Introduction
Terpenes are widespread natural compounds involved
in various biological activities, playing a significant role in
human medicine. They include triterpenes, saponines and other
triterpenoids, representing one of numerous classes of natural
compounds. The main groups of triterpenes and saponines are
represented by tetracyclic derivatives of protostane, cycloartane,
dammarane, euphane and pentacyclic derivatives of ursane,
gammacerane, lupane and hopane (1).
Pentacyclic triterpenes are secondary metabolites that are based on
a 30-carbon skeleton and synthesized in many plants by the
cyclization of squalene (2). These
are widely distributed in fruit peel, leaves and stem bark
(3). It has been demonstrated that
several triterpenes have the ability to induce cancer cell
apoptosis and prevent oxidative stress, inflammation and
hypertension (4,5).
Pomolic acid (PA) is a pentacyclic triterpene
isolated from the flowers of Osmanthus fragrans var.
aurantiacus Makino that are found in China, Japan and the
southern portion of Korea. The flowers are used by the Chinese to
give an aroma to tea or wine and in cosmetics for hair and skin
(6,7). Also, dried flowers have
neuroprotective, free radical scavenging and anti-oxidative effects
(8). PA inhibited the growth of
leukemia cell line HL-60 and induced mitochondria-dependent
apoptotic cell death (9). PA has
been shown to induce apoptosis by activating the caspase cascade
through loss of the mitochondrial transmembrane potential
(ΔΨm), independently of anti-apoptotic Bcl-2 expression
in leukemia cells (9,10). PA is suggested to overcome multidrug
resistance mediated by overexpression of anti-apoptotic Bcl-2
proteins (10,11). PA also showed anti-proliferative
activities against human gastric adenocarcinoma (MK-1), human
uterine carcinoma (HeLa) and murine melanoma (B16F10) cells
(12). However, the apoptotic
mechanisms of PA in human carcinoma cells were not investigated in
detail. In our preliminary experiment on cytotoxic effects, PA
reduced the viabilities of human ovarian adenocarcinoma SK-OV-3,
human colon carcinoma HCT-116, human breast adenocarcinoma MCF-7,
SK-BR-3 and human melanoma SK-MEL-5 cells. The greatest inhibition
of PA was observed in SK-OV-3 cells (data not shown). To the best
of our knowledge, the apoptotic effect of PA on SK-OV-3 cells has
not yet been examined. Thus, in this study, we report the
PA-induced apoptosis and its molecular mechanism in human ovarian
adenocarcinoma SK-OV-3 cells.
Materials and methods
Ethical considerations
The study was approved by the Kyung Hee University
Institutional Animal Care and Use Committee (Yongin, Korea).
Extraction and isolation of pomolic
acid
The dried and powdered flowers (800 g) of
Osmanthus fragrans var. aurantiacus Makino, were
extracted with 80% aqueous methanol (MeOH) (3 liters x3). Extracts
were partitioned with ethyl acetate (EtOAc; 3 liters x3) and
H2O (3 liters). The soluble fraction of EtOAc extract
(30 g) was subjected to SiO2 column (8x15 cm)
chromatography (c.c.), eluted with n-hexane-EtOAc (5:1 → 1:1, 5
liters of each) and monitored using thin-layer chromatography (TLC)
to produce 30 fractions (OSE-1 to OSE-30). Non-soluble precipitate
(OSE-P, 2.69 g) of EtOAc extracts was applied to the
octadecylsilica gel (ODS) c.c. and eluted with MeOH-H2O
(4:1, 18 liters) to produce 8 fractions (OSE-P-1 to OSE-P-8).
OSE-P-4 [Ve/Vt (elution volume/total volume) 0.27–0.38, 224 mg] was
subjected to SiO2 c.c. and eluted with
CHCl3-MeOH (25:1, 2.1 liters) to give 14 fractions
(OSE-P-4-1 to OSE-P-4-14). The subfraction OSE-P-4-4 yielded 20 mg
PA (Ve/Vt 0.21–0.28, ODS TLC Rf 0.21,
MeOH-H2O=13:1).
Cell culture
Human ovarian adenocarcinoma SK-OV-3 cells were
obtained from the Korea Cell Line Bank (KCLB, Seoul, Korea). Cells
were grown at 37°C with 5% CO2 in RPMI-1640 medium with
10% (v/v) fetal bovine serum (FBS) and 1% (v/v)
penicillin-streptomycin. All cell culture media and reagents were
purchased from Thermo Scientific Hyclone (Waltham, MA, USA).
Cytotoxic assay
The cytotoxicity of PA was measured using an MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,
Sigma, St. Louis, MO, USA] colorimetric assay. Cells were seeded
onto 96-well plates at a density of 1x104 cells/well in
100 μl RPMI-1640 supplemented with 10% (v/v) FBS. Following 24 h
incubation at 37°, the cells were treated with serum-free
RPMI-1640, containing various concentrations of PA. Following a
further 24h incubation, 50 μl MTT [5 mg/ml in phosphate-buffered
saline, (PBS)] was added to each well. The cells were incubated at
37°C for 2 h. Following removal of the medium, the cells were
treated with 100 μl dimethyl sulfoxide (DMSO) for 5 min and then
the optical density was measured using a microplate reader
(Bio-Tek, Winooski, VT, USA) at 550 nm. Cell viability was
calculated as a percentage of viable cells in the PA-treated group
(5, 15, 25 and 50 μM) vs. a control group using the following
equation: Cell viability (%) = [(ODCompound -
ODBlank)/(ODContol - ODBlank)] ×
100.
Annexin V assay
Modulation of phosphatidylserine externalization
during apoptosis was identified using annexin V conjugated with the
fluorescent dye FITC. SK-OV-3 cells were seeded onto 6-well plates
at a density of 3x105 cells/well. Following treatment
with 25 μM PA for 6 h, the cells were stained with annexin V-FITC
conjugate and then imaged under x40 objective magnification using a
confocal laser scanning microscope (LSM 510 Meta, Carl Zeiss,
Oberkochen, Germany).
Cell cycle analysis
SK-OV-3 cells were seeded onto 6-well plates at a
density of 3x105 cells/well. Following dose-dependent
treatment with PA for various times up to 12 h, cells were
collected and washed twice with ice-cold PBS. Cell pellets were
fixed in 70% (v/v) cold ethanol overnight at −20°C. Fixed cells
were centrifuged, washed and resuspended in 100 μl PBS, then mixed
with 100 μl RNase A (1 mg/ml, Sigma) and incubated for 30 min at
37°. The cells were stained by adding 400 μl propidium iodide (PI;
50 μg/ml, Sigma). After filtering through a nylon mesh (40 μm), the
DNA content of stained cells was analyzed using the FACSVantage SE
and CellQuest program (BD Biosciences, San Jose, CA, USA).
Mitochondrial membrane potential
(ΔΨm) assay
SK-OV-3 cells treated with 25 μM PA for 3 or 6 h
were labeled for 30 min with 0.1 μM tetramethylrhodamine ethyl
ester (TMRE; Sigma), a cationic, lipophilic dye that accumulates in
the negatively charged mitochondrial matrix and collected by
trypsinization. The fluorescence intensity was monitored at 582 nm
(FL2 channel) by FACSVantage SE (BD Biosciences).
Real-time polymerase chain reaction (PCR)
analysis
Total RNA was isolated using an RNeasy Mini kit
(Qiagen, Hilden, Germany) according to the manufacturer’s protocol.
DNase I (Takara Bio Inc., Shiga, Japan)-treated total RNA was
transcribed into cDNA using an ImProm-II Reverse Transcription
System (Promega, Madison, WI, USA). Quantitative real-time PCR was
performed using the ABI prism 7000 sequence detection system
(Applied Biosystems, Foster City, CA, USA). The reaction was
carried out in a total volume of 20 μl containing 10 μl 2X SYBR
Premix EX Taq™ (Takara Bio Inc.), 1 μl each oligonucleotide primer
(10 μM) and 1 μl DNA. The sequences of the sense and antisense
primers for death receptor (DR) 5, DR4 and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were as follows: DR5 sense:
5′-TGC ACC ACG ACC AGA AAC AC-3′; DR5 antisense: 5′-ATC ACC GAC CTT
GAC CAT CC-3′; DR4 sense: 5′-AGG GTC TCA GAG GAG GAG GC-3′; DR4
antisense: 5′-GGA GTC AAA GGG CAC GAT GT-3′; GAPDH sense: 5′-AGG
AGG CAT TGC TGA TGA TC-3′; GAPDH antisense: 5′-AGT GAG GGT CTC TCT
CTT CC-3′. Following completion of PCR, the data were analyzed
using ABI prism 7000 sequence detection system software. The
expression of mRNA was normalized with GAPDH.
Western blot analysis
After seeding onto 6-well plates at a density of
3x105 cells/well, cells were treated with 25 μM PA for
various time periods up to 12 h, then lysed with RIPA buffer
(Thermo Fisher Scientific Inc., Rockford, IL, USA) supplemented
with a protease inhibitor cocktail (Roche, Basel, Switzerland).
Protein concentrations were determined using an RC/DC Bio-Rad assay
kit (Bio-Rad, Hercules, CA, USA) following the manufacturer’s
instructions. Protein samples were separated using electrophoresis
on 10–15% polyacrylamide-SDS gel. The electrophoresized proteins on
the gel were transferred onto a polyvinylidene fluoride (PVDF)
membrane (Pall Life Sciences, Port Washington, NY, USA), blocked
with 5% (w/v) skimmed milk (BD Biosciences), incubated with
anti-human cleaved caspase-3 and -8, anti-human caspase-9 (Cell
Signaling Technology Inc., Danvers, MA, USA), anti-human DR4 and
DR5 (Imgenex, San Diego, CA, USA), anti-human PARP and anti-human
α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then
probed with horseradish peroxidase-conjugated anti-rabbit or mouse
IgG antibody (GE Healthcare Life Sciences, Stockholm, Sweden). The
membrane was detected using an enhanced chemiluminescent western
blotting detection system (GE Healthcare Life Sciences).
Statistical analysis
All data are presented as mean ± standard deviation
(SD). Student’s t-test was used to compare different data groups.
P<0.05, P<0.01 and P<0.001 were considered to indicate
statistically significant differences.
Results
PA demonstrates dose-dependent cytotoxic
effects
PA was isolated from flowers of Osmanthus
fragrans var. aurantiacus Makino (Fig. 1A). Identification of the structure
was confirmed on the basis of several spectroscopic analyses,
including infrared (IR), 1H- and 13C-nuclear
magnetic resonance (NMR) and 2D-NMR (correlation spectroscopy,
COSY; heteronuclear single quantum coherence, HSQC and
heteronuclear multiple-bond correlation spectroscopy, HMBC) (data
not shown). To evaluate the cytotoxic effect of PA on SK-OV-3
cells, cells were treated with different concentrations (5, 15, 25
and 50 μM) of PA for 24 h and cell viabilities were measured using
an MTT assay. PA dose-dependently inhibited the viability of
SK-OV-3 cells (Fig. 1B). When 50 μM
PA was treated for 24 h, the viability of SK-OV-3 cells decreased
to 10.3% of that for untreated control cells. IC50 (50%
inhibitory concentration) value occurred between 15 and 25 μM.
PA induces apoptosis in SK-OV-3
cells
To determine whether the cytotoxic effect of PA was
caused by apoptosis, PA-treated SK-OV-3 cells were immunostained
with annexin V-FITC conjugate and monitored under a confocal
microscope (Fig. 2A). Annexin
V-FITC-stained cells were observed in PA-treated cells, but not in
an untreated control group. Cell cycle analysis was performed to
determine the sub-G1 apoptotic population of PA-treated SK-OV-3
cells. Cells were treated with different concentrations (5, 15, 25
and 50 μM) of PA for 6 or 12 h and their DNA contents were analyzed
using flow cytometry following propidium iodide (PI) staining. As
shown in Fig. 2B and C, the number
of cells in the sub-G1 population increased in a time- and
dose-dependent manner. Following 6 h incubation with 5, 15, 25 and
50 μM PA, the sub-G1 populations increased to 0.6, 1.8, 9.8 and
19.8%, respectively. The sub-G1 populations of cells treated with
5, 15, 25 and 50 μM PA for 12 h increased to 1.01, 7.41, 13.01 and
33.93%, respectively. Taken together, these results indicate that
PA induces apoptosis in SK-OV-3 cells.
 | Figure 2Annexin V and flow cytometric analysis
of PA-treated SK-OV-3 cells. (A) PA increased apoptotic cells
immunostained with annexin V-FITC. Cells were treated with 25 μM PA
for 6 h and stained with annexin V-FITC, then imaged under x40
objective magnification using a confocal microscope (bar=20 μm).
DIC represents the differential interference contrast. (B) PA
induced apoptosis in a dose and time-dependent manner. Cells were
treated with different concentrations (0, 5, 15, 25 and 50 μM) of
PA for 6 h or 12 h, then sub-G1 populations were measured using a
flow cytometer after staining with PI. The sub-G1 populations of
cells treated 25 μM of PA for 6 h or 12 h are represented as a
diagram. FL1-A indicates fluorescence 1 area for the measurement of
the PI fluorescence intensity (excitation, 488 nm; emission, 530
nm). (C) Three independent experiments of (B) were performed and
are represented as a bar diagram. PA, pomolic acid; SK-OV-3 cells,
human ovarian adenocarcinoma cells; PI, propidium iodide. |
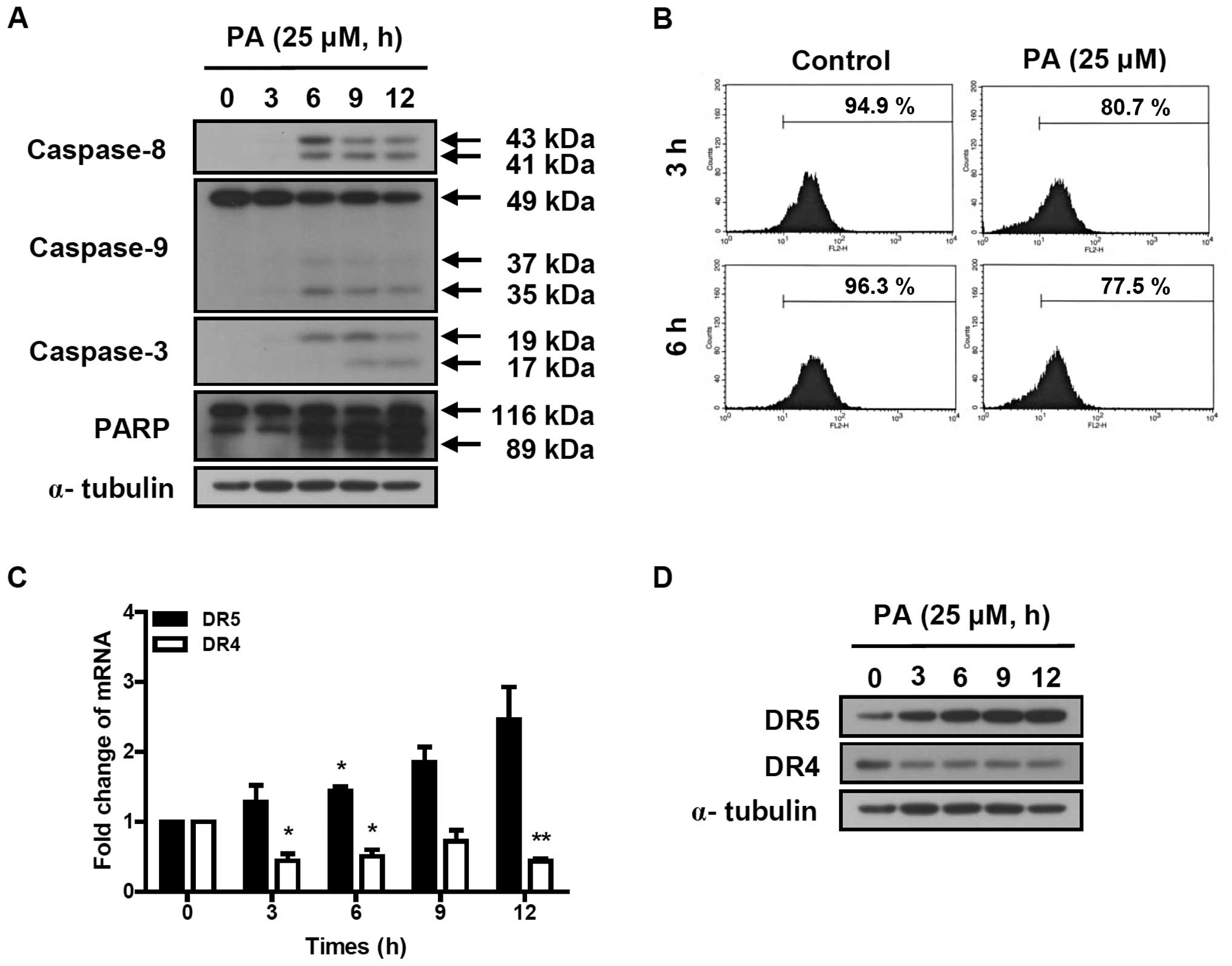
PA induces the activation of the caspase
cascade
The activation of the caspase cascade was determined
in SK-OV-3 cells treated with 25 μM PA for indicated times (3, 6, 9
and 12 h). Cleaved caspase-8 (41 and 43 kDa) and caspase-3 (17 and
19 kDa) were increased by the treatment of PA (Fig. 3A). PA induced the reduction of
procaspase-9 (49 kDa) and increased the cleaved caspase-9 (35 and
37 kDa). PA also induced the cleavage of poly ADP-ribose polymerase
(PARP). These results indicate that PA induces apoptosis in SK-OV-3
cells via the activation of the caspase cascade.
PA reduces the mitochondrial
transmembrane potential (ΔΨm) and increased the
expression of DR5 in SK-OV-3 cells
To determine the effect of PA on mitochondrial
transmembrane potential (ΔΨm), SK-OV-3 cells were
treated with 25 μM PA for 3 or 6 h and labeled with TMRE. The
fluorescence intensity was measured using flow cytometry. The
number of TMRE-stained cells showing fluorescence intensities up to
10 were 80.7 and 77.5% following 3 and 6 h incubation of 25 μM PA,
respectively (Fig. 3B). This means
that the treatment of 25 μM PA for 3 and 6 h reduced the
ΔΨm by 15 and 19.5%, respectively, compared to
PA-nontreated cells. This indicates that PA induces the loss of
mitochondrial transmembrane potential, resulting in the activation
of caspase-9.
To investigate the effect of PA on death
receptor-induced extrinsic apoptosis pathway, the expression levels
of DR4 and DR5 were analyzed using real-time PCR and western blot
analysis. As shown in Fig. 3C, DR5
transcript was time-dependently increased in PA-treated SK-OV-3
cells, resulting in 1.3, 1.7, 2.3 and 2.5-fold increases following
3, 6, 9 and 12 h incubation with 25 μM PA, respectively. The
protein levels of DR5, based on densitometry (Fig. 3D), increased 3.5, 5.1, 6 and
6.1-fold, respectively, following 3, 6, 9 and 12 h incubation with
25 μM PA. However, DR4 transcript and protein levels decreased in
PA-treated SK-OV-3 cells. This indicates that PA increases
expression of DR5, resulting in the activation of caspase-8, a
caspase cascade mediator of the death receptor-induced extrinsic
apoptosis pathway.
Discussion
PA inhibited the growth of leukemia HL-60 cells
(9) and showed anti-proliferative
activities in several human carcinoma cells including human gastric
adenocarcinoma (MK-1), human uterine carcinoma (HeLa) and murine
melanoma (B16F10) cells (12).
Although mitochondria-dependent apoptotic cell death was evaluated
in leukemia HL-60 cells, PA-induced apoptotic mechanisms of human
carcinoma cells were not investigated in detail. In a preliminary
experiment, we determined the cytotoxic effect of PA on the
viabilities of human carcinoma cells. The greatest inhibition of PA
was observed in SK-OV-3 cells (data not shown). We chose SK-OV-3
cells to investigate PA-induced apoptotic mechanisms of human
carcinoma cells. In this study, we examined the effect of PA on the
viability of human ovarian adenocarcinoma SK-OV-3 cells and
investigated the mechanisms involved in PA-induced apoptosis. PA
dose-dependently inhibited the viability of SK-OV-3 cells (Fig. 1B) and induced apoptosis, which was
characterized by detection of cell surface annexin V and sub-G1
apoptotic cell populations (Fig.
2).
The caspase-3 activation cascade played a central
role in apoptotic mechanisms (13)
as determined in PA-treated SK-OV-3 cells. Cleaved caspase-8, -9
and -3 levels increased following treatment with PA (Fig. 3A). Our findings indicate that PA
activates two major apoptotic pathways, intrinsic and extrinsic, as
is the case for other reported triterpenoids, to induce apoptosis
through mitochondria-mediated intrinsic and death receptor-induced
extrinsic pathways (14,15). PA has been reported to induce
apoptosis by activation of caspase-9 and -3 through the loss of
mitochondrial transmembrane potential (ΔΨm) in leukemia
cells (9). In SK-OV-3 cells, PA was
also found to reduce the mitochondrial transmembrane potential
(ΔΨm) (Fig. 3B). This
implies that PA induces apoptosis in SK-OV-3 cells via a
mitochondria-mediated intrinsic pathway.
In addition, PA induced activation of caspase-8, a
caspase cascade mediator of the death receptor-induced extrinsic
pathway. This indicates that PA induces apoptosis through the death
receptor-induced extrinsic pathway. Recently, triterpenoids such as
celastrol and ursolic acid have been suggested to potentiate tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced
apoptosis through upregulation of DR4 and/or DR5 (16,17).
In our previous study, 3-O-acetyloleanolic acid was also
shown to induce apoptosis in HCT-116 cells, which is mediated by
upregulation of DR5 (18). TRAIL is
a TNF family member showing anti-tumor activity in various cancer
cell types as well as minimal cytotoxicity to normal cells and
tissues (19). The binding between
TRAIL and the two death receptors DR4 (TRAILR1/APO-2) and DR5
(TRAILR2/KILLER) leads to oligomerization of the death receptors
and activation of the death receptor-induced extrinsic pathway
(20). DR5 and DR4 signaling
induces apoptosis via caspase-8-mediated activation of the caspase
cascade (21,22). PA increased the expression of DR5,
increasing induction of the caspase-8-mediated extrinsic pathway.
To the best of our knowledge, this is the first report showing that
PA induces apoptosis in SK-OV-3 cells as well as upregulation of
TRAIL signaling-related DR5 in PA-mediated apoptosis.
In conclusion, our results demonstrated for the
first time PA-induced apoptotic mechanisms in human ovarian
adenocarcinoma SK-OV-3 cells. Our findings suggest that PA
increases expression of TRAIL signaling-related death receptor DR5
and induces apoptosis in SK-OV-3 cells via the
mitochondria-mediated intrinsic and the death receptor-induced
extrinsic pathway.
Acknowledgements
This study was supported by grants
from the Basic Science Research Program through the National
Research Foundation of Korea (NRF) funded by the Ministry of
Education, Science and Technology (2012001575) and from Kyung Hee
University in 2010 (KHU-20110257).
References
|
1
|
Patocka J: Biologically active pentacyclic
triterpenes and their current medicine signification. J Appl
Biomed. 1:7–12. 2003.
|
|
2
|
Phillips DR, Rasbery JM, Bartel B and
Matsuda SP: Biosynthetic diversity in plant triterpene cyclization.
Curr Opin Plant Biol. 9:305–314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jager S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various plants
- rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009.PubMed/NCBI
|
|
4
|
Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS,
Son HC and Kim KW: Intracellular Ca2+ release mediates
ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J
Cancer. 73:725–728. 1997.
|
|
5
|
Yamaguchi Y, Yamada K, Yoshikawa N,
Nakamura K, Haginaka J and Kunitomo M: Corosolic acid prevents
oxidative stress, inflammation and hypertension in SHR/NDmcr-cp
rats, a model of metabolic syndrome. Life Sci. 79:2474–2479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duke JA and Ayensu ES: Medicinal Plants of
China. 2. Reference Publications Inc; Algonac, MI, USA: pp.
3811985
|
|
7
|
Hedrick UP: Sturtevant’s Edible Plants of
the World. Dover Publications; Mineola, NY, USA: pp. 6861972
|
|
8
|
Lee HH, Lin CT and Yang LL:
Neuroprotection and free radical scavenging effects of Osmanthus
fragrans. J Biomed Sci. 14:819–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandes J, Weinlich R, Castilho RO,
Kaplan MAC, Amarante-Mendes GP and Gattass CR: Pomolic acid
triggers mitochondria dependent apoptotic cell death in leukemia
cell line. Cancer Lett. 219:49–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandes J, Weinlich R, Castilho RO,
Amarante-Mendes P and Gattass CR: Pomolic acid may overcome
multidrug resistance mediated by overexpression of anti-apoptotic
Bcl-2 proteins. Cancer Lett. 245:315–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vasconcelos FC, Gattass RR, Rumjanek VM
and Maia RC: Pomolic acid-induced apoptosis in cells from patients
with chronic myeloid leukemia exhibiting different drug resistance
profile. Invest New Drugs. 25:525–533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshida M, Fuchigami M, Nagao TT, et al:
Antiproliferative constituents from umbelliferase plants VII.
Active triterpenes and rosmarinic acid from Centella
asiatica. Biol Pharm Bull. 28:173–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng TS, Schlosser SF, Dao T, Hingorani
R, Crispe IN, Boyer JL and Flavell RA: Caspase-3 controls both
cytoplasmic and nuclear events associated with Fas-mediated
apoptosis in vivo. Proc Natl Acad Sci USA. 95:13618–13623.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee B, Lee DY, Yoo KH, Baek NI, Park JH
and Chung IS: Calenduloside E 6’-methyl ester induces apoptosis in
CT-26 mouse colon carcinoma cells and inhibits tumor growth in a
CT-26 xenograft animal model. Oncol Lett. 4:22–28. 2012.
|
|
16
|
Sung B, Park B, Yadav VR and Aggarwal BB:
Celastrol, a triterpene, enhances TRAIL-induced apoptosis through
the down-regulation of cell survival proteins and up-regulation of
death receptors. J Biol Chem. 285:11498–11507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prasad S, Yadav VR, Kannappan R and
Aggarwal BB: Ursolic acid, a pentacyclin triterpene, potentiates
TRAIL-induced apoptosis through p53-independnet up-regulation of
death receptors. J Biol Chem. 286:5546–5557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo KH, Park JH, Cui EJ, et al:
3-O-Acetyloleanolic acid induces apoptosis in human colon carcinoma
HCT-116 cells. Phytother Res. View
Article : Google Scholar : 2012.PubMed/NCBI
|
|
19
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene
Therapy. 12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wajant H, Gerspach J and Pfizenmaier K:
Tumor therapeutics by design: targeting and activation of death
receptors. Cytokine Growth Factor Rev. 16:55–76. 2005. View Article : Google Scholar : PubMed/NCBI
|