Introduction
Osteosarcoma arises from mesenchymal tissues and is
the most common primary malignant bone tumor in children and
adolescents (1,2). Since osteosarcoma is highly aggressive
and commonly metastasizes to the lung, patients with metastatic or
recurrent osteosarcoma usually have an extremely poor prognosis
(2–5). Although the diagnosis and treatment of
osteosarcoma have been improved significantly during the past 30
years (3,6), approximately 30–40% of patients
experience osteosarcoma relapse, mostly within the first three
years after diagnosis. Common factors, such as demographics (age
and gender), tumor size, site and stage have been used for the
prognosis of advanced osteosarcoma, but the results are difficult
to reproduce due to the usage of various techniques and units for
measurement. Furthermore, tumor-related metastasis and
chemotherapeutic response are significant prognostic factors, but
they usually occur at the late stage of osteosarcoma (1–4).
Therefore, there is an urgent need for the discovery of new
reliable and efficient biomarkers for the prognosis of
osteosarcoma.
Extracellular matrix metalloproteinase inducer
(EMMPRIN, also known as CD147, EMMPRIN/CD147) is a highly
glycosylated transmembrane protein and is abundantly expressed on
the membrane surface of various tumor cells, including non-small
cell lung cancer (NSCLC) (7),
macrophage-like lymphoid neoplasm P388D1 cells (8), thyroid carcinoma (9), primary cutaneous malignant melanoma
(10), intrahepatic
cholangiocarcinoma (11),
colorectal/gastric cancer (12),
renal cell carcinoma (13),
prostate cancer (14), cervical
cancer (15), epithelial ovarian
cancer (16) and breast carcinoma
(17). EMMPRIN/CD147 promotes
survival, invasion and metastasis of tumor cells through multiple
pathways and mechanisms, including the functional loss of p53, a
tumor suppressor (18), upregulated
expression of vascular endothelial growth factor (VEGF) (13,19–21),
disruption of transforming growth factor-β1 (TGF-β1), a
growth-modulating factor (22), and
regulation of the urokinase-type plasminogen activation (uPA)
system of serine proteases (23).
Therefore, EMMPRIN/CD147 has been suggested potentially as a
prognostic biomarker for certain types of tumors and as a
therapeutic target (24). However,
the prognostic value of EMMPRIN/CD147 in human osteosarcoma remains
to be elucidated.
The aim of present study was to examine whether
EMMPRIN/CD147 could be expressed in osteosarcoma tissues and to
analyze the potential association of the levels of EMMPRIN/CD147
expression with clinicopathological characteristics and survival
outcome in Chinese patients with osteosarcoma.
Materials and methods
Cell lines and cultures
Human osteosarcoma cell lines (Saos-2, U-2OS and
MG-63), human malignant melanoma cell line A375 and human
osteoblast cell line Hob were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in
Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen, Carlsbad,
CA, USA), supplemented with 20 U/ml penicillin, 100 μg/ml
streptomycin (Sigma, Beijing, China), and 10% heat-inactivated
fetal bovine serum (FBS; Biowhittaker, Walkersville, MD, USA) at
37°C in a humidified incubator supplied with 5%
CO2(25).
Western blot analysis
Western blot analysis was performed as described
previously (26). Briefly,
individual osteosarcoma cell lines grown to confluency were
harvested and lysed in buffer containing 50 mmol/l Tris-HCl (pH
8.0), 150 mmol/l NaCl, 100 μg/ml
phenylmethan-sulfonylfluoride and 1% Triton X-100 for 30 min on
ice, followed by centrifugation. After quantification of protein
concentrations, equal amounts (50 μg/lane) of cell lysates
were separated by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a nitrocellulose
membrane. Subsequently, the membrane was blocked with 5% non-fat
dry milk in TBST [10 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl and
0.05% Tween-20] at room temperature for 1 h. The proteins were
probed with rabbit anti-human EMMPRIN/CD147 antibodies (1:1,000;
Boster, Wuhan, China) or control anti-glyceraldehyde-3-phosphate
dehydrogenase antibodies (anti-GAPDH, Sigma) at 4°C overnight. The
bound antibodies were detected with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit antibodies (1:10,000; Boster) and
visualized using the SuperSignal West Femto trial kit (Pierce
Biotechnology, Rockford, IL, USA) (27), according to the manufacturer’s
instructions.
ELISA
Human osteosarcoma cell lines (Saos-2, U-2OS and
MG-63), human malignant melanoma cell line A375 and human
osteoblast cell line Hob were harvested and after being
centrifuged, cell pellets were weighed and re-suspended in an equal
volume of lysis buffer. Following centrifugation, the
concentrations of EMMPRIN/CD147 in the supernatants were determined
by ELISA using the EMMPRIN/CD147 ELISA kit, according to the
manufacturer’s instructions (Biosensis, Thebarton, Australia), as
reported in a previous study (28).
The concentrations of cellular EMMPRIN/CD147 were determined
according to the standard curve and expressed as μg/mg wet
cells.
Research subjects
Written informed consent was obtained from
individual participants and the experimental protocol was approved
by the Institution Research Board of The First Affiliated Hospital
of China Medical University (Liaoning, China). A total of 55
patients with osteosarcoma were collected from the in-patient
service at the Department of Orthopaedics (The First Affiliated
Hospital of China Medical University) between 1997 and 2003 for
this retrospective study. These patients had been diagnosed with
osteosarcoma at stage IIA or above, according to the
clinicopathological classification of diagnostic standards
(29,30). The patients were intravenously
administered neoadjuvant chemotherapy of one cycle of 8
g/m2 methotrexate for one day, and 120 mg/m2
doxorubicin and 75 mg/m2 cisplatin daily for three
consecutive days for two cycles consecutively. Subsequently, these
patients were subjected to surgical resection of the tumor, and
their surgical margins were classified, according to the Enneking
scoring system, as highly malignant intracompartmental osteogenic
sarcomas (IIA), extracompartmental lesions (IIB) or osteogenic
sarcomas with manifestation of metastases (III) (29,30).
Following surgery, specimens were collected from individual
patients and evaluated for their diagnosis and adequacy of the
surgical margins. Surgical resection of a tumor with radical or
wide margins was considered as adequate, whereas those with
marginal or intralesional margins were classified as inadequate.
The patients received postoperative chemotherapy with two cycles of
methotrexate, doxorubicin and cisplatin, as described above, while
35 patients with a poor histological response to preoperative
chemotherapy, based on the percentage of tumor cell necrosis in the
surgical specimens (31), were
treated with the medicines described above and two cycles of 15
g/m2 ifosfamide daily for five consecutive days.
Patients were followed up and clinical data were updated for more
than 5 years. Tumor recurrence at any site or mortality were
defined as an adverse event. The clinicopathological features of
the osteosarcoma patients are shown in Table I.
 | Table ICorrelation between EMMPRIN/CD147
expression and clinicopathological characteristics. |
Table I
Correlation between EMMPRIN/CD147
expression and clinicopathological characteristics.
| | EMMPRIN/CD147
| |
|---|
| Parameter | n (%) | Negative/weak |
Moderate/strong | P-value |
|---|
| Total | 55 (100) | 25 | 30 | |
| Gender | | | | 0.162 |
| Male | 32 (58) | 12 | 20 | |
| Female | 23 (42) | 13 | 10 | |
| Age (years) | | | | 0.495 |
| ≤18 | 37 (65) | 18 | 19 | |
| >18 | 18 (35) | 7 | 11 | |
| Anatomical
location | | | | 0.539 |
| Tibia/femur | 35 (67) | 17 | 18 | |
| Elsewhere | 20 (33) | 8 | 12 | |
| Tumor size
(cm3) | | | | 0.637 |
| ≤50 | 37 (31) | 16 | 21 | |
| >50 | 18 (69) | 9 | 9 | |
| Histological
subtype | | | | 0.451 |
| Osteoblastic | 25 (45) | 9 | 16 | |
|
Chondroblastic | 20 (24) | 10 | 10 | |
| Fibroblastic | 9 (29) | 5 | 4 | |
| Not
specified | 1 (2) | 1 | 0 | |
| Pathological
classification | | | | 0.003a |
| IIA | 18 (33) | 14 | 4 | |
| IIB | 20 (36) | 7 | 13 | |
| III | 17 (31) | 4 | 13 | |
| Treatment | | | | 0.580 |
| Limb salvage | 33 (64) | 14 | 19 | |
| Amputation | 22 (36) | 11 | 11 | |
| Percentage of dead
cells | | | | 0.001 |
| <90% | 35 (67) | 10 | 25 | |
| ≥90% | 20 (33) | 15 | 5 | |
| Surgical
margins | | | | 0.707 |
| Adequate | 53 (96) | 24 | 19 | |
| Inadequate | 2 (4) | 1 | 1 | |
Immunohistochemistry
The cellular expression of EMMPRIN/CD147 in surgical
tumor tissues from patients was detected by immunohistochemistry
analysis, as described previously (32,33)
using the Streptavidin-Avidin-Biotin-Peroxidase Complex (SABC). The
specimen tissue sections at 4 μm were treated with 3%
H2O2 for 10 min at room temperature. The
sections were blocked with 5% bovine serum albumin (Zhongshan,
Beijing, China) in PBS solution for 20 min and probed with rabbit
anti-human EMMPRIN/CD147 antibodies (1:300; Boster) at 4°C for 12
h. After washing, the bound antibodies were detected with
biotinylated goat anti-rabbit IgG (1:100) and SABC complex at 30°C
for 20 min. The immunoreactivity was visualized by
3,3-diaminobenzidine tetrachloride (DAB) and counterstained with
hematoxylin, then examined under a light microscope (CX41; Olympus,
Tokyo, Japan) at ×400 magnification. In addition, the
formalin-fixed, paraffin-embedded tumor sections at 4 μm
were stained with hematoxylin and eosin (H&E) for histological
diagnosis.
The negative controls for immunohistochemistry
included using PBS control (without the primary EMMPRIN/CD147
antibody), and 15 non-tumor rib bone tissue samples from patients
who had undergone surgery. As a positive control for CD147
expression, immunostaining was performed on a sample of prostate
cancer tissue with known strong expression of CD147, which had been
used in a previous study (34). The
quality of staining was evaluated using consecutive control
sections. The cells stained positively by anti-EMMPRIN/CD147 were
assessed, as previously reported (35,36).
Briefly, the number of positively stained cells and total number of
cells in a given area were evaluated by two pathologists in a
blinded manner. If the amount of positively stained cells was ≤5%
the tissue was considered as negative (−); 6–25%, weak (+); 26–50%,
moderate (++); and ≥51%, strong (+++). A total of at least 400
cells from five areas of individual tissue samples were
evaluated.
Survival analysis
The overall survival of individual patients was
defined from the day of surgery up to the last follow-up
examination or death of the patient. Data from patients who had
survived at the end of observation period were censored at their
last follow-up visit. Individuals who succumbed to other diseases
unrelated to osteosarcoma or survived at the end of the observation
period were considered a censoring event. Event-free survival was
calculated from the date of initial diagnosis. Overall survival and
event-free survival curves were plotted for each group of patients,
according to the levels of EMMPRIN/CD147 expression in the surgical
specimen tissues.
Statistical analysis
Data shown are the real case number and percentage
for each group. All of the patients were stratified according to
individual parameters, and the difference in the frequency of cases
between groups was calculated by the χ2 test or Fisher’s
exact test. The difference in overall and event-free survival of
each group of patients was analyzed by the log-rank test and the
mean survival time between groups was calculated by Student’s
t-test, followed by analysis of the 95% confidence interval (CI).
The Cox proportional hazards model was used for multivariate
analysis. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
EMMPRIN/CD147 is expressed by human
osteosarcoma cell lines
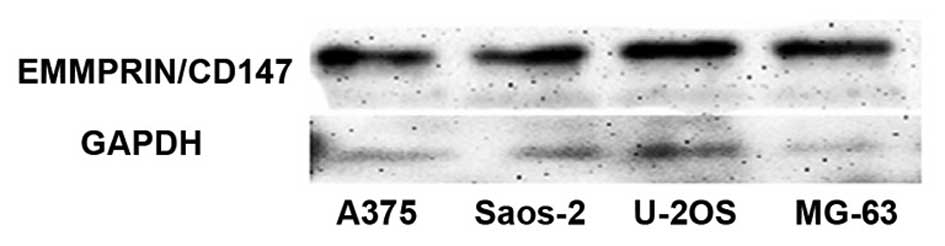
The human osteosarcoma cells lines, Saos-2, U-2OS
and MG-63, and human malignant melanoma cell line, A375, as well as
human non-tumor osteoblast cell line, Hob, were cultured in
vitro and harvested. After lysis, the cell lysates were
separated by SDS-PAGE and the expression of EMMPRIN/CD147 was
characterized by western blot assays (Fig. 1). There was no detectable
EMMPRIN/CD147 expression in human non-tumor osteoblast cell line,
Hob (data not shown). However, high levels of EMMPRIN/CD147
expression were observed in all of the three osteosarcoma cell
lines tested and human malignant melanoma cell line, A375, and the
relative levels of EMMPRIN/CD147 to the control GAPDH were
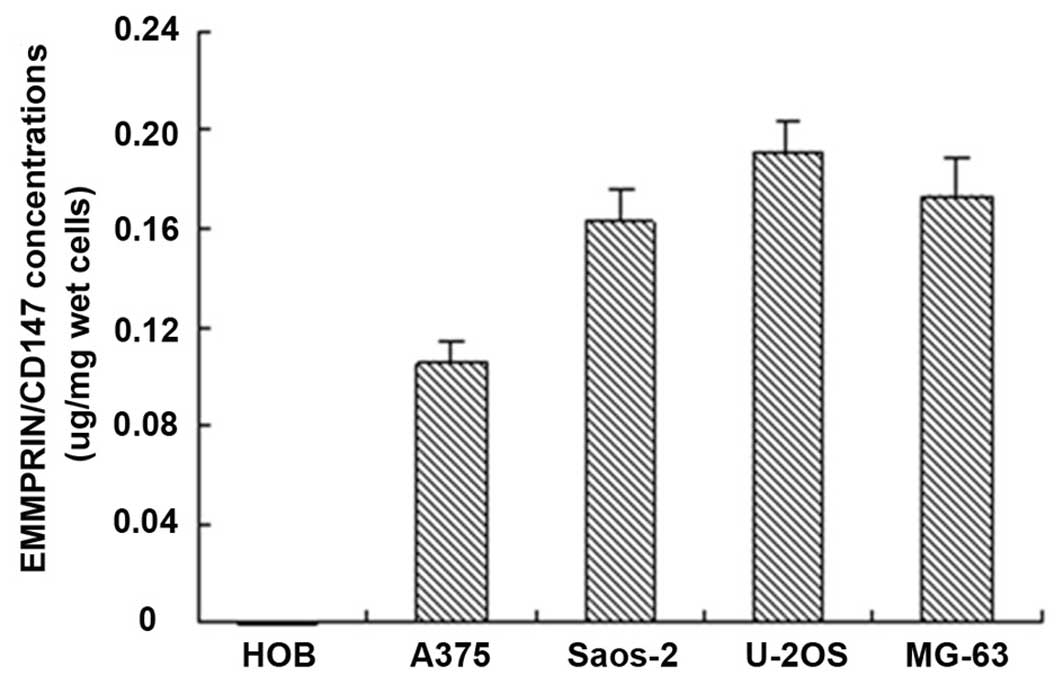
undistinguishable among these cell lines. Furthermore, the ELISA
analysis also indicated that EMMPRIN/CD17 was expressed in the
osteosarcoma cells and malignant melanoma cell line A375 (Fig. 2). These data demonstrated that the
EMMPRIN/CD147 was highly expressed in human osteosarcoma cells.
EMMPRIN/CD147 is expressed in human
osteosarcoma tumor tissues
Next, we examined whether EMMPRIN/CD147 was
expressed in spontaneously developed human osteosarcoma tissues.
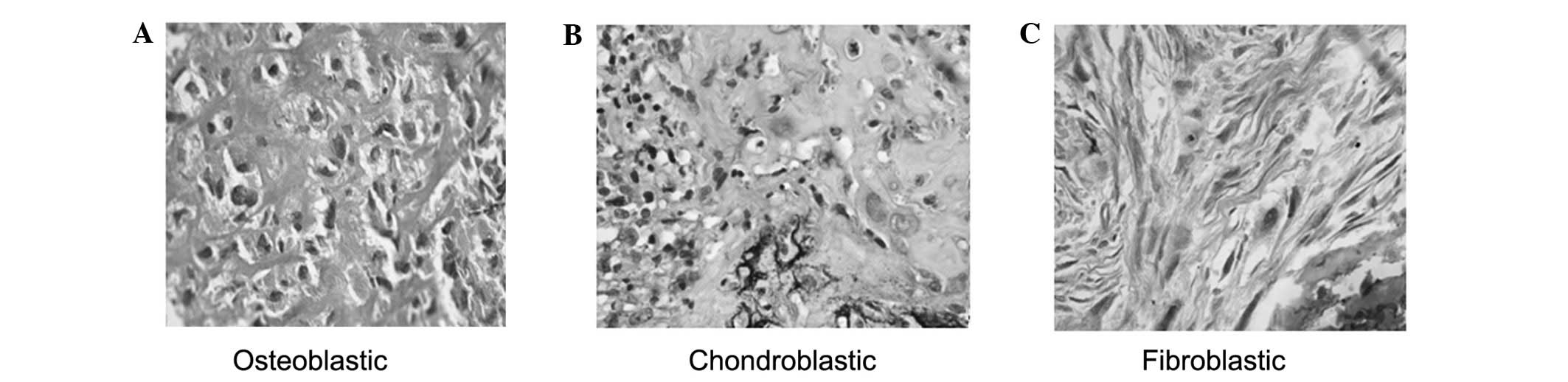
Specimens from 55 patients with osteosarcoma were collected and
were defined as osteoblastic (25/55; Fig. 3A), chondroblastic (20/55; Fig. 3B), fibroblastic (9/55; Fig. 3C), and non-specific osteosarcoma
(1/55), based on histological examinations. The demographic and
clinicopathogenic characteristics are presented in Table I. Pathological classification
identified those patients with osteosarcoma at stage IIA or above.
These osteosarcomas were characterized by the presence of osteoid
(bone formation) within the tumor tissues. The tumor cells were
very polymorphic (anaplastic), similar to giant cells with numerous
atypical mitoses or multinucleated osteoclast-like giant cells
(Fig. 3A–C), similar to those
observed in previous reports (29,30).
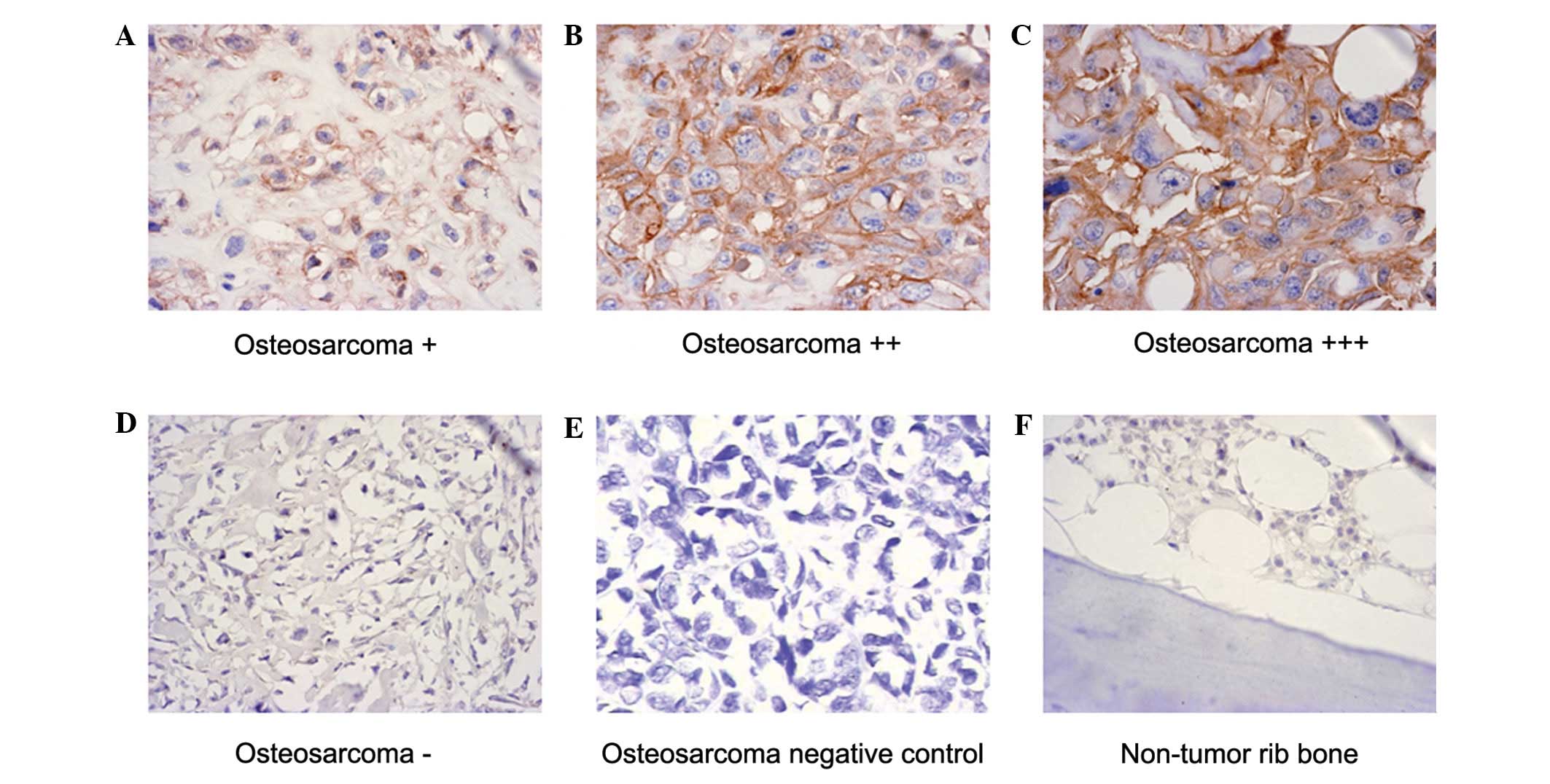
Immunohistochemical analyses revealed that 45 of the
55 osteosarcoma tissue samples were positive for anti-EMMPRIN/CD147
staining (Fig. 4A–C), but negative
in 10 out of 55 osteosarcoma tissue samples and negative controls
(Fig. 4D and E) as well as
non-tumor rib bone tissue samples (Fig.
4F). The relative levels of EMMPRIN/CD147 expression in the
osteosarcoma tissues were classified as negative/weak (25/55) or
moderate/high (30/55), respectively (Table I). Furthermore, immunoreactivity
against EMMPRIN/CD147 was observed predominantly in the membrane
and cytoplasm of osteosarcoma tumor cells, but not in the nucleus
of tumor cells or in surrounding stromal cells (Fig. 4A–C). In addition, the intensity of
anti-EMMPRIN/CD147 staining was notably higher in osteosarcoma
cells than in the bone giant cell tumors and non-cancerous adjacent
tissues. Notably, stratification of each measure indicated that the
EMMPRIN/CD147 immunostaining intensity was closely associated with
the pathological degree. Indeed, 13 out of 17 or 20 patients with
osteosarcoma at stage III or IIB had strong staining of
anti-EMMPRIN/CD147, while only four out of 18 patients with
osteosarcoma at stage IIA were strongly positive for
anti-EMMPRIN/CD147 (P<0.05; Table
I). However, the intensity of anti-EMMPRIN/CD147 staining was
not associated with other parameters. Therefore, EMMPRIN/CD147 was
expressed in the majority of human osteosarcoma tissues and the
relative levels of EMMPRIN/CD147 expression were associated
positively with the pathogenic degree in human osteosarcoma.
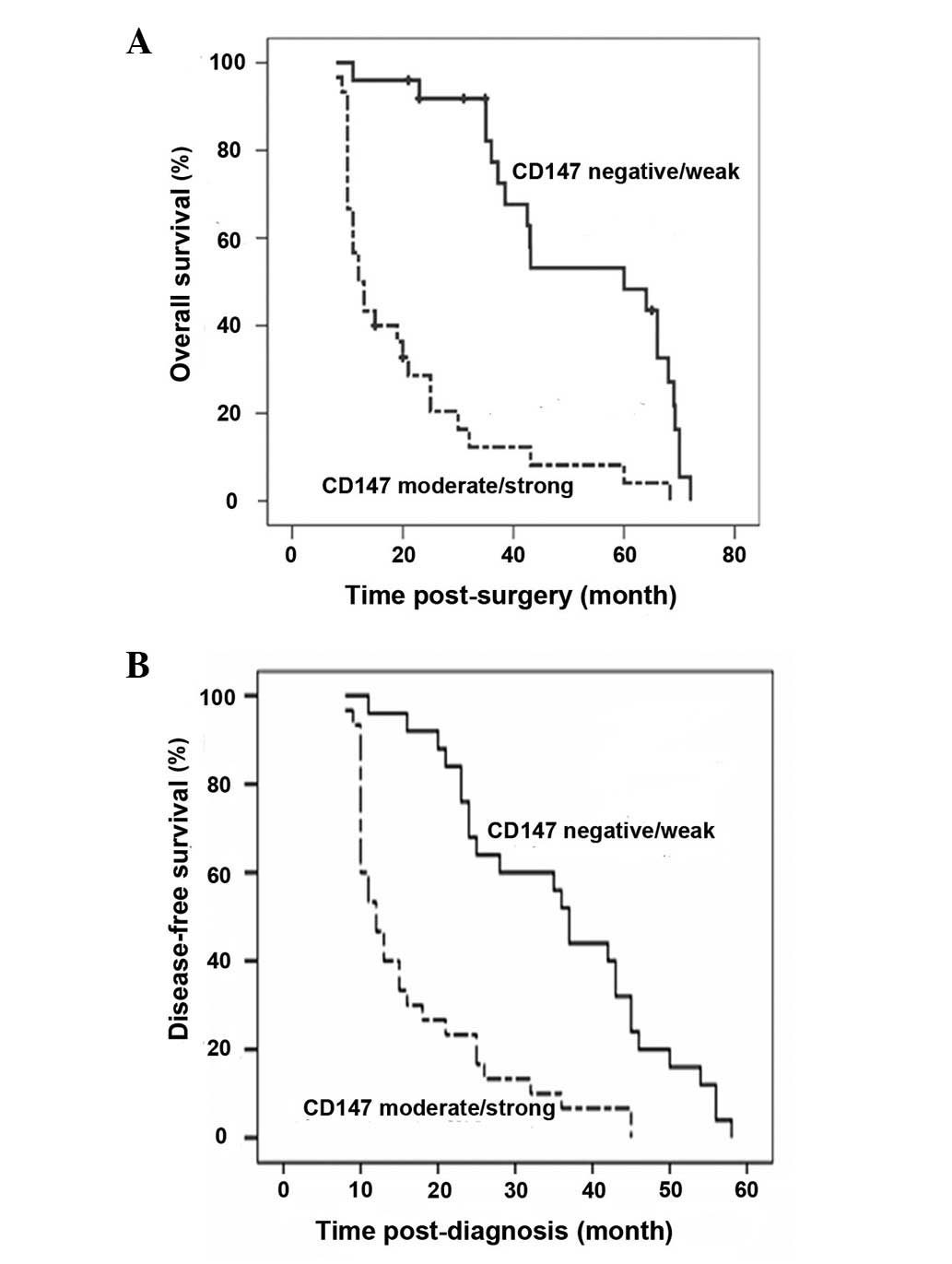
High levels of EMMPRIN/CD147 expression
are associated negatively with the survival period of osteosarcoma
patients
We followed up all patients for a mean period of 32
months (8–72 months) and found that 42 patients (42/55) succumbed
to the osteosarcoma without other evident diseases during the
follow-up period. The mean survival time for the patients with
osteosarcoma at stage IIB/III was 33 months, which was
significantly shorter than that of patients with tumors at stage
IIA (P<0.05). A multivariate analysis (Cox regression model)
revealed that the tumor pathological degree (IIA vs. IIB/III)
(P=0.001; 95% CI, 5.004–85.535) and EMMPRIN/CD147 expression
(P=0.002; 95% CI, 1.810–13.238), but not gender, age, tumor
location, treatment or histological subtype, were significantly
associated with the overall survival (Fig. 5A; Table
I). Notably, patients with osteosarcoma that had
moderate/strong EMMPRIN/CD147 expression displayed significantly
shorter periods of overall survival, compared with those with
negative/weak EMMPRIN/CD147-expressing tumors (Fig. 5A; Table
I; P<0.05). A similar pattern of event-free survival was
observed in these two groups of patients (Fig. 5B). Therefore, the expression of
EMMPRIN/CD147 is likely to be a negative prognostic factor for the
survival of patients with osteosarcoma.
Discussion
Osteosarcoma is the most common primary malignant
bone tumor in children and adolescents, and has an extremely poor
prognosis in patients due to fast metastasis and tumor recurrence
(2–6), although significant improvements in
the prognosis of localized osteosarcoma have been noted over the
past 30 years (3,6). We found that 42 out of 55 patients
succumbed to osteosarcoma during the 5-year follow-up, supporting
the notion that osteosarcoma has an extremely poor prognosis in
patients. The value of currently used prognostic factors, including
chemotherapy response, tumor volume, older age, gender and
p-glycoprotein expression remains in debate. Our data demonstrated
that EMMPRIN/CD147 was expressed in human osteosarcoma cell lines
and osteosarcoma tissues. The relative levels of EMMPRIN/CD147
expression in human osteosarcoma tissues were positively correlated
with the pathological degree, but negatively correlated with the
survival period. Therefore, our findings suggest that the levels of
EMMPRIN/CD147 expression may be used as one prognostic factor for
estimating the survival of osteosarcoma patients in clinics.
EMMPRIN/CD147 is a highly glycosylated transmembrane
protein belonging to the immunoglobulin superfamily 34 and is
widely presented and/or overexpressed on the membrane surface of
various malignant tumor cells (7,8,10–17).
Due to its close association with pathological classification and
overall survival analysis, EMMPRIN/CD147 has been potentially
suggested as a prognostic factor in certain types of malignant
tumors (7,8,10,12–15,17,21–24).
In the present study, our results indicated that EMMPRIN/CD147 was
expressed not only in all three human osteosarcoma cell lines, but
also in the cell membrance and cytoplasm of osteosarcoma tissues
from the majority of patients pathologically diagnosed with
osteosarcoma at grade IIA or above. These results indicated that
EMMPRIN/CD147 may be one of the prognostic factors for human
osteosarcoma. In addition, our data indicated that the levels of
EMMPRIN/CD147 expression were significantly associated with the
pathological degree of osteosarcoma, but not with age, tumor size,
location, gender, treatment or histological subtype. These findings
are consistent with a recent study (33) and further support the notion that
the pathological stage and EMMPRIN/CD147 expression may be ideal
prognostic factors for human osteosarcoma.
Our findings indicate that the expression of
EMMPRIN/CD147 was closely associated with the pathological degree
of osteosarcoma, suggesting that EMMPRIN/CD147 may be significant
in the development and progression of osteosarcoma. Previous
studies have shown that EMMPRIN/CD147, through multiple pathways
and mechanisms, stimulates adjacent fibroblasts to produce matrix
metalloproteinases, and thus promotes survival, invasion and
metastasis of tumor cells (13,18–23,36).
Indeed, EMMPRIN/CD147 expression is correlated significantly with
the stage of clinicopathology in thyroid carcinoma (9), adenocarcinomas (7), esophageal squamous cell carcinomas
(37) and prostate cancer (14). Furthermore, treatment with
anti-EMMPRIN/CD147 antibody delays the formation of tumors in
animal models (38). In addition,
treatment with EMMPRIN/CD147-specific RNA interference inhibits the
tumorigenicity and metastasis of human lymphoid neoplasms, oral
squamous carcinoma, prostate carcinoma and bladder carcinoma cells,
increasing their sensitivity to chemotherapeutic drugs (8,39,40).
These findings have indicated that RMMPRIN/CD147 may be an
important molecule in tumor progression and an attractive target
for antitumor treatment. Hence, EMMPRIN/CD147 may be one
therapeutic candidate target for the treatment of osteosarcoma in
clinics.
In conclusion, our data indicated that high levels
of EMMPRIN/CD147 were expressed in human osteosarcoma cells and
tissues. The levels of EMMPRIN/CD147 expression were positively
correlated with the clinicopathological degree of osteosarcoma and
negatively correlated with the survival of osteosarcoma patients.
Therefore, EMMPRIN/CD147 expression may be used as a potential
prognostic marker and therapeutic target for the intervention of
human osteosarcoma. We recognize the limitation of the small sample
size used in the present study and advise that further studies of
the dynamic expression of EMMPRIN/CD147 with a bigger population of
osteosarcoma patients are warranted.
Acknowledgements
The authors would like to acknowledge
Medjaden for their great help in preparing the manuscript.
References
|
1
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davicioni E, Wai DH and Anderson MJ:
Diagnostic and prognostic sarcoma signatures. Mol Diagn Ther.
12:359–374. 2008. View Article : Google Scholar
|
|
3
|
Fagioli F, Biasin E, Mereuta OM, et al:
Poor prognosis osteosarcoma: new therapeutic approach. Bone Marrow
Transplant. 41(Suppl 2): S131–S134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakamoto A and Iwamoto Y: Current status
and perspectives regarding the treatment of osteo-sarcoma:
chemotherapy. Rev Recent Clin Trials. 3:228–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang LL: Biology of osteogenic sarcoma.
Cancer J. 11:294–305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multi-disciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar
|
|
7
|
Hakuma N, Betsuyaku T, Kinoshita I, et al:
High incidence of extracellular matrix metalloproteinase inducer
expression in non-small cell lung cancers. Association with
clinicopathological parameters. Oncology. 72:197–204. 2007.
View Article : Google Scholar
|
|
8
|
Jia L, Wei W, Cao J, Xu H, Miao X and
Zhang J: Silencing CD147 inhibits tumor progression and increases
chemosensitivity in murine lymphoid neoplasm P388D1 cells. Ann
Hematol. 88:753–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aratake Y, Marutsuka K, Kiyoyama K, et al:
EMMPRIN (CD147) expression and differentiation of papillary thyroid
carcinoma: implications for immunocytochemistry in FNA cytology.
Cytopathology. 21:103–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen T and Zhu J: Evaluation of EMMPRIN
and MMP-2 in the prognosis of primary cutaneous malignant melanoma.
Med Oncol. 27:1185–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Tu Z, Du S, Wang Y and Wang Q:
Expression of matrix metalloproteinase 2 and extracellular matrix
metalloproteinase inducer are unfavorable postoperative prognostic
factors in intrahepatic cholangiocarcinoma. Pathol Oncol Res.
16:47–53. 2010. View Article : Google Scholar
|
|
12
|
Buergy D, Fuchs T, Kambakamba P, et al:
Prognostic impact of extracellular matrix metalloprotease inducer:
immunohistochemical analyses of colorectal tumors and
immunocytochemical screening of disseminated tumor cells in bone
marrow from patients with gastrointestinal cancer. Cancer.
115:4667–4678. 2009. View Article : Google Scholar
|
|
13
|
Liang YX, He HC, Han ZD, et al: CD147 and
VEGF expression in advanced renal cell carcinoma and their
prognostic value. Cancer Invest. 27:788–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han ZD, Bi XC, Qin WJ, et al: CD147
expression indicates unfavourable prognosis in prostate cancer.
Pathol Oncol Res. 15:369–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju XZ, Yang JM, Zhou XY, Li ZT and Wu XH:
EMMPRIN expression as a prognostic factor in radiotherapy of
cervical cancer. Clin Cancer Res. 14:494–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sillanpaa S, Anttila M, Suhonen K, et al:
Prognostic significance of extracellular matrix metalloproteinase
inducer and matrix metalloproteinase 2 in epithelial ovarian
cancer. Tumour Biol. 28:280–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JM, O’Neill P, Jin W, et al:
Extracellular matrix metalloproteinase inducer (CD147) confers
resistance of breast cancer cells to anoikis through inhibition of
Bim. J Biol Chem. 281:9719–9727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Evans B, O’Neill P, et al: A role
for p53 in the regulation of extracellular matrix metalloproteinase
inducer in human cancer cells. Cancer Biol Ther. 8:1722–1728. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bougatef F, Quemener C, Kellouche S, et
al: EMMPRIN promotes angiogenesis through hypoxia-inducible
factor-2alpha-mediated regulation of soluble VEGF isoforms and
their receptor VEGFR-2. Blood. 114:5547–5556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang Y, Nakada MT, Kesavan P, et al:
Extracellular matrix metalloproteinase inducer stimulates tumor
angiogenesis by elevating vascular endothelial cell growth factor
and matrix metalloproteinases. Cancer Res. 65:3193–3199. 2005.
|
|
21
|
Zheng HC, Takahashi H, Murai Y, et al:
Upregulated EMMPRIN/CD147 might contribute to growth and
angiogenesis of gastric carcinoma: a good marker for local invasion
and prognosis. Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duivenvoorden WC, Hirte HW and Singh G:
Transforming growth factor beta1 acts as an inducer of matrix
metalloproteinase expression and activity in human
bone-metastasizing cancer cells. Clin Exp Metastasis. 17:27–34.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quemener C, Gabison EE, Naimi B, et al:
Extracellular matrix metalloproteinase inducer up-regulates the
urokinase-type plasminogen activator system promoting tumor cell
invasion. Cancer Res. 67:9–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riethdorf S, Reimers N, Assmann V, et al:
High incidence of EMMPRIN expression in human tumors. Int J Cancer.
119:1800–1810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang P, Xu TY, Guan YF, Su DF, Fan GR and
Miao CY: Perivascular adipose tissue-derived visfatin is a vascular
smooth muscle cell growth factor: role of nicotinamide
mononucleotide. Cardiovasc Res. 81:370–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Xu TY, Guan YF, et al:
Nicotinamide phosphoribosyltransferase protects against ischemic
stroke through SIRT1-dependent adenosine monophosphate-activated
kinase pathway. Ann Neurol. 69:360–374. 2011. View Article : Google Scholar
|
|
27
|
Wang P, Guan YF, Du H, Zhai QW, Su DF and
Miao CY: Induction of autophagy contributes to the neuroprotection
of nicotinamide phosphoribosyltransferase in cerebral ischemia.
Autophagy. 8:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang P, Zhang RY, Song J, et al: Loss of
AMP-activated protein kinase-alpha2 impairs the insulin-sensitizing
effect of calorie restriction in skeletal muscle. Diabetes.
61:1051–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.PubMed/NCBI
|
|
30
|
Enneking WF, Spanier SS and Goodman MA:
Current concepts review. The surgical staging of musculoskeletal
sarcoma. J Bone Joint Surg Am. 62:1027–1030. 1980.PubMed/NCBI
|
|
31
|
Picci P, Bacci G, Campanacci M, et al:
Histologic evaluation of necrosis in osteosarcoma induced by
chemotherapy. Regional mapping of viable and nonviable tumor.
Cancer. 56:1515–1521. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang P, Yang FJ, Du H, et al: Involvement
of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in
brain fat mass- and obesity-associated (FTO) downregulation during
energy restriction. Mol Med. 17:523–532. 2011.PubMed/NCBI
|
|
33
|
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S and
Chen X: VEGF and EMMPRIN expression correlates with survival of
patients with osteosarcoma. Surg Oncol. 20:13–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Madigan MC, Kingsley EA, Cozzi PJ,
Delprado WJ, Russell PJ and Li Y: The role of extracellular matrix
metalloproteinase inducer protein in prostate cancer progression.
Cancer Immunol Immunother. 57:1367–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishibashi Y, Matsumoto T, Niwa M, et al:
CD147 and matrix metalloproteinase-2 protein expression as
significant prognostic factors in esophageal squamous cell
carcinoma. Cancer. 101:1994–2000. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nabeshima K, Iwasaki H, Koga K, Hojo H,
Suzumiya J and Kikuchi M: Emmprin (basigin/CD147): matrix
metalloproteinase modulator and multifunctional cell recognition
molecule that plays a critical role in cancer progression. Pathol
Int. 56:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng MF, Tzao C, Tsai WC, et al:
Expression of EMMPRIN and matriptase in esophageal squamous cell
carcinoma: correlation with clinicopathological parameters. Dis
Esophagus. 19:482–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dean NR, Newman JR, Helman EE, et al:
Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of
head and neck cancer. Clin Cancer Res. 15:4058–4065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuang YH, Chen X, Su J, et al: RNA
interference targeting the CD147 induces apoptosis of multi-drug
resistant cancer cells related to XIAP depletion. Cancer Lett.
276:189–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han ZD, He HC, Bi XC, et al: Expression
and clinical significance of CD147 in genitourinary carcinomas. J
Surg Res. 160:260–267. 2010. View Article : Google Scholar : PubMed/NCBI
|