Introduction
Metastases of malignancies to the parotid region are
relatively infrequent (21–42% of all malignant tumors) and
originate primarily from head and neck squamous cell carcinoma and
melanoma of the skin (1).
Metastases of an infraclavicular origin are rare (0.16–4%)
(1,2).
Renal cell carcinoma (RCC) is known for its high
propensity for early metastasis and ≤1/3 of patients diagnosed with
RCC present with metastatic disease at the time of diagnosis
(3). Although RCC may metastasize
to any organ system, its metastasis to the maxillofacial area is a
relatively rare phenomenon. This study describes a patient whose
first clinical presentation of RCC was parotid region metastasis.
To the best of our knowledge, this case is the first documented
example of such large metastasis of RCC.
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Medical College of Xi’an Jiaotong
University, Shaan’xi, Xi’an, China, and written informed consent
was obtained from the patient.
Case report
A 44-year-old female visited our hospital due to the
presence of a painless mass in her parotid region. The patient
denied all genitourinary symptoms and was otherwise well.
Laboratory studies revealed normal hemogram, urinalysis and blood
ureanitrogen results. The results of a complete ear, nose and
throat examination were also normal. The head and neck computed
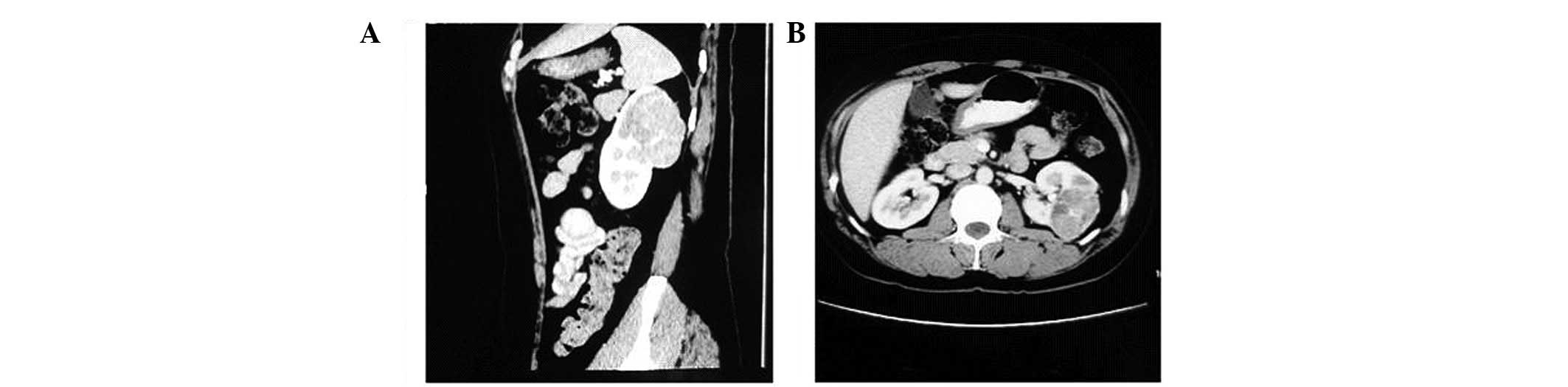
tomography (CT) scan revealed a 4×2.5-cm mass in the right parotid
region accompanied by an osteolytic destruction of the right
mandibular branch (Fig. 1A). A
roentgenogram of the chest was observed to be normal. Further
examination by an abdominal CT scan revealed a 6.5-cm mass in the
left kidney, suggesting RCC (Fig.
2), and a mass in the right iliac area accompanied by a
slightly osteolytic destruction of the right iliac bone (Fig. 3A). A radical nephrectomy was
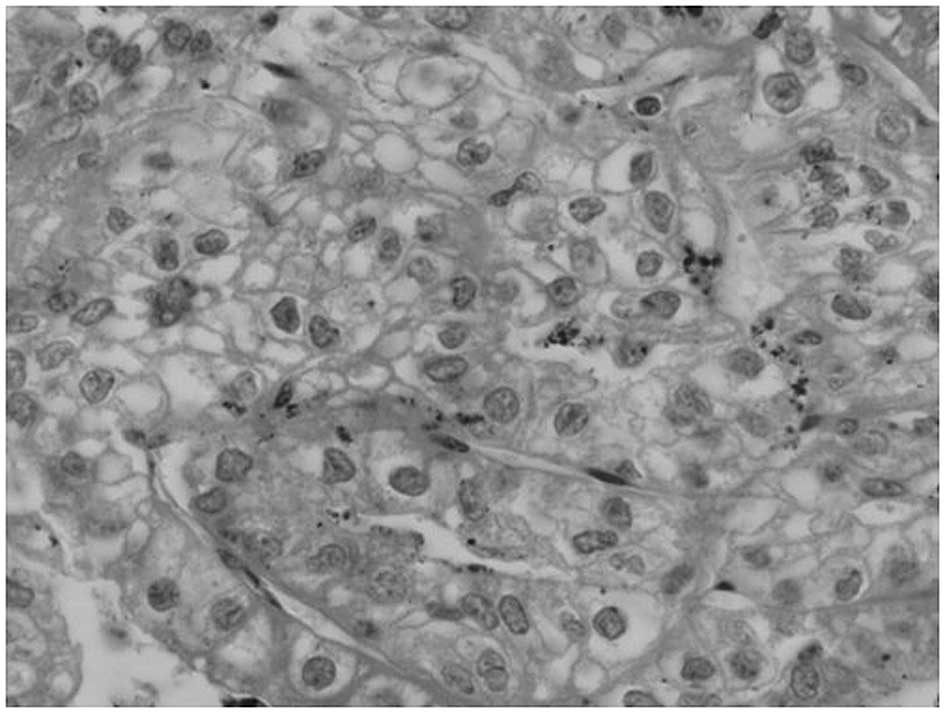
performed. The histological analysis revealed a renal clear cell
carcinoma (Fig. 4) and confirmed
the renal tumor to be the primary neoplasm. The patient was then
discharged from the hospital, as she did not accept any further
treatment.
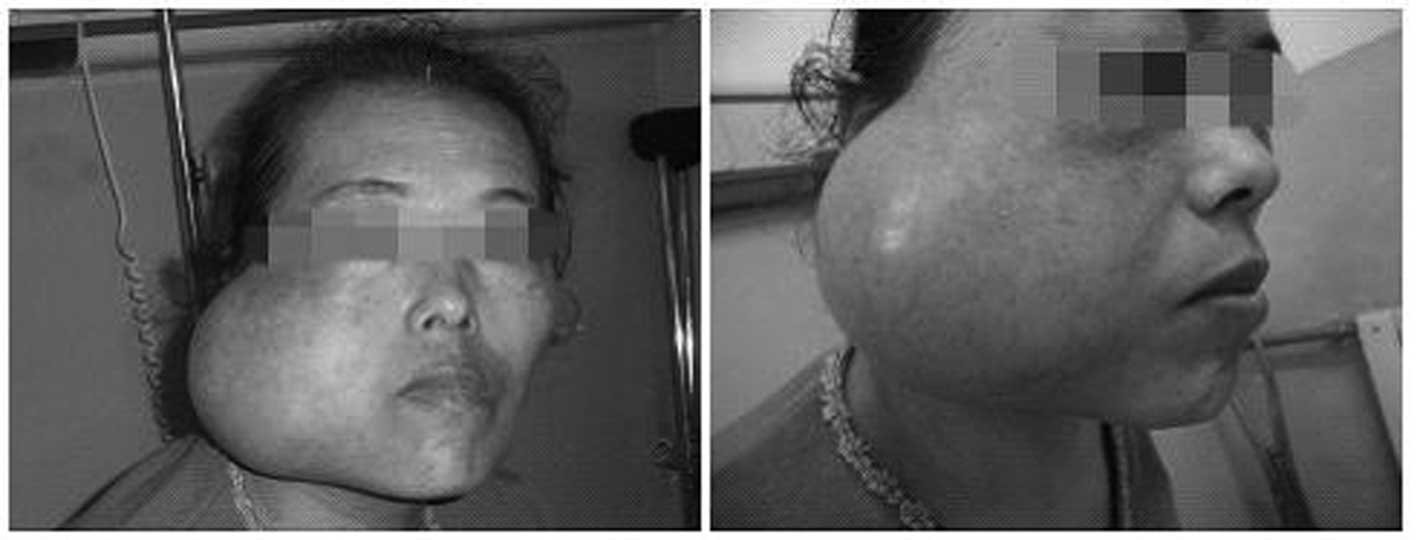
After seven months, the patient presented with the
mass in the right parotid region that had enlarged to a ball-like
size and was causing a difficulty in opening the mouth. On physical
examination, positive findings consisted of a firm mass measuring
∼12×13 cm in the parotid region (Fig.
5). The mass demonstrated no tenderness but had local heat. The
CT scan revealed that the masses in the right parotid region and
the right iliac area had enlarged to 12.5 and 13.5 cm,
respectively, and were accompanied by serious osteolytic
destruction of the right mandibular branch and the right iliac
bone, respectively (Figs. 1B and
3B). Furthermore, liver metastasis,
multiple bone metastases and bilateral multiple metastatic lesions
in the lungs were identified. The patient was administered
interleukin (IL)-2 and bisphosphonates, and received local
radiotherapy to the right parotid region and pelvic cavity.
However, after three weeks, oral mucositis occurred. Due to
unbearable pain, the patient did not accept further radiotherapy.
Therefore, targeted therapy with sunitinib was recommended;
however, the patient refused this treatment due to its high cost.
The patient was treated with Chinese medicine and supportive care.
Further follow-up continues.
Discussion
In adults, RCC constitutes 2–3% of all malignancies,
accounting for ∼90% of all primary renal tumors, with an incidence
peak in the sixth decade of life (4,5). RCC
are hypervascular tumors with a high expression of vascular
endothelial growth factor (VEGF), the VEGF receptor (VEGFR), the
platelet-derived growth factor (PDGF) receptor and basic fibroblast
growth factor (bFGF) (6). Cancer
cells are also known to have a good adaptive potential in a diverse
array of microenvironments, giving rise to the high metastatic
potential of RCC (7). Additionally,
as the kidney receives ∼25% of the circulating blood volume per
minute, the main mechanism of systemic metastases of RCC is
hematogenous metastasis. The most common sites for RCC metastasis
are the lungs (45.2%) and bone (29.5%), followed by the lymph nodes
(21.8%) and the liver (20.3%) (8).
Metastasis to the head and neck region are less common, comprising
14–16% of all cases (9). The
current patient who initially presented with metastasis to the
parotid region, which then developed into systemic multiple
metastases, was a rare case.
Throughout the past decade, improvements in the
understanding of the molecular pathways implicated in the
pathogenesis of RCC has led to a marked expansion in the treatment
options available to patients with metastatic RCC (mRCC).
Previously, systemic treatment was limited to cytokine therapy with
IL-2 or interferon (IFN), as mRCC is largely resistant to
chemotherapy (10). However, in
patients with mRCC, cytokine therapy is correlated with low
response and high toxicity rates (10). In recent years, identification of
VEGF, VEGFR and the mammalian target of rapamycin (mTOR) as
dysregulated signaling pathways in the development and progression
of RCC has led to the rapid development of novel molecular targeted
therapies. Thus far, six targeted therapies, including sorafenib,
sunitinib, bevacizumab (in combination with IFN-γ), temsirolimus,
everolimus and pazopanib, have been evaluated in randomized,
controlled phase III clinical trials of patients with mRCC and
approved by the US Food and Drug Administration (FDA) for the
management of mRCC (11). Each of
these new agents has demonstrated a significant clinical benefit
and fewer detrimental side-effects, leading to a better quality of
life for patients (11–17). New targeted agents with novel
mechanisms of action are also being studied, including histone
deacetylase inhibitors, angiopoietin/tyrosine protein kinase
receptor (TIE-2) inhibitors and carbonic anhydrase IX inhibitors
(11).
Recent developments have raised the question of
whether patients benefit most from combinatorial or sequential
therapy of targeted agents. At present, sequential therapy with
targeted agents is the standard of care (18). Combinatorial therapy strategies have
not yet been demonstrated to be beneficial, with a number of
combinations exhibiting excessive toxicity with marginal or
inferior efficacy compared with that observed with the sequential
use of agents (19). Ongoing
investigations concerning the biomarkers that predict severe
adverse events and the response of an individual patient to
different targeted therapies will lead to a more personalized
approach to treating mRCC (20,21).
Additionally, novel immunological therapies are currently being
developed to treat mRCC, including those that block cytotoxic
T-lymphocyte antigen 4 (CTLA4) (11). Despite the recent progress, mRCC
remains a disease with no curative therapy and further research is
required.
References
|
1
|
Vara A, Madrigal B, Pérez del Río MJ, et
al: Parotid metastasis from renal clear cell adenocarcinoma. An
unusual site for metastasis. Urol Int. 61:196–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pisani P, Angeli G, Krangli M and Pia F:
Renal carcinoma metastasis to the parotid gland. J Laryngol Otol.
104:352–354. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leibovich BC, Pantuck AJ, Bui MH, et al:
Current staging of renal cell carcinoma. Urol Clin North Am.
30:481–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karumanchi SA, Merchan J and Sukhatme VP:
Renal cancer: molecular mechanisms and newer therapeutic options.
Curr Opin Nephrol Hypertens. 11:37–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suh JH, Oak T, Ro JY, et al:
Clinicopathologic features of renal cell carcinoma in young adults:
a comparison study with renalcell carcinoma in older patients. Int
J Clin Exp Pathol. 2:489–493. 2009.PubMed/NCBI
|
|
6
|
Mena AC, Pulido EG and Guillén-Ponce C:
Understanding the molecular-based mechanism of action of the
tyrosine kinase inhibitor: Sunitinib. Anticancer Drugs. 21(Suppl
1): S3–S11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miah MS, White SJ, Oommen G, et al: Late
simultaneous metastasis of renal cell carcinoma to the
submandibular and thyroid gland seven years after radical
nephrectomy. Int J Otolaryngol. 2010:6980142010.PubMed/NCBI
|
|
8
|
Bianchi M, Sun M, Jeldres C, et al:
Distribution of metastatic sites in renal cell carcinoma: a
population-based analysis. Ann Oncol Ann Oncol. 23:973–980. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkis P, Bou-Malhab F and Mouaccadieh L:
Solitary laryngeal metastasis from clear cell carcinoma of the
kidney: Clinical case and review of the literature. Prog Urol.
22:307–309. 2012.(In French).
|
|
10
|
Oudard S, George D, Medioni J and Motzer
R: Treatment options in renal cell carcinoma: past, present and
future. Ann Oncol. 18(Supl 10): x25–x31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matrana MR, Atkinson B, Jonasch E and
Tannir NM: Emerging targeted therapies in metastatic renal cell
carcinoma. Curr Clin Pharmacol. 6:189–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients withmetastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Escudier B, Bellmunt J, Négrier S, et al:
Final results of the phase III, randomized, double-blind AVOREN
trial of first-line bevacizumab (BEV) plus interferon-alpha 2a
(IFN) in metastatic renal cell carcinoma (mRCC). J Clin Oncol
(Meeting Abstracts). 27(Suppl): 15S2009.
|
|
14
|
Sternberg CN, Davis ID, Mardiak J, et al:
Pazopanib in locally advanced or metastatic renal cell carcinoma:
results of a randomized phase III trial. J Clin Oncol.
28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hudes G, Carducci M, Tomczak P, et al:
Global ARCC Trial: Temsirolimus, interferon alfa, or both for
advanced renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007.
View Article : Google Scholar
|
|
16
|
Escudier B: Sorafenib for the management
of advanced renal cell carcinoma. Expert Rev Anticancer Ther.
11:825–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Motzer RJ, Escudier B, Oudard S, et al
RECORD 1 Study Group: Phase 3 trial of everolimus for metastatic
renal cell carcinoma: Final results and analysis of prognostic
factors. Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guide-lines in Oncology™. Kidney Cancer.
V.2.2010. Available at http://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf,
accessed March 26, 2010.
|
|
19
|
Hutson TE: Targeted therapies for the
treatment of metastatic renal cell carcinoma: clinical evidence.
Oncologist. 16(Suppl 2): 14–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizuno R and Oya M: Biomarkers of response
to molecular targeted therapy in renal cell carcinoma. Gan To
Kagaku Ryoho. 38:1088–1091. 2011.(In Japanese).
|
|
21
|
Eisengart LJ, MacVicar GR and Yang XJ:
Predictors of response to targeted therapy in renal cell carcinoma.
Arch Pathol Lab Med. 136:490–495. 2012. View Article : Google Scholar : PubMed/NCBI
|