Introduction
The matrix metalloproteinases (MMPs) are a large
family of highly homologous zinc-dependent endopeptidases, which
play a fundamental role in extracellular matrix degradation and
remodeling (1). MMPs are ubiquitous
in tissues and biological fluids and are produced by a wide variety
of normal and tumoral cells. MMPs are synthesized as inactive
proenzymes and require activation through a proteolytic mechanism;
therefore, they are found within tissues and plasma as inactive
precursors, active enzymes or inactive enzymes bound to specific
inhibitors (2).
Extracellular matrix digestion and remodeling are
required by tumor cells in order to migrate into the stroma and
therefore these events are fundamental steps in tumor local
invasion; furthermore, MMPs partially participate in tumor
neoangiogenesis. Consequently, MMPs produced by either tumor cells
or host peri-tumoral cells are directly involved in the stimulation
of tumor invasion and metastasis (3,4). MMPs
are classified depending on the specificity of the degrading
substrate, specifically MMPs 2 and 9 are named type IV
collagenases, or alternatively gelatinase A and B, respectively.
Their degrading substrates are gelatine, the denatured form of
collagen, and type IV collagen, the main component of the basement
membrane. MMP 3 is also known as stromelysin I and plays a key role
in the tumor growth, as it degrades interstitial type I and III
collagens. Many MMPs, in particular MMPs 2 and 9, are easily
detected and quantified in body fluids. Several studies have been
performed on the evaluation of plasma MMP concentrations in
correlation with different diseases, including diabetes,
atherosclerosis, inflammation and cancer. Of these studies, one was
performed on breast cancer and revealed that the concentration of
total MMP 2 and 9 was significantly higher in the plasma of
patients with breast cancer compared with patients with benign
breast diseases and the control groups (5). Another study performed on breast
cancer demonstrated that the MMP 2 serum level was higher in
patients positive for the estrogen receptor; furthermore, this
study showed that a lower MMP 2 level was a predictor of
disease-free and overall survival (6). Positive expression of MMPs 3, 2 and 9
has been reported to be correlated with the progression of breast
cancer in a rat model and their levels were significantly higher in
the metastatic brain tissue of rats with breast cancer compared
with normal brain tissue (7).
However, to date no study evaluating the concentration of MMP 3 in
the plasma of tumor patients has been performed.
For this reason, we analyzed the plasma
concentration of MMPs 2, 3 and 9, comparing patients with breast
adenocarcinomas with patients with breast fibroadenomas or normal
controls. Plasma was collected 24 h before and 96 h after surgery
and the concentration of each MMP was compared before and after
surgery. Furthermore, the values of each MMP were correlated with
biological and clinical parameters of the patients.
Materials and methods
Serum samples
Peripheral venous blood samples were collected from
patients with breast neoplasia who underwent surgery at the
Department of Surgical Sciences (University Sapienza, Rome, Italy).
Informed consent was obtained from all patients. This study
received the positive approval of the Ethic Committee of the
Faculty of Medicine of the University Sapienza of Rome, Italy.
Blood sampling was performed 24 h before and 96 h
after surgery. The venous blood was collected in tubes with
EDTA-anticoagulants and were then centrifuged at 4,000 × g for 10
min. The plasma was recovered, aliquoted and stored at −80°C until
analysis.
Enzyme-linked immunosorbent assay
(ELISA)
Plasma concentrations of MMPs were evaluated by the
ELISA technique utilizing commercially available kits. For MMPs 2
and 9, the Quantikine ELISA kits (R&D Systems, Minneapolis, MN,
USA) were used and for MMP 3 the Human Biotrak Assay (Amersham
Pharmacia Biotech, Amersham, UK) was used. All plasma samples and
the negative controls and the samples for the standard curve were
measured in duplicate and the mean was calculated. The ELISA method
was performed according to the supplier’s instructions and the
sample concentrations were obtained from the standard curve. The
absorbances were measured on a microplate reader at 450 nm.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). The Mann-Whitney U test for unpaired samples, the
Kruskal-Wallis test for multiple comparisons and the Spearman’s
rank correlation coefficent were used (SPSS 13.01 for Windows, SPSS
Inc., Chicago, IL, USA; GraphPad Prism 4.0, GraphPad Software Inc.,
San Diego, CA, USA). P<0.05 (two-sided) was considered to
indicate a statistically significant result.
Results
A group of 80 patients with primary breast
neoplasia, 50 with carcinoma and 30 with fibroadenoma, were
selected for the present study. The clinical and biological data of
all carcinoma patients were obtained and are listed in Table I; T and N factors, histological
grade, Ki67 levels and status of estrogen and progesterone
receptors and Her2/neu were considered.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | n | % |
|---|
| Total | 80 | |
| Diagnosis | | |
| Breast cancer | 50 | |
| Breast
fibroadenoma | 30 | |
| T factor | | |
| T1 | 28 | 56 |
| T2 | 15 | 30 |
| T3 | 6 | 12 |
| T4 | 1 | 2 |
| N factor | | |
| N− | 34 | 68 |
| N+ | 16 | 32 |
| Grading | | |
| G1 | 6 | 12 |
| G2 | 17 | 34 |
| G3 | 27 | 54 |
| Ki67 (cut-off
10%) | | |
| Negative | 18 | 36 |
| Positive | 32 | 64 |
| Estrogen receptor
(cut-off 10%) | | |
| Negative | 18 | 36 |
| Positive | 32 | 64 |
| Progesterone receptor
(cut-off 10%) | | |
| Negative | 28 | 56 |
| Positive | 22 | 44 |
| Her2/neu | | |
| Negative (score
0–1) | 36 | 72 |
| Positive (score
2–3) | 14 | 28 |
Comparison of plasmatic MMPs 2, 3 and 9
between breast cancer and fibroadenoma
Plasma was collected from each patient 24 h before
and 96 h after surgery and the concentrations of MMPs 2, 3 and 9
were quantified by ELISA. The mean values were then calculated for
each group of patients, i.e., carcinomas and fibroadenomas. The
mean concentrations of each MMP from the plasma collected before
surgery in carcinomas were compared with those of the
fibroadenomas. A significant difference was found in MMP 2
concentraion, with values of 257±17 ng/ml for carcinomas versus
174±12 ng/ml for fibroadenomas; this increase of plasma MMP 2 in
carcinoma patients compared with fibroadenoma patients was
statistically significant (P<0.01; Fig. 1). The difference between carcinoma
and fibroadenoma tissues was not significant for MMPs 3 and 9. The
MMP 3 concentration was 110±7.6 ng/ml in the carcinomas and 101±5.5
ng/ml in the fibroadenomas; the MMP 9 concentration was 65±5.2
ng/ml in the carcinomas and 54±9.2 ng/ml in the fibroadenomas
(Fig. 1).
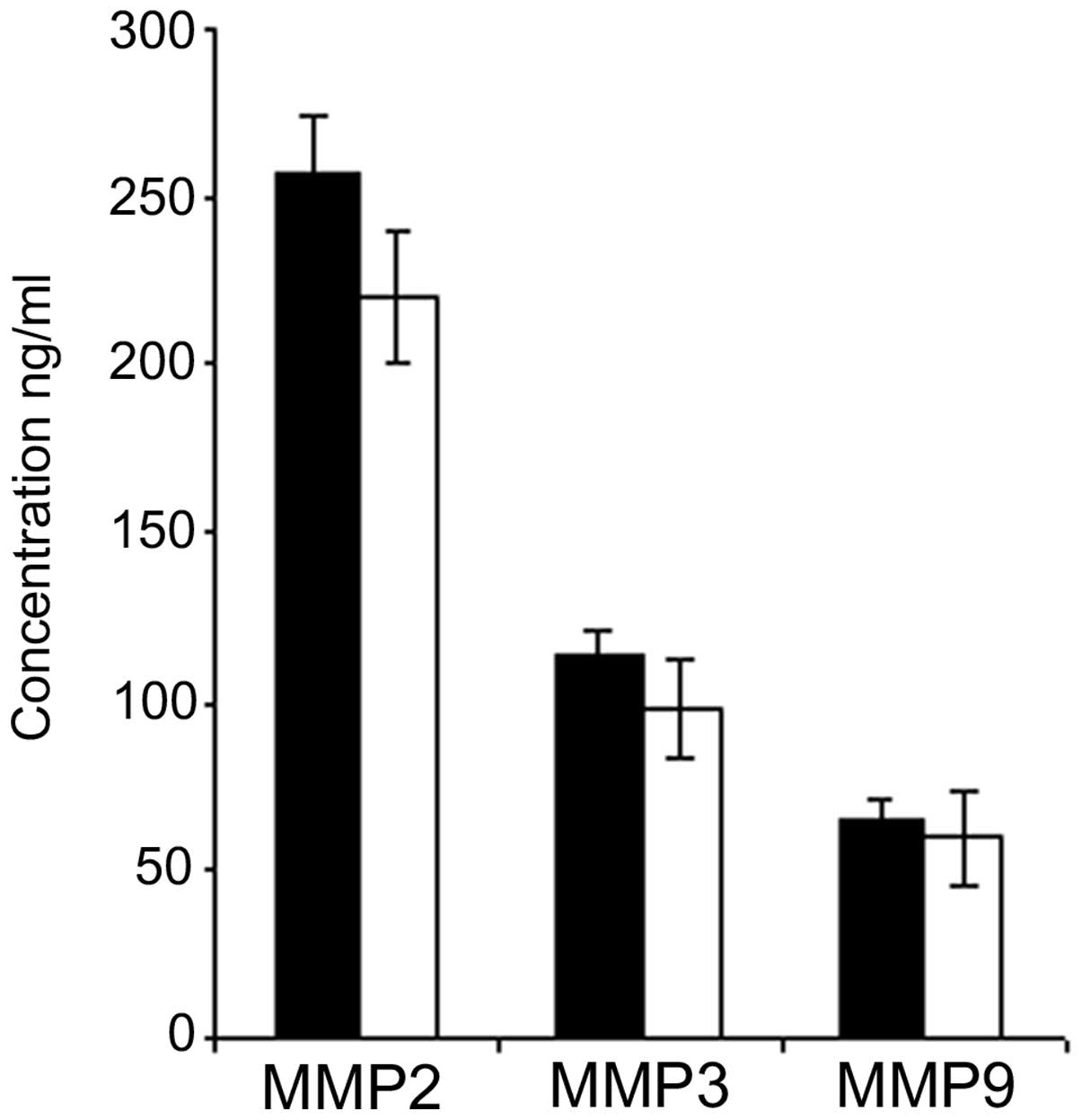
Comparison of plasmatic MMPs 2, 3 and 9
in breast cancer before and after surgery
The group of carcinoma patients was then analyzed in
order to compare the mean values for each MMP obtained before and
after surgery. The plasma concentration of each MMP slightly
decreased 96 h after surgery; however the difference between the
pre- and post-surgery values was not statistically significant for
the three MMPs (Fig. 2).
Correlation between plasmatic MMPs 2, 3
and 9 and clinicobiological parameters of breast cancer
We next investigated any possible correlation among
pre-surgical MMP 2, 3 and 9 plasma concentrations and the clinical
and biological parameters of carcinoma patients: T and N values,
grade, Ki67 level and status of estrogen and progesterone receptors
and Her2/neu. The statistical analysis revealed a significant
direct correlation between MMP 2 and G value (P<0.01) and
between MMP 9 and G value (P<0.05; Table II). No further significant
correlation was identified between the other carcinoma parameters
analyzed and MMP 2, 3 and 9 concentration. In conclusion, increased
concentrations of MMPs 2 and 9 were correlated significantly with
the histological grade of the tumor, meaning that a less
differentiated breast cancer should be characterized by higher
levels of plasma MMPs 2 and 9 .
 | Table IICorrelations between pre-surgical MMPs
2, 3 and 9 and clinical parameters. |
Table II
Correlations between pre-surgical MMPs
2, 3 and 9 and clinical parameters.
| Clinical
parameters | MMP 3 basal | MMP 2 basal | MMP 9 basal |
|---|
| er | | | |
| PC | 0.301 | −0.052 | 0.390 |
| Sig. (2-code) | 0.061 | 0.734 | 0.004 |
| N | 40 | 45 | 37 |
| pgr | | | |
| PC | −0.082 | −0.062 | 0.017 |
| Sig. (2-code) | 0.615 | 0.687 | 0.922 |
| N | 40 | 45 | 37 |
| erbB | | | |
| PC | 022 | 0.028 | −0.090 |
| Sig. (2-code) | 891 | 0.857 | 0.596 |
| N | 40 | 44 | 37 |
| ki67 | | | |
| PC | 0.030 | −0.308 | −0.082 |
| Sig. (2-code) | 0.854 | 0.390 | 0.630 |
| N | 40 | 45 | 37 |
| T | | | |
| PC | −0.008 | −0.049 | −0.039 |
| Sig. (2-code) | 0.957 | 0.741 | 0.813 |
| N | 43 | 48 | 39 |
| N | | | |
| PC | 0.098 | −0.105 | −0.010 |
| Sig.
(2-code) | 0.531 | 0.478 | 0.950 |
| N | 43 | 48 | 39 |
| G | | | |
| PC | 0.031 | 0.369a | 0.324b |
| Sig.
(2-code) | 0.842 | 0.010 | 0.044 |
| N | 43 | 48 | 39 |
| MMP 3 basal | | | |
| PC | 1 | 0.050 | 0.426a |
| Sig.
(2-code) | | 0.756 | 0.009 |
| N | 45 | 41 | 37 |
| MMP 2 basal | | | |
| PC | 0.050 | 1 | 0.023 |
| Sig.
(2-code) | 0.756 | | 0.889 |
| N | 41 | 48 | 39 |
| MMP 9 basal | | | |
| PC | 0.426a | 0.023 | 1 |
| Sig.
(2-code) | 0.009 | 0.889 | |
| N | 37 | 39 | 41 |
Discussion
The role played by MMPs in several steps of cancer
local invasion and metastasis is already well known: the increase
in levels of these enzymes within the tumor site is due either to
tumor cells or to peri-tumoral activated fibroblasts, as well as to
inflammatory cells (8–10). In particular, MMP 2 has been studied
for its involvement in breast cancer progression, and the positive
immunostaining of the enzyme has been proposed to be a marker of
aggressiveness in breast carcinoma (11); however, it has been demonstrated
that blocking MMP 2 secretion and activation may reduce the risk of
breast cancer metastasis (12). The
concentration of specific MMPs has also been described to be
enhanced in the serum of tumoral patients, including patients with
breast neoplasia (13), and the
combined determination of MMP 2, MMP 9 and TIMP1 has been proposed
as a significant marker in blood plasma for bladder cancer
diagnosis (14). Our data show
that, among the three MMPs analyzed, the plasma MMP 2 level was
significantly higher in breast carcinoma patients than in
fibroadenoma patients. This result agrees with those of a previous
study, which reported that blood levels of MMP 2 were higher in
breast cancer patients than in healthy blood donors (15). Furthermore, an elevated activity of
pro-MMP 2 and pro-MMP 9 has been described in the serum of mammary
neoplasia patients and has been correlated with tumor size and
lymph node status, indicating the usefulness of these enzymes as
staging markers for breast cancer (16,17).
Our results demonstrate that the plasma
concentration of all three MMPs decreaseed 96 h after surgery, but
the variations of these values were not statistically significant.
These data may be explained by the fact that 96 h is too soon after
the surgery to observe the total recovery of normal MMPs plasma
concentration. In fact, the local and systemic inflammation
reactions that follow surgery determine an activation of normal
cells with an increase of MMPs, therefore a significant decrease of
MMPs serum levels becomes evident only 1 month after surgery
(18,19).
Further data were obtained with regard to the
correlation of MMP 2 and 9 plasma concentrations with the G value
of the tumor. Poorly differentiated tumors correspond to an
advanced stage of neoplasia and are classified with a high value of
grading (G3). This stage of tumor is usually characterized by
higher invasive potential compared with well-differentiated tumors
(low grading values of G1 and G2), which usually correspond to
tumors with better prognosis.
An important feature of the tumoral invasive
phenotype is the capability to produce lytic enzymes, such as MMPs;
therefore, an increase in these enzymes may correlate with a more
advanced and invasive tumor. Our results supported this theory,
with higher MMP 2 and 9 plasma concentrations correlating with a
less differentiated cancer phenotype, as indicated by a high
grading. A previous study described that the expression levels of
MMPs 8, 10, 12 and 27 in breast cancer tissue were correlated with
tumor grade, since they were higher in G3 compared with G2 cancers
(20).
A study concerning MMP 2 and 9 immunohistochemical
expression performed on patients with lymph node-negative breast
cancer demonstrated that the co-expression of the two MMPs is an
unfavourable prognostic factor for relapse-free survival (21). MMP 2-positive immunoreactivity in
lymph node-positive breast cancer has been reported to be
correlated with unfavourable prognosis and increased risk of
recurrence in both pre- and postmenopausal patients (22,23).
A recent study described a direct correlation of
immunohistochemical expression between MMP 2 and cyclooxygenase-2
(COX-2) in invasive ductal breast carcinoma tissues (24); however, the expression of COX-2 may
be associated with increased angiogenesis and lymph node
metastasis, and it is also correlated with high histological grade
and large tumor size in breast cancer (25). Taken together, this information
indicates that a direct link exists between MMP 2 and COX-2 and
that they may both be markers of cell invasion and poor prognosis
in breast cancer (26). Our data
regarding the correlation between plasma MMP 2 and 9 values and
tumor histological grade also suggest the consideration of these
parameters as molecular markers of tumor invasion, as has been
already indicated for COX-2 (27).
These data confirm the role of certain MMPs in the
development and progression of breast cancer and are in agreement
with recent clinical research proposing the use of new synthetic
metalloproteinase blockers in anticancer therapy, including
batimastat, marimastat and tetracycline derivates (12). Therefore, these findings suggest the
value of the evaluation of plasma MMP 2 and 9 as additional markers
for the prognosis of breast cancer.
References
|
1
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanemaaijer R, Verheijen JH, Maguire TM,
Visser H, Toet K, McDermott E, et al: Increased gelatinase-A and
gelatinase-B activities in malignant vs. benign breast tumors. Int
J Cancer. 2:204–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liotta LA: Tumor invasion and metastases -
role of the extracellular matrix: Rhoads Memorial Award lecture.
Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
4
|
Lynch CC and Matrisan LM: Matrix
metalloproteinases in tumor-host cell communication.
Differentiation. 70:561–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Somiari SB, Shriver CD, Heckman C, Olsen
C, Hu H, Jordan R, Arciero C, Russell S, Garguilo G, Hooke J and
Somiari RI: Plasma concentration and activity of matrix
metalloproteinase 2 and 9 in patients with breast disease, breast
cancer and at risk of developing breast cancer. Cancer Lett.
233:98–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leppä S, Saarto T, Vehmanen L, Blomqvist C
and Elomaa I: A high serum matrix metalloproteinase-2 level is
associated with an adverse prognosis in node-positive breast
carcinoma. Clin Cancer Res. 10:1057–1063. 2004.PubMed/NCBI
|
|
7
|
Mendes O, Kim HT and Stoica G: Expression
of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat
model. Clin Exp Metastasis. 22:237–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baker EA, Stephenson TJ, Reed MW and Brown
NJ: Expression of proteinases and inhibitors in human breast cancer
progression and survival. Mol Pathol. 55:300–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bodey B, Bodey B Jr, Siegel SE and Kaiser
HE: Matrix metalloproteinases in neoplasm-induced extracellular
matrix remodelling in breast carcinoma. Anticancer Res.
21:2021–2028. 2001.PubMed/NCBI
|
|
10
|
González LO, Pidal I, Junquera S, Corte
MD, Vásquez J, Rodríguez JC, Lamelas ML, Merino AM, García-Muñíz JL
and Vizoso FJ: Overexpression of matrix metalloproteinases and
their inhibitors in mononuclear inflammatory cells in breast cancer
correlates with metastasis-relapse. Br J Cancer. 97:957–963.
2007.PubMed/NCBI
|
|
11
|
Talvensaari-Mattila A, Pääkkö P, Höyhtyä
M, Blanco-Sequeiros G and Turpeenniemi-Hujanen T: Matrix
metalloproteinase-2 immunoreactive protein: a marker of
aggressiveness in breast carcinoma. Cancer. 83:1153–1162. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
13
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 type IV collagenases in serum of patients with
pleural effusions. Int J Oncol. 26:1363–1368. 2005.PubMed/NCBI
|
|
14
|
Staack A, Badendieck S, Schnorr D, Loening
SA and Jung K: Combined determination of plasma MMP2, MMP9, and
TIMP1 improves the non-invasive detection of transitional cell
carcinoma of the bladder. BMC Urol. 6:192006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Incorvaia L, Badalamenti G, Rini G, Arcara
C, Fricano S, Sferrazza C, Di Trapani D, Gebbia N and Leto G:
MMP-2, MMP-9 and activin A blood levels in patients with breast
cancer or prostate cancer metastasis to the bone. Anticancer Res.
27:1519–1525. 2007.PubMed/NCBI
|
|
16
|
Jinga DC, Blidaru A, Condrea I, Ardeleanu
C, Dragomir C, Szegli G, Stefanescu M and Matache C: MMP-9 and
MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast
cancer: correlations with prognostic factors. J Cell Mol Med.
10:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stankovic C, Konjevic G, Gopcevic K, Jovic
V, Inic M and Jurisic V: Activity of MMP-2 and MMP-9 in sera of
breast cancer patients. Pathol Res Pract. 206:241–247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quaranta M, Daniele A, Coviello M, Venneri
MT, Abbate I, Caringella ME, Di Tardo S, Divella R, Trerotoli P, Di
Gennaro M, Schittulli F, Fransvea E and Giannelli G: MMP-2, MMP-9,
VEGF and CA 15.3 in breast cancer. Anticancer Res. 27:3593–3600.
2007.
|
|
19
|
Ranuncolo SM, Armanasco E, Cresta C, Bal
De Kier Joffe E and Puricelli L: Plasma MMP-9 (92 kDa-MMP) activity
is useful in the follow-up and in the assessement of prognosis in
breast cancer patients. Int J Cancer. 106:745–751. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Köhrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines: New
findings and review of the literature. BMC Cancer.
9:1882009.PubMed/NCBI
|
|
21
|
Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS,
Di GH, Liu G, Li FM, Ou ZL, Jie C, Shen ZZ and Shao ZM: Prognostic
value of matrix metalloproteinases (MMP-2 and MMP-9) in patients
with lymph node-negative breast carcinoma. Breast Cancer Res Treat.
88:75–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Talvensaari-Mattila A, Pääkkö P and
Turpeenniemi-Hujanen T: MMP-2 positivity and age less than 40 years
increases the risk for recurrence in premenopausal patients with
node-positive breast carcinoma. Breast Cancer Res Treat.
58:287–293. 1999.PubMed/NCBI
|
|
23
|
Talvensaari-Mattila A, Pääkko P,
Blanco-Sequeiros G and Turpeenniemi-Hujanen T: Matrix
metalloproteinase-2 (MMP-2) is associated with the risk for a
relapse in postmenopausal patients with node-positive breast
carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer
Res Treat. 65:55–61. 2001. View Article : Google Scholar
|
|
24
|
Atik E, Akansu B, Bakaris S and Aban N:
Expression of cyclooxygenase-2 and its relation to histological
grade, inducible nitric oxide synthase, matrix metalloproteinase-2,
CD-34, Caspase-3, and CD8 in invasive ductal carcinoma of the
breast. Saudi Med J. 31:130–134. 2010.
|
|
25
|
Nassar A, Radhakrishnan A, Cabrero IA,
Cotsonis G and Cohen C: COX-2 expression in invasive breast cancer:
correlation with prognostic parameters and outcome. Appl
Immunohistochem Mol Morphol. 15:255–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sivula A, Talvensaari-Mattila A, Lundin J,
Joensuu H, Haglund C, Ristimaki A and Turpeenniemi-Hujanen T:
Association of cyclooxygenase-2 and matrix metalloproteinase-2
expression in human breast cancer. Breast Cancer Res Treat.
89:215–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de la Torre J, Sabadell MD, Rojo F, Lirola
JL, Salaricru S, Reventos J, Ramón y Cajal S and Xercavins J:
Cyclo-oxygenase type 2 is dysregulated in breast ductal carcinoma
in situ and correlates with poor outcome. Eur J Obstet Gynecol
Reprod Biol. 151:72–76. 2010.PubMed/NCBI
|