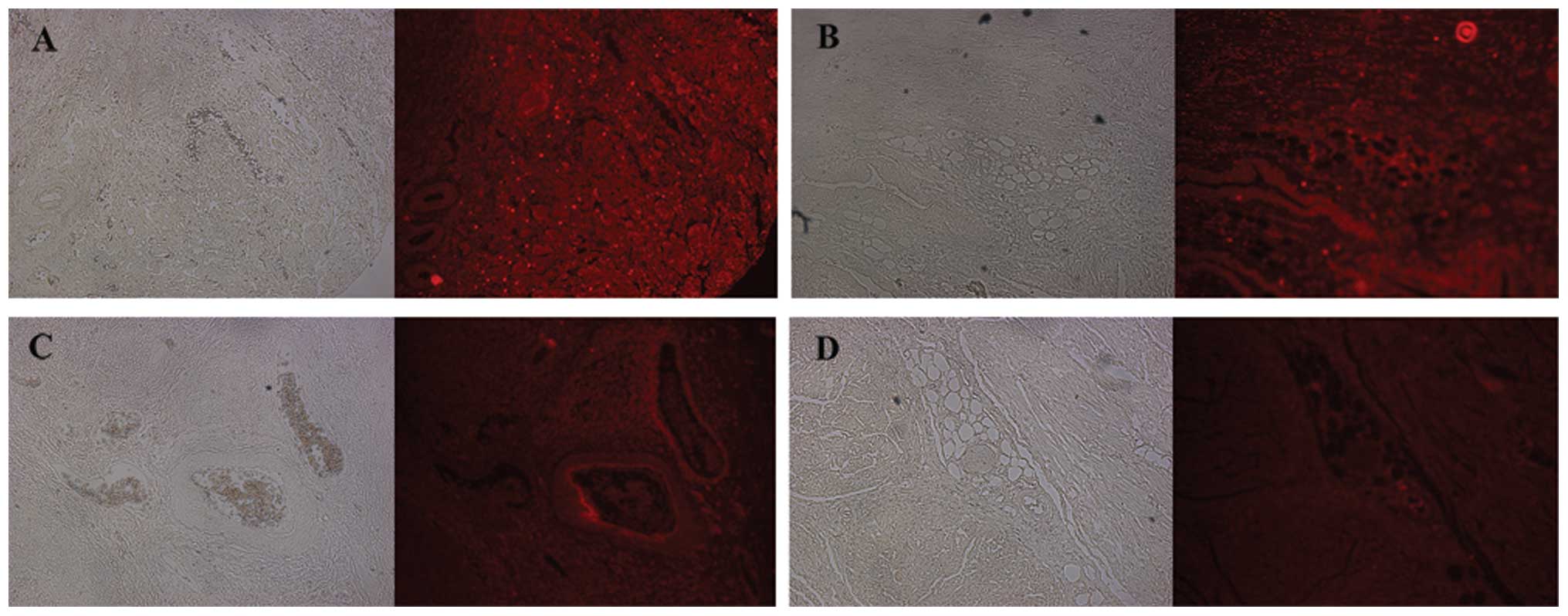
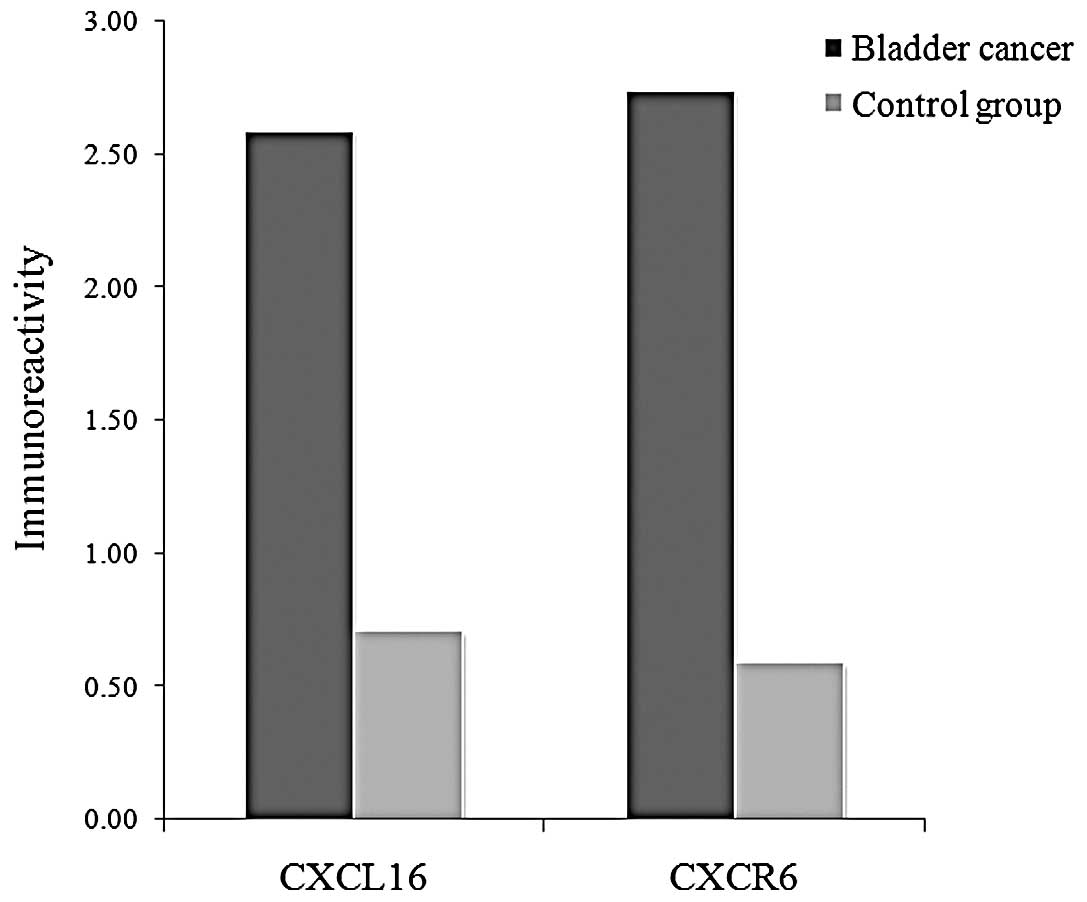
The positive CXCL16 and CXCR6 immunoreactivity was
higher in the bladder cancer group than the control group (P=0.021
and P=0.007, respectively, Fig. 2).
The mean IHC staining scores of CXCL16 were 2.59 and 0.71 in the
bladder cancer group and the control group, respectively. The mean
IHC staining scores of CXCR6 were 2.74 and 0.59, respectively
(Fig. 3).
The mean IHC scores of CXCL16/CXCR6 were 2.40/2.61
and 3.53/3.76 in patients without/with perineural invasion;
2.00/2.26 and 3.44/3.59 in patients with nuclear grade 1 and 2 vs.
grade 3 patients; 1.68/1.86 and 3.32/3.46 in low-grade vs.
high-grade patients, 2.27/2.50 and 3.00/3.08 in patients with
non-muscle-invasive vs. muscle-invasive cancer, 2.32/2.50 and
3.36/3.46 in patients without/with carcinoma in situ, and
2.16/2.44 and 3.35/3.27 in patients without/with lymphovascular
invasion, respectively (Table
I).
 | Table IImmunohistochemical staining of
CXCL16/CXCR6 according to clinical variables. |
Table I
Immunohistochemical staining of
CXCL16/CXCR6 according to clinical variables.
| CXCL16
| CXCR6
|
|---|
| Variable | Mean expression | P-value | Mean expression | P-value |
|---|
| PNI | | | | |
| Negative | 2.40 | 0.017 | 2.61 | 0.007 |
| Positive | 3.53 | | 3.76 | |
| Nuclear grade
(1973) | | | | |
| 1 and 2 | 2.00 | <0.001 | 2.26 | <0.001 |
| 3 | 3.44 | | 3.59 | |
| Nuclear grade
(2004) | | | | |
| Low | 1.68 | <0.001 | 1.86 | <0.001 |
| High | 3.32 | | 3.46 | |
| T stage | | | | |
|
Non-muscle-invasive | 2.27 | 0.003 | 2.50 | 0.019 |
|
Muscle-invasive | 3.00 | | 3.08 | |
| CIS | | | | |
| Negative | 2.32 | <0.001 | 2.50 | <0.001 |
| Positive | 3.36 | | 3.46 | |
| LVI | | | | |
| Negative | 2.16 | <0.001 | 2.44 | 0.001 |
| Positive | 3.35 | | 3.27 | |
Correlation between IHC staining scores
and clinical variables in bladder cancer
The IHC staining score of CXCL16 was correlated with
the 1973 WHO grade (P=0.008), 2004 WHO grade (P<0.001),
pathological T stage (P<0.001), presence of perineural invasion
(P=0.008), presence of lymphovascular invasion (P<0.001),
presence of positive surgical margin (P=0.033), presence of
ureteral invasion (P=0.033) and prostatic stromal invasion
(P<0.001). The IHC staining of CXCR6 was correlated with the
1973 WHO grade (P<0.001), 2004 WHO grade (P<0.001),
pathological T stage (P=0.001), presence of perineural invasion
(P=0.001), presence of lymphovascular invasion (P=0.005), presence
of ureteral invasion (P<0.001), numbers of positive lymph nodes
(P=0.018) and prostatic stromal invasion (P=0.001; Table II).
 | Table IICorrelation between
immunohistochemical staining of CXCL16/CXCR6 and clinical
characteristics. |
Table II
Correlation between
immunohistochemical staining of CXCL16/CXCR6 and clinical
characteristics.
| Characteristic | No. of
patients | Statistical
analysis | CXCL16 | CXCR6 |
|---|
| 1973 WHO grade | 133 | Pearson
correlation | 0.510 | 0.463 |
| P-value | 0.008a | 0.000b |
| 2004 WHO grade | 90 | Pearson
correlation | 0.485 | 0.482 |
| P-value | 0.000b | 0.000b |
| T stage | 146 | Pearson
correlation | 0.322 | 0.277 |
| P-value | 0.000b | 0.001b |
| CIS | 42 | Pearson
correlation | 0.244 | 0.229 |
| P-value | 0.119 | 0.146 |
| PNI | 51 | Pearson
correlation | 0.370 | 0.459 |
| P-value | 0.008a | 0.001b |
| LVI | 124 | Pearson
correlation | 0.339 | 0.249 |
| P-value | 0.000b | 0.005a |
| Ureteral
invasion | 147 | Pearson
correlation | 0.176 | 0.296 |
| P-value | 0.033a | 0.000b |
| SM | 146 | Pearson
correlation | 0.177 | 0.155 |
| P-value | 0.033a | 0.062 |
| Positive LN | 101 | Pearson
correlation | 0.159 | 0.234 |
| P-value | 0.113 | 0.018a |
| Presence of
PCA | 88 | Pearson
correlation | 0.395 | 0.346 |
| P-value | 0.000b | 0.001b |
In multivariate analysis, the IHC staining of CXCL16
was correlated with the 2004 WHO grade and lymphovascular invasion
(P=0.021 and P=0.011, respectively), CXCR6 was correlated with the
1973 WHO grade (P=0.001), 2004 WHO grade (P<0.001), pathological
T stage (P=0.002) and perineural invasion (P=0.031).
Prognostic significance of IHC staining
scores
There were no significant differences between the
IHC staining scores of CXCL16/CXCR6 and 5-year OS (P=0.292 and
P=0.202, respectively). For CXCL16, statistical difference between
patients with score 0 and 6 could not be obtained since there were
very few patients with a score of 0 (4 patients) or 6 (4 patients).
However, 50% of OS showed that higher CXCL16 or CXCR6 IHC staining
scores were related to shorter survival duration, which did not
show statistical significance.
Discussion
Transitional cell carcinoma of the bladder is the
most frequent tumor of the urinary tract. Despite advances in early
detection and therapy, bladder cancer remains a life threatening
disease due to the high occurrence of locally advanced or distant
metastases. Radical cystectomy is the gold standard treatment for
muscle-invasive bladder cancer. However, at least 50% of patients
with locally advanced disease are expected to develop systemic
progression within 3 years (2).
Patients with advanced bladder cancer are commonly treated with
systemic chemotherapy. Analyzing survival rates from standard
chemotherapy schedules such as M-VAC (methotrexate, vinblastine,
adriamycin and cisplatin) and GC (gemcitabine and cisplatin),
results are somewhat discouraging (11). Saxman et al(12) reported that only 5 (3.7%) of 133 of
the patients who underwent treatment with M-VAC were alive at the
6-year follow-up duration. This report demonstrated that patients
with distant metastases are not cured by systemic chemotherapy.
Lehmann et al(13)
demonstrated that the GC regimen did not extend overall or
progression-free survival more than M-VAC. These reports
demonstrated that it is the metastases rather than the primary
bladder cancer that cause most cancer-related mortalities; however,
there has been little progress in identifying molecules which were
associated metastases. To improve the diagnosis and therapy of
localized and metastatic bladder cancer, the identification of
molecules involved in the development and progression of this
disease is a high priority.
Cancer arises from genetic mutations and epigenetic
events in the proliferating cells and changes in the tumor’s
microenvironment that includes capillaries, smooth muscle cells,
fibroblasts and inflammatory cells (14). In 1863, Virchow focused on
leukocytes in tumors and suggested that cancer begins at sites
associated with chronic inflammation (15). Nonetheless, immunologists have
generally considered leukocytes as agents with the potential to
limit or eliminate cancers (16).
Metastasis is not a simple phenomenon, but a highly complicated
process composed of a series of molecular events (17). Several molecular families have been
identified to play pivotal roles in cancer metastases. Recently,
emerging evidence suggests that a third family, the chemokines and
their receptors, are involved in organ-specific metastasis.
Chemokines are small, secreted peptides that control the adhesion
and trans-endothelial migration of leukocytes, lymphocytes and
monocytes, particularly during immune and inflammatory reactions,
which were initially researched for their role in the regulation of
leukocyte trafficking to inflammatory sites. The chemokine
superfamily consists of nearly 50 cytokine members and 20 chemokine
receptors (18,19), both involved with enhancing the
immunity of tumor-associated antigen, regulating new blood vessel
formation, promoting cancer cell proliferation and directing cancer
cell metastasis to different destinations. Chemokines and their
receptors have also been reported for their significant roles in
tumor metastasis, recurrence and angiogenesis in previous studies
(20). The interaction of these
soluble chemokines with their specific, transmembrane G
protein-coupled receptors mediates their biological effects.
Several recent studies have shown that cancer cells
show tumor specific, nonrandom patterns of chemokine receptors and
that signaling through these receptors is crucial for chemotactic
migration, invasion and metastasis (17). Retz et al(11) showed that bladder cancer cells
express CXCR4 progressively with advanced tumorigenesis and that
this receptor interacts with CXCL12 to mediate tumor chemotaxis and
invasion through connective tissue. These properties identify CXCR4
as a potential target for the attenuation of bladder cancer
metastases. During metastasis of prostate cancer CXCR4 protein
expression increases in primary and metastatic lesions (21) while the level of CXCL12 was
accordingly elevated in the bone (17). There is some indication that a
similar correlation may exist between CXCR6 and CXCL16. In fact, Hu
et al(22) found that both
CXCR6 and CXCR4 are expressed in similar proportions in malignant
prostate cancer tissue and benign prostate hyperplasia tissue. In
addition, CXCR6 and CXCR4 both show increased expression in
malignant tissue. Their corresponding ligands, CXCL16 and CXCL12,
were also both found in the human bone tissues. Therefore, it
appears both the CXCL12/CXCR4 pathway and CXCL16/CXCR6 pathway are
involved in prostate cancer cell migration. CXCL12 and CXCL16
induce migration of prostate cancer cells in an independent
extracellular manner. However, this does not preclude the
possibility that the two ligands are part of a greater chemokine
and chemokine receptor network that mediates cancer cell metastasis
and invasion, and that ligands CXCL16 and CXCL12 cooperate or even
compete with each other (22).
However, there is no study that has investigated the interaction of
CXCR6 and its ligand CXCL16 in bladder cancer.
Retz et al(11) demonstrated high mRNA levels of CXCR4
in invasive and locally advanced bladder cancer, and IHC analysis
of a case of bladder cancer was consistent with the mRNA expression
data for CXCR4. In current study, the expression of mRNA of CXCR6
in bladder cancer tissues was higher than in the control group;
however, the expression of mRNA of CXCL16 in bladder cancer tissues
was lower than in the control group. This result indicates that
malignant bladder tissue itself is more likely associated with
CXCR6.
The significance of the role of chemokines should be
determined in a large cohort of bladder tumor patients undergoing
long-term observation for disease outcome. Such a study will help
determine the prognostic value of mRNA and protein expression of
chemokines. In this study, the expression of CXCL16/CXCR6
demonstrated a correlation with survival in bladder cancer
patients. Notably, a recent study investigating tissue samples from
clear cell renal cell carcinoma found a significant correlation
between strong specific chemokine staining and poor tumor-specific
survival using multivariate analysis (23). Therefore, monitoring chemokine
expression in patients with bladder cancer may improve current
tumor staging systems and provide new concepts for adjuvant
chemotherapy.
The role for CXCL16/CXCR6 in cancer progression and
metastasis has been elucidated through efforts to examine CXCL16
and CXCR6 mRNA in both benign and cancer cell lines in prostate
cancer. In a study of prostate cell lines, Ha et al(17) evaluated CXCL16 and CXCR6 mRNA
expression both in a benign cell line (PrEC) and in cancer cell
lines (LNCaP and PC3). Through real-time PCR, they showed higher
concentrations of CXCL16/CXCR6 mRNA levels in the cancer cell
lines. However, it remains unknown exactly how CXCL16 and CXCR6
contribute to cancer metastasis and invasion. Lu et
al(24) evaluated the role of
CXCL16/CXCR6 in cancer aggressiveness. They showed that among
prostate cancer cell lines, the more aggressive lines C4-2B and PC3
expressed higher levels of CXCL16/CXCR6 mRNA than the less
aggressive LNCaP. Additional studies of actual prostate tissue have
similarly supported the existence of a positive correlation between
CXCL16/CXCR6 expression and cancer aggressiveness (17). An IHC study by Wang et
al(24) demonstrated that CXCR6
expression, which showed strong epithelial staining, was correlated
with Gleason score, whereas the expression of CXCL16 was not
correlated with Gleason score. Similarly, in the current
experiment, the mRNA expression of CXCR6 in the bladder cancer
group was higher than that in the control group, whereas CXCL16 was
not. Coupled with their previous cell line studies, this result
appears to corroborate the results of Ha et al(17) that CXCL16 and total CXCR6 mRNA
expression are higher in more aggressive cancer cases. Other recent
findings from studies of prostate cancer tissue have provided
further confirmation that the CXCL16/CXCR6 expression level is
closely associated with high malignant features, as observed with
the Gleason grade and tumor stage in prostate cancer (25). Metastatic bone marrow, but not the
liver and lung tissues, expressed higher levels of CXCL16 and total
CXCR6 mRNA than the primary prostate cancer tissues, implying that
the metastatic process was correlated with chemokines and its
receptors (17). Soluble CXCL16
induces the migration of CXCR6-expressing cancer cells and enhances
the proliferation of these cancer cells with CXCR6 expression in
vitro.
Since CXCL16 and CXCR6 are co-expressed in cancer
cells, as shown in the present study, it is difficult to
discriminate between their individual roles in cancer formation and
metastasis. Recent studies have suggested that interaction between
CXCR6 and CXCL16 influences angiogenesis (26). Angiogenesis, in turn, is critical to
cancer cell proliferation as it increases blood flow to cancer
tissue, further promoting cell growth. It has long been documented
that many chemokines are responsible for regulating angiogenesis.
In particular, angiogenesis is usually enhanced by chemokines
positive for the glutamate-leucin-arginine (ELR) tripeptide motif
(ELR+) and inhibited by ELR chemokines (27). However, Wang et al(5) found that CXCL16, though an ELR
chemokine, promoted angiogenesis in vitro. The authors
induced CXCR6 overexpression in PC3 and C4-2B prostate cancer cell
lines, and observed increased IL-6 and IL-8 secretion and further
angiogenesis. When they suppressed secretion by limiting CXCR6
expression, angiogenesis slowed accordingly. In vivo,
significant new blood vessel formations occurred in tumors from
C4-2B cells with CXCR6 overexpression, whereas tumors from C4-2B
cells with reduced CXCR6 expression showed suppressed angiogenesis.
Wang et al(5) also observed
a similar role of CXCR6 in the growth and invasion of the actual
prostate cancer cells. Using CXCR6 siRNA transfection in
vitro, they found that decreased CXCR6 expression resulted in a
reduction of invasion of prostate cancer cell lines PC3 and C4-2B
stimulated by sCXCL16. Similarly, reduced expression of CXCR6 in
cells limited cancer cell invasion. As expected, tumors generated
from C4-2B cells with CXCR6 overexpression were significantly
larger than those from control cells, while tumor growth was
significantly suppressed in tumors established from cells with
reduced CXCR6 by CXCR6-siRNA. These data suggest that the
interaction between CXCR6 and soluble CXCL16 promotes the
proliferation and invasion of cancer cells. Experiments in other
cancer tissues have also shown that soluble CXCL16 induces
migration and proliferation of cancer cells. These cancer tissues
include pancreatic ductal adenocarcinoma cancer (28), schwannomas (29), renal cancer (30) and nasopharyngeal carcinoma (31). In conclusion, the present study
revealed that the expression of CXCL16/CXCR6 was correlated with
aggressive behaviors of bladder cancer and survival. Based on our
results, the CXCL16/CXCR6 axis appears to be important in the
progression of bladder cancer and is a potential therapeutic
target.
Acknowledgements
This study was supported by a grant
from the Korean Health Technology R&D Project, Ministry of
Health and Welfare, Republic of Korea (A070001), This study was
supported by a grant from the National R&D Program for Cancer
Control, Ministry for Health, Welfare and Family affairs, Republic
of Korea (0920050).
References
|
1
|
Latini DM, Lerner SP, Wade SW, Lee DW and
Quale DZ: Bladder cancer detection, treatment and outcomes:
Opportunities and challenges. Urology. 75:334–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein JP, Lieskovsky G, Cote R, et al:
Radical cystectomy in the treatment of invasive bladder cancer:
Long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
3
|
Nishizawa K, Nishiyama H, Oishi S, et al:
Fluorescent imaging of high-grade bladder cancer using a specific
antagonist for chemokine receptor CXCR4. Int J Cancer.
127:1180–1187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eisenhardt A, Frey U, Tack M, Rosskopf D,
Lummen G, Rubben H and Siffert W: Expression analysis and potential
functional role of the CXCR4 chemokine receptor in bladder cancer.
Eur Urol. 47:111–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Lu Y, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the
AKT/mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi K, Hojo S, Akashi T, Yasumoto K
and Saiki I: Chemokine receptors in cancer metastasis and cancer
cell-derived chemokines in host immune response. Cancer Sci.
98:1652–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hojo S, Koizumi K, Tsuneyama K, et al:
High-level expression of chemokine CXCL16 by tumor cells correlates
with a good prognosis and increased tumor-infiltrating lymphocytes
in colorectal cancer. Cancer Res. 67:4725–4731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ludwig A, Schulte A, Schnack C, et al:
Enhanced expression and shedding of the transmembrane chemokine
CXCL16 by reactive astrocytes and glioma cells. J Neurochem.
93:1293–1303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scholz F, Schulte A, Adamski F, et al:
Constitutive expression and regulated release of the transmembrane
chemokine CXCL16 in human and murine skin. J Invest Dermatol.
127:1444–1455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tabata S, Kadowaki N, Kitawaki T, et al:
Distribution and kinetics of SR-PSOX/CXCL16 and CXCR6 expression on
human dendritic cell subsets and CD4+ T cells. J Leukoc Biol.
77:777–786. 2005.PubMed/NCBI
|
|
11
|
Retz MM, Sidhu SS, Blaveri E, et al: CXCR4
expression reflects tumor progression and regulates motility of
bladder cancer cells. Int J Cancer. 114:182–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saxman SB, Propert KJ, Einhorn LH, et al:
Long-term follow-up of a phase iii intergroup study of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 15:2564–2569.
1997.PubMed/NCBI
|
|
13
|
Lehmann J, Retz M and Stockle M: Is there
standard chemotherapy for metastatic bladder cancer? Quality of
life and medical resources utilization based on largest to date
randomized trial. Crit Rev Oncol Hematol. 47:171–179. 2003.
|
|
14
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006.
|
|
17
|
Ha HK, Lee W, Park HJ, Lee SD, Lee JZ and
Chung MK: Clinical significance of CXCL16/CXCR6 expression in
patients with prostate cancer. Mol Med Report. 4:419–424.
2011.PubMed/NCBI
|
|
18
|
Luster AD: Chemokines-chemotactic
cytokines that mediate inflammation. N Engl J Med. 338:436–445.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Struyf S, Proost P and Van Damme J:
Regulation of the immune response by the interaction of chemokines
and proteases. Adv Immunol. 81:1–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun YX, Wang J, Shelburne CE, et al:
Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers
(PCa) in vivo. J Cell Biochem. 89:462–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu W, Zhen X, Xiong B, Wang B, Zhang W and
Zhou W: CXCR6 is expressed in human prostate cancer in vivo and is
involved in the in vitro invasion of PC3 and LNCap cells. Cancer
Sci. 99:1362–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Staller P, Sulitkova J, Lisztwan J, Moch
H, Oakeley EJ and Krek W: Chemokine receptor CXCR4 downregulated by
von Hippel-Lindau tumour suppressor pVHL. Nature. 425:307–311.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu Y, Wang J, Xu Y, et al: CXCL16
functions as a novel chemotactic factor for prostate cancer cells
in vitro. Mol Cancer Res. 6:546–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darash-Yahana M, Gillespie JW, Hewitt SM,
et al: The chemokine CXCL16 and its receptor, CXCR6, as markers and
promoters of inflammation-associated cancers. PLoS One.
4:e66952009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng L, Chen N, Li Y, Zheng H and Lei Q:
CXCR6/CXCL16 functions as a regulator in metastasis and progression
of cancer. Biochim Biophys Acta. 1806:42–49. 2010.PubMed/NCBI
|
|
27
|
Belperio JA, Keane MP, Arenberg DA, et al:
CXC chemokines in angiogenesis. J Leukoc Biol. 68:1–8. 2000.
|
|
28
|
Gaida MM, Gunther F, Wagner C, et al:
Expression of the CXCR6 on polymorphonuclear neutrophils in
pancreatic carcinoma and in acute, localized bacterial infections.
Clin Exp Immunol. 154:216–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Held-Feindt J, Rehmke B, Mentlein R, et
al: Overexpression of CXCL16 and its receptor CXCR6/bonzo promotes
growth of human schwannomas. Glia. 56:764–774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gutwein P, Schramme A, Sinke N, et al:
Tumoural CXCL16 expression is a novel prognostic marker of longer
survival times in renal cell cancer patients. Eur J Cancer.
45:478–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ou DL, Chen CL, Lin SB, Hsu CH and Lin LI:
Chemokine receptor expression profiles in nasopharyngeal carcinoma
and their association with metastasis and radiotherapy. J Pathol.
210:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|