Introduction
Sarcomatoid variant of urothelial carcinoma (SV-UC)
is a rare variant of UC accounting for approximately 0.3% of all
bladder malignancies (1). SV-UC is
characterized by the presence of biphasic malignant neoplastic
components exhibiting morphological and/or immunohistochemical
evidence of epithelial and mesenchymal differentiation (2). The malignant mesenchymal component of
SV-UC is usually composed of undifferentiated high-grade spindle
cell neoplasm often resembling ‘malignant fibrous histiocytoma’,
with the presence or absence of heterologous elements, including
osteosarcoma, chondrosarcoma, rhabdomyosarcoma and liposarcoma
(2). The sarcomatoid areas may
merge with foci of overlying urothelial carcinoma in situ or
conventional invasive UC.
The cytological features of SV-UC are not well known
and only one cytological analysis of SV-UC has been previously
reported (3). The present case
study includes the first analysis of cytological features from a
series of SV-UC cases and discusses possible differential
diagnostic considerations. This study was approved by the Ethics
Committee of Shiga University of Medical Science. Informed consent
was obtained from the patients.
Patients and methods
Case reports
Case 1
A 64-year-old Japanese male presented with gross
hematuria. Cystoscopy revealed multiple polypoid masses with
ulceration in the bladder. Biopsies from these polypoid masses and
subsequent total cystourethrectomy were performed. Following
surgery, chemotherapy was administered. No recurrence or metastases
were observed 6 months following surgery.
Case 2
An 80-year-old Japanese male presented with gross
hematuria. Cystoscopy revealed a pedunculated papillary tumor in
the bladder and tumor resection using a cystoscopy was performed.
Two months following the initial procedure, second-look cystoscopy
identified no residual tumor. No recurrence or metastases were
observed 8 months following the initial cystoscopy.
Case 3
A 75-year-old Japanese male presented with
persistent lower abdominal pain. Computed tomography demonstrated
multiple tumorous lesions in the liver and hydronephrosis of the
left kidney. Cystoscopy revealed an ulcerated polypoid tumor in the
bladder and tumor resection using a cystoscopy was performed. A
metastatic bladder tumor in the liver was clinically suspected and
chemotherapy was administered.
Cytological analysis
Urine specimens from patients diagnosed
histopathologically with SV-UC were retrieved. Six urine specimens
from three patients were available in this study (two, one and
three samples from case 1, 2 and 3, respectively). The specimens
were voided urine samples obtained prior to surgical procedure or
cystoscopy. Cytological specimens were Papanicolaou-stained and
analyzed for cytological features, including background, number of
neoplastic cells, cellular arrangement, cell size and shape,
cellular border and nuclear features.
Histological analysis
Tissues from cystoscopic or surgical resections were
fixed by formalin and embedded in paraffin. Tissue sections were
stained with hematoxylin and eosin and subjected to
immunohistochemistry using an autostainer (XT system Benchmark,
Ventana Medical System, Tucson, AZ, USA) according to the
manufacturer’s instructions.
Results
Cytological findings
Cytological features of the 3 cases are summarized
in Table I.
 | Table ICytological features of the
sarcomatoid variant of urothelial carcinoma. |
Table I
Cytological features of the
sarcomatoid variant of urothelial carcinoma.
| Case | Background | Number of neoplastic
cells | Cellular
arrangement | Cell size | Cell shape | Nuclear features | Spindle-shaped
atypical cells |
|---|
| 1 | Necrotic | Abundant | Single >> small
cluster | Large | Round to
polygonal | Large round to oval
with coarse chromatin and occasional prominent nucleoli. | Present |
| 2 | Necrotic | Few | Single | Large | Round to
polygonal | Large round to oval
with coarse chromatin and inconspicuous nucleoli | Absent |
| 3 | Necrotic | Abundant | Single > small
cluster | Large | Round to
polygonal | Large round to oval
with coarse chromatin and occasional prominent nucleoli | Absent |
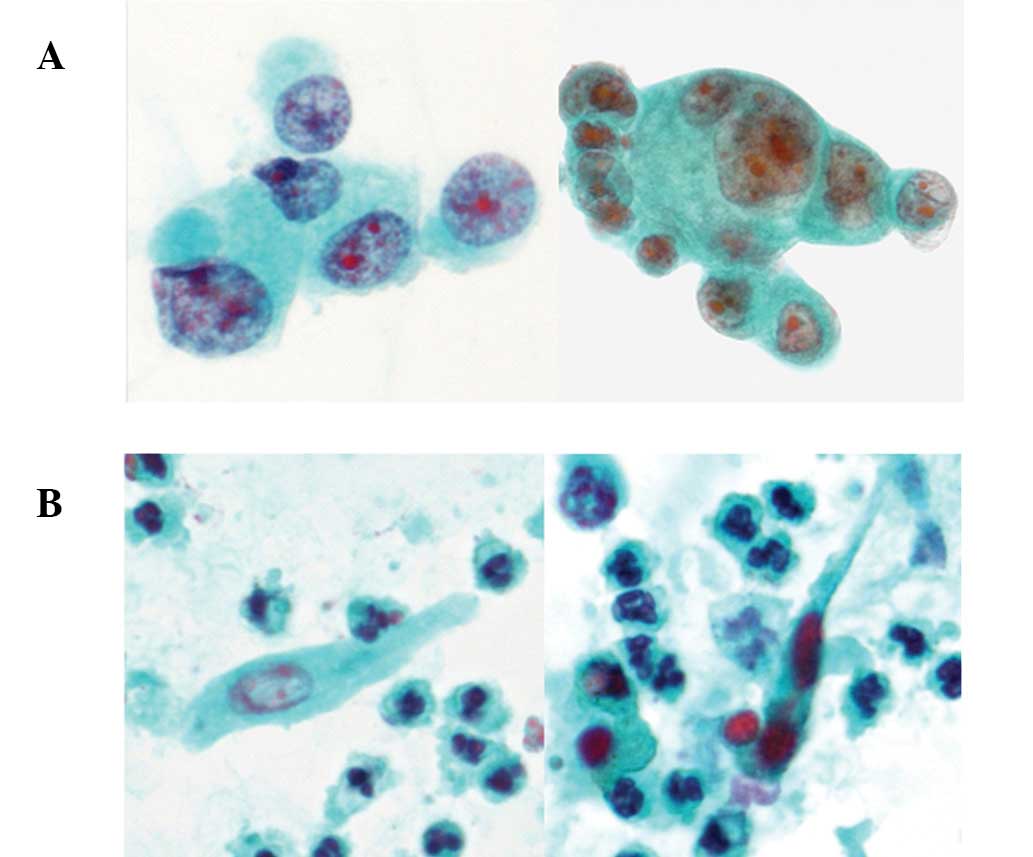
Case 1
Two cytological specimens revealed abundant single
tumor cells and a small number of tumor cell clusters in a necrotic
background. Tumor cells were large-sized and round to polygonal in
shape with ill-defined cell borders. The cells had a high
nuclear/cytoplasmic (N/C) ratio and enlarged round to oval nuclei
containing coarse chromatin and occasional prominent nucleoli
(Fig. 1A). In addition, a few
spindle-shaped atypical cells with enlarged oval nuclei containing
coarse chromatin and dense cytoplasm were also observed in one
specimen (Fig. 1B).
Case 2
One cytological specimen revealed a small number of
single tumor cells, which were large-sized and round to polygonal
in shape with ill-defined cell borders, in a necrotic background.
The cells had a high N/C ratio and enlarged round to oval nuclei
containing coarse chromatin and inconspicuous nucleoli. Tumor cell
clusters and atypical spindle cells were not observed.
Case 3
Three cytological specimens revealed abundant single
tumor cells and a limited number of small clusters of tumor cells
in a necrotic background. Cells were large-sized and round to
polygonal in shape with ill-defined cell borders. The cells had a
high N/C ratio and enlarged round to oval nuclei containing coarse
chromatin and occasional prominent nucleoli. No atypical spindle
cells were observed.
Histopathological findings
Clinicopathological and immunohistochemical features
of the 3 cases are summarized in Table
II.
 | Table IIClinicopathological and
immunohistochemical features of the sarcomatoid variant of
urothelial carcinoma. |
Table II
Clinicopathological and
immunohistochemical features of the sarcomatoid variant of
urothelial carcinoma.
| Case | Age/Gender | Chief complaint | Histopathological
features | Heterologous
component | Depth | Immunohistochemical
features
|
|---|
| Sarcomatoid
component | Conventional UC
component |
|---|
| 1 | 64/M | Gross hematuria | Sarcomatoid component
>> conventional high-grade UC | Present (RD) | pT2b | CK (−), VM (+) desmin
(+, RD) | CK(+), VM (−) |
| 2 | 80/M | Gross hematuria | Conventional
high-grade UC >>sarcomatoid component | Absent | ≥pT2 | CK (−), VM (+) | CK(+), VM (−) |
| 3 | 75/M | Abdominal pain | Sarcomatoid component
>> conventional high-grade UC | Absent | pT1 | CK (−), VM (+) | CK(+), VM (−) |
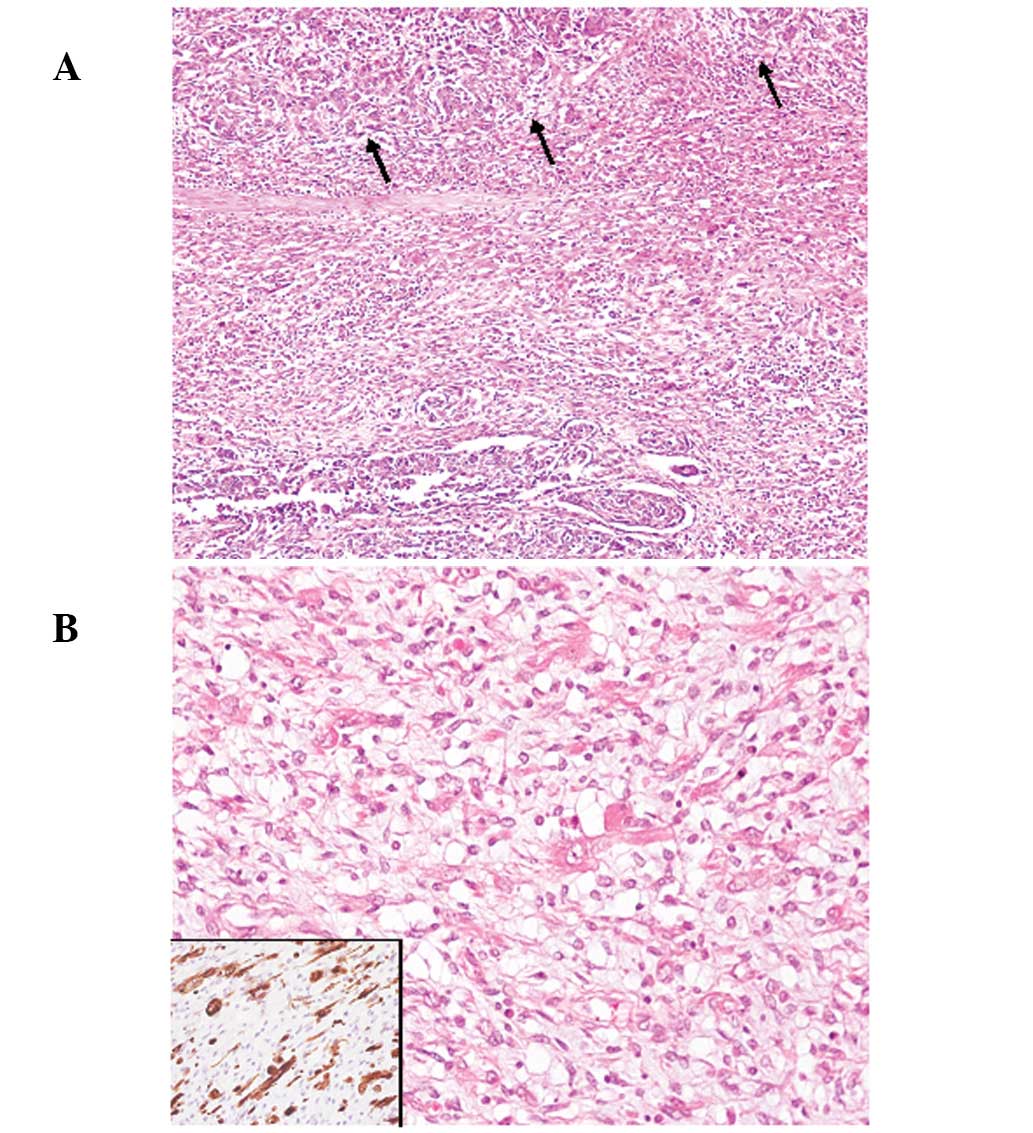
Case 1
Macroscopically, multiple polypoid lesions composed
of proliferating atypical spindle-shaped tumor cells (Fig. 2A), with ulceration were present in
the bladder. Tumor cells had hyperchromatic large nuclei with
nucleoli and specific spindle-shaped tumor cells were observed to
have rich eosinophilic cytoplasms and striation, which were
immunohistochemically positive for desmin, indicative of presence
of the rhabdomyosarcomatous component (Fig. 2B). The tumor was comprised of ∼70%
of the component and the residual area was conventional high-grade
UC, which was largely present on the surface of the tumor (Fig. 2A). The tumor had invaded into the
deeper portion of the muscular layer of the bladder (pT2b).
Case 2
The papillary tumor was largely composed of
conventional invasive high-grade UC. In addition, proliferating
spindle-shaped tumor cells with hyperchromatic large nuclei were
observed (∼20% of the tumor). No heterologous component was
identified. The tumor had invaded into the muscular layer
(>pT2).
Case 3
The polypoid tumor was composed of proliferating
atypical spindle-shaped cells containing large nuclei with nucleoli
(∼80% of the tumor). The conventional invasive high-grade UC
component was also identified, largely on the tumor surface. No
heterologous component was observed. The tumor had invaded into the
subepithelial connective tissue, however, muscular invasion was not
observed (pT1).
Discussion
Cytological examination of urine specimens is
important for the detection, diagnosis and follow-up of patients
with UC. It is well recognized that cytological examination of
urine specimens is highly sensitive for the detection of
conventional high-grade UC (4).
Moreover, the cytological features of rare histopathological
variants of UC, including micropapillary and nested, were
previously described (5–8). Although one cytological study of SV-UC
has been previously reported (3),
the cytological features of a series of SV-UC have yet to be
described.
The present study revealed four cytological features
of SV-UC: i) Tumor cells were abundant in a necrotic background and
single tumor cells were predominant, although small clusters of
tumor cells were occasionally present; ii) tumor cells were
large-sized and round to polygonal in shape with ill-defined cell
borders; iii) tumor cells had a high N/C ratio and enlarged round
to oval nuclei containing coarse chromatin and occasional nucleoli.
iv) Spindle-shaped atypical cells were rarely identified.
Cytological observations in a previous report on
SV-UC were consistent with features i), ii) and iii) from the
present study (3). However, these
cytological features are indistinguishable from those of
conventional invasive high-grade UC. We hypothesize that tumor
cells with features i), ii) and iii) may have originated from the
conventional high-grade UC component of SV-UC, which usually
contains conventional invasive UC and UC in situ components,
particularly on the tumor surface. These components were present in
all cases of this series, although it is unclear whether they were
present in the previous report (3).
The sarcomatoid component of SV-UC is generally present in the
deeper portion of the tumor, therefore, the detection frequency of
the sarcomatoid component in the voided cytological specimen may be
low, as demonstrated in the present case study, in which
spindle-shaped atypical cells representing the sarcomatoid
component were observed in only one specimen.
In the present series, 5/6 specimens (cases 1 and 3)
were initially diagnosed as malignant (UC) and the remaining
specimen (case 2) was suspected to be malignant (suspicious for UC)
due to a limited number of atypical cells. However, sarcomatoid
component was not reported in any of the cases. The cytodiagnosis
of SV-UC may be extremely difficult, however, cytodiagnosis of
malignancy may prove possible due to the presence of a conventional
UC component.
Cytological differential diagnosis of SV-UC includes
UC accompanied by a squamous cell carcinoma (SCC) component and
malignant mesenchymal tumors, including leiomyosarcoma and
rhabdomyosarcoma. UC is occasionally identified to include a SCC
component and pure SCC of the urinary bladder is rare. In voided
urine specimens, atypical parakeratotic cells with high N/C ratio
and enlarged hyperchromatic nuclei are observed in moderately to
poorly differentiated SCC and in well differentiated cases, the
presence of squamous cells demonstrating definite malignant
features may be rare, although anucleated squamous and atypical
parakeratotic cells have been previously observed (9). Spindle-shaped atypical cells are
present in the urine specimen of UC with SCC and pure SCC cases, as
well as SV-UC (9,10). However, parakeratotic atypical
squamous cells are present in UC with SCC and pure SCC cases
(10), but not SV-UC, although
SV-UC and UC with SCC contains a conventional high-grade UC
component in urine specimens. These observations may facilitate
clinical differentiation of SV-UC from UC with SCC. Leiomyosarcoma
and rhabdomyosarcoma must also be included in differential
diagnosis of SV-UC as these tumors reveal spindle-shaped tumor
cells as well (11,12). However, due to the lack of a
conventional high-grade UC component in leiomyosarcoma and
rhabdomyosarcoma, differentiation from these tumors is simple and
rapid.
References
|
1
|
Torenbeek R, Blomjous CE, de Bruin PC,
Newling DW and Meijer CJ: Sarcomatoid carcinoma of the urinary
bladder. Clinicopathologic analysis of 18 cases with
immunohistochemical and electron microscopic findings. Am J Surg
Pathol. 18:241–249. 1994. View Article : Google Scholar
|
|
2
|
Lopez-Beltran A, Sauter S, Gasser T, et
al: Infiltrating urothelial carcinoma. World Health Organization
Classification of Tumours. Pathology and Genetics of Tumours of the
Urinary System and Male Genital Organs. Eble JN, Sauter G, Epstein
JI and Sesterhenn IA: IARC Press; Lyon: pp. 93–109. 2004
|
|
3
|
Iwa N, Ito S, Takegaki Y, et al: Cytologic
features of sarcomatoid carcinoma of the urinary bladder: a case
report. Diagn Cytopathol. Sep 26–2011.(Epub ahead of print).
|
|
4
|
Brown FM: Urine cytology. It is still the
gold standard for screening? Urol Clin North Am. 27:25–37.
2000.PubMed/NCBI
|
|
5
|
Zhu B, Rohan SM and Lin X: Urine
cytomorphology of micropapillary urothelial carcinoma. Diagn
Cytopathol. May 24–2012.(Epub ahead of print).
|
|
6
|
Nicolas MM, Jagirdar JS, Arisco AM and
Valente PT: Micropapillary carcinoma of the urinary bladder: report
of a case and review of its cytologic features. Diagn Cytopathol.
39:784–787. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakuma T, Furuta M, Mimura A, Tanigawa N,
Takamizu R and Kawano K: Urine cytology of micropapillary carcinoma
of the urinary bladder. Diagn Cytopathol. 39:852–856. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cardillo M, Reuter VE and Lin O: Cytologic
features of the nested variant of urothelial carcinoma. A study of
seven cases. Cancer Cytopathol. 99:23–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raab SS: Urine cytology. Diagnostic
Cytopathology. Gray W and Kocjan G: 3rd edition. Churchill
Livingstone; Philadelphia, PA: pp. 398–401. 2010
|
|
10
|
Owens CL and Ali SZ: Atypical squamous
cells in exfoliative urinary cytology: clinicopathologic
correlates. Diagn Cytopathol. 33:394–398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hemachandran M, Nada R and Rajwanshi A:
Leiomyosarcoma of the urinary bladder: a diagnostic challenge in
urine cytology. Diagn Cytopathol. 31:281–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mincione GP and Grechi G: Urinary cytology
of rhabdomyosarcoma in children. Report of two cases located in the
urinary bladder and in the prostate. Pathologica. 75:797–801.
1983.PubMed/NCBI
|