Introduction
Esophageal cancer is the sixth leading cause of
cancer-related mortality worldwide, but it is also the least
studied type of tumor. There is an exceedingly high incidence of
esophageal squamous cell carcinoma (ESCC) in Asian countries,
particularly in north and central China.
Although 90% of cancer mortalities are caused by
metastasis, the mechanisms of metastasis remain poorly defined.
Consequently, a better understanding of metastasis offers promise
for the development of improved cancer therapies (1–3).
Deterioration of cell-cell and cell-extracellular matrix (ECM)
adhesions is often observed in tumor cells, and this may be
associated with the invasion and metastasis of cells into
surrounding tissues and blood vessels. Epithelial cadherin
(E-cadherin) is thought to mediate cell-cell adhesion, and this
protein plays a critical role in cancer invasion and metastasis.
E-cadherin complexes with other submembraneous cytosolic proteins,
including E-catenin and β-catenin, and these catenins mediate the
connection of E-cadherin to actin filaments. Altered expression of
the E-cadherin/β-catenin complex is associated with
de-differentiation, invasion and metastasis of tumors (4).
Serum response factor (SRF) is a member of the
highly conserved MADS (MCM1, Agamous, Deficiens, SRF) box family of
transcription factors which regulates the expression of immediate
early genes, such as c-fos, muscle-specific genes, and genes
involved in cytoskeleton regulation, motility and adhesion
(5). A number of researchers have
reported that SRF is highly expressed in tumors, including
colorectal cancer (6),
hepatocelluar carcinoma (7,8), breast cancer (5) and thyroid carcinoma (9). Overexpression of SRF in hepatocellular
carcinoma and breast cancer accelerates cell migration and
invasion, and there is a subsequent acquisition of mesenchymal
phenotypes due to the expression of a mesenchymal marker (vimentin)
(5,10). Furthermore, overexpression of SRF in
colorectal cancer has been reported to decrease E-cadherin
expression and increase nuclear β-catenin expression (6).
Expression of SRF in ESCC and its role in the
modulation of the E-cadherin/β-catenin complex have not been
investigated. In this study, we examined the expression of SRF in
ESCC. We also examined the effect of the downregulation of SRF by
RNA interference (RNAi) on the proliferation and invasion of
Eca-109 cells via altered expression of SRF, E-cadherin and
β-catenin.
Materials and methods
Tissue sample collection
We retrospectively studied ESCC specimens from
surgical resections taken between 2009 and 2011 at Tangshan
People’s Hospital, China. These patients (n=73) did not receive any
preoperative adjuvant radiation or chemotherapy. All research
involving human participants was approved in writing by the
patients studied and the ethics committee at Hebei Medical
University.
Immunohistochemistry for SRF
Paraffin-embedded sections were permeabilized with
0.2% Triton and blocked with 5% bovine serum albumin (BSA) in 0.1 M
phosphate-buffered saline (PBS) for 30 min to reduce nonspecific
binding, followed by incubation with primary antibodies against SRF
(sc-335; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
biotinylated secondary antibody and ABC reagent (Boshide Bio, Inc.,
Wuhan, China). Immunoreactivity was visualized with DAB. Staining
was scored independently by two observers, and a high level of
concordance (90%) was achieved. When the observers disagreed, the
slides were reviewed to arrive at a consensus.
Clear nuclear SRF staining in tumor cells was
defined as SRF-positive (6). For
assessment of SRF proteins, two scores were assigned to each core:
i) staining intensity was scored as 0 (absent), 1 (weak), 2
(moderate) or 3 (strong); and ii) the percentage of positively
stained epithelial cells was scored as 0 (<10% positive), 1
(10–30%), 2 (31–70%) or 3 (>70%). An overall protein expression
score was calculated by multiplying the intensity and positivity
scores (overall score range, 0–9), and further simplified by
dichotomization to negative (≤3) or positive (≥4).
SRF silencing
Cells were transfected with small interfering RNA
(siRNA) against SRF using the Lipofectamine 2000 transfection
reagent according to the manufacturer’s instructions (Shanghai
GenePharma Co., Ltd, China). siRNA with the following sequences
were obtained from GenePharma: i) siRNA-SRF-1107: sense:
5′-GCAAGGCACUGAUUCAGA CTT-3′ and antisense: 5′-GUCUGAAUCAGUGCCUUG
CTT-3′ (in our preliminary experiment, we found that siRNA-SRF 1107
had a higher effect than others measured by real-time PCR and
western blot analysis); ii) Negative-siRNA: sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. Briefly, 1×105 human ESCC
Eca-109 cells (Cell Resource Center, Shanghai Life Sciences
Institute, Chinese Academy of Sciences) per well were plated in
6-well plates and cultured to reach 80% confluence. Cells were then
transfected with siRNA using transfection reagent in serum-free
medium.
Cell lines and proliferation assay
Eca-109 cells were maintained in a 5% CO2
atmosphere at 37°C in Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal bovine serum (FBS). The cells were divided
into four groups: i) control: serum-free; ii) siRNA-SRF:
siRNA-SRF+RNAi-mate; iii) negative control: siRNA-negative
control+RNAi-mate; and iv) mock transfection: RNAi-mate. All
experiments were performed in triplicate.
The Eca-109 cells were seeded in parallel into
96-well tissue culture plates at a density of 5×103
cells per well in full growth medium (DMEM plus 10% FBS). Cells
were incubated overnight, then quiesced in serum-free medium for 12
h before treatment with siRNA-SRF. After treatment for 48 h, the
medium was removed and the cells were incubated with a 10% cell
counting kit (CCK)-8 (Dojindo, Kumamoto, Japan) for 4 h at 37°C.
Cells were counted with a microELISA plate reader at 450 nm.
Cell cycle analysis
Cells were trypsinized, washed once with ice-cold
PBS and fixed with 70% ethanol at −20°C overnight. After washing
twice with PBS, cells were stained with 10 μg/ml propidium
iodide (Sigma, St. Louis, MO, USA) containing 1 mg/ml RNase A
(Sigma) at 37°C for 20 min in the dark and analyzed with a
FACSCalibur flow cytometer and CellQuest software.
Cell invasion assays
Cell invasion assays were performed using a 24-well
Transwell migration chamber (Corning Life Sciences, Acton, MA,
USA). The upper and lower chambers were separated by a
polyvinyl-pyrrolidone-free polycarbonate membrane with an
8-μm pore size. Cells (4×104 per well) were
suspended in serum-free medium and placed in the upper chamber.
Medium containing 2% FBS was used as the chemoattractant source.
Twelve hours later, cells on the upper surface of the filter were
wiped with a cotton swab. Cells on the lower surface of the filters
were fixed and stained with Giemsa. Cells that migrated to the
lower surface of the filter were counted under a light microscope
at ×200 magnification in ten randomly selected fields per well.
Western blot analysis
The total protein content of cells and lung tissue
lysed by RIPA (ZO2338A, Aidlab; Beijing, China) was quantified with
the BCA assay (PC0020, Solarbio; Beijing, China). Proteins (70
μg/lane) were separated in 10% gel by SDS-PAGE and
electrotransferred to a nitrocellulose membrane (Solarbio).
Membranes were blocked with 5% non-fat milk and incubated overnight
at 4°C with the primary antibody [anti-SRF; anti-E-cadherin
(sc-7870); anti-β-catenin (sc-7870); anti-cyclin D1 (sc-8376);
anti-β-actin (sc-47778); Santa Cruz Biotechnology] followed by
alkaline phosphatase-conjugated secondary antibodies (E030220,
E020210; Earthox, San Francisco, CA, USA). Target bands were
visualized by the addition of BCIP/NET (E116; Amresco, Solon, OH,
USA). Results were normalized with β-actin and expressed as the
fold change from specific bands in the control group.
Quantitative real time polymerase chain
reaction (PCR) for SRF
The following oligonucleotide primers specific for
human genes were used in this study: SRF, sense
5′-CTTAACATGGCATCTTCGACACT-3′ and antisense
5′-CTTAACCTCTAATCCCCATTGCT-3′; GAPDH, sense
5′-GGGAAACTGTGGCGTGAT-3′ and antisense 5′-TGGGTGTCGCTGTTGAAGT-3′.
Total RNA was extracted from cells using TRIzol reagent (15596-026,
Invitrogen Life Technologies, Carlsbad, CA, USA), and cDNA was
generated from 1 μg RNA using a random hexamer and the
Omniscript RT kit (c28025-032, Invitrogen). Real-time PCR was
performed as described in the PCR core kit of SBYR-Green
(c11733-038, Invitrogen). The data were analyzed using the ΔΔCt
method and presented as arbitrary units.
Statistical analysis
Values are expressed as mean ± SEM. Comparisons
between multiple independent groups were conducted using one-way
ANOVA followed by post-hoc analysis with the Brown-Forsythe test
and SPSS 13.0. P<0.05 was considered to indicate a statistically
significant difference.
Results
SRF, E-cadherin and β-catenin protein
expression in ESCC and lymph node metastatic foci and their
clinical characteristics
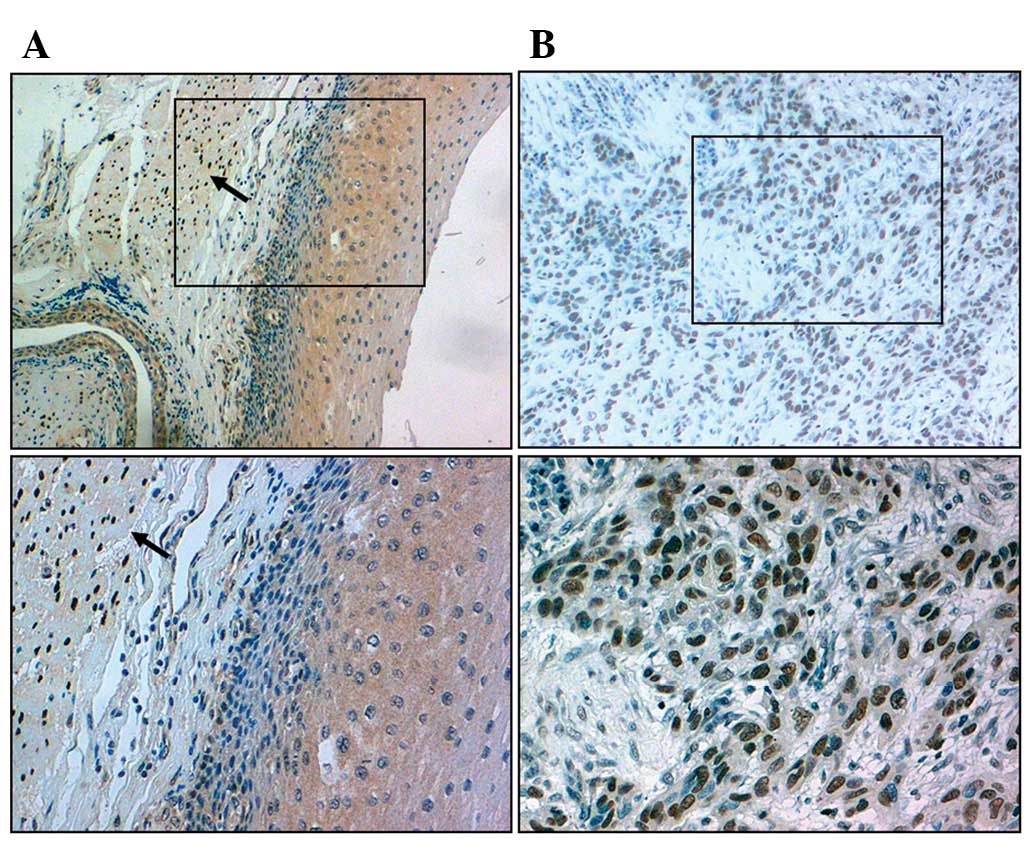
SRF protein positive detection rates in ESCC tissues
(47.95%; 42/73) were higher than those of normal controls (20.00%;
6/30; χ2=12.037, P<0.05; Fig. 1). We also evaluated possible
correlations between the expression of SRF and α-smooth muscle
actin (α-SMA) in tumor cells with the clinicopathological
characteristics of ESCC, including gender, age, tumor diameter,
histological grade, lymph node metastasis and depth of invasion.
SRF expression in the tumor cells was associated with poor
differentiation, deep invasion and lymph node metastasis (Table I, P<0.05).
 | Table ISerum response factor (SRF) expression
in relation to clinicopathological features in esophageal squamous
cell carcinoma. |
Table I
Serum response factor (SRF) expression
in relation to clinicopathological features in esophageal squamous
cell carcinoma.
| Clinicopathological
features | | SRF
| | | α-SMA
| | |
|---|
| n | + | − | % | χ2 | P-value | + | − | % | χ2 | P-value |
|---|
| Gender | | | | | | | | | | | |
| Male | 55 | 31 | 24 | 56.36 | 0.125 | 0.724 | 25 | 30 | 54.55 | 0.554 | 0.457 |
| Female | 18 | 11 | 7 | 61.11 | | | 10 | 8 | 55.56 | | |
| Age (years) | | | | | | | | | | | |
| ≥60 | 47 | 28 | 19 | 59.57 | 0.225 | 0.635 | 26 | 21 | 55.32 | 2.875 | 0.090 |
| <60 | 26 | 14 | 12 | 53.85 | | | 9 | 17 | 34.62 | | |
| Diameter | | | | | | | | | | | |
| ≥5 cm | 40 | 25 | 15 | 62.50 | 0.893 | 0.345 | 22 | 18 | 55.00 | 1.765 | 0.184 |
| <5 cm | 33 | 17 | 16 | 51.51 | | | 13 | 20 | 39.39 | | |
| Differentiation | | | | | | | | | | | |
| High+moderate | 22 | 8 | 14 | 36.36 | 5.777 | 0.016 | 12 | 10 | 54.55 | 0.550 | 0.485 |
| Low | 51 | 34 | 17 | 66.67 | | | 23 | 28 | 45.10 | | |
| Depth | | | | | | | | | | | |
| ≤ Muscular
layer | 25 | 7 | 18 | 28.00 | 13.574 | 0.000 | 7 | 18 | 28.00 | 6.060 | 0.014 |
| ≥ Adventitia | 48 | 35 | 13 | 72.92 | | | 28 | 20 | 58.33 | | |
| Lymphatic
metastasis | | | | | | | | | | | |
| Positive | 42 | 30 | 12 | 71.43 | 7.815 | 0.005 | 25 | 17 | 59.52 | 5.313 | 0.021 |
| Negative | 31 | 12 | 19 | 38.71 | | | 10 | 21 | 32.26 | | |
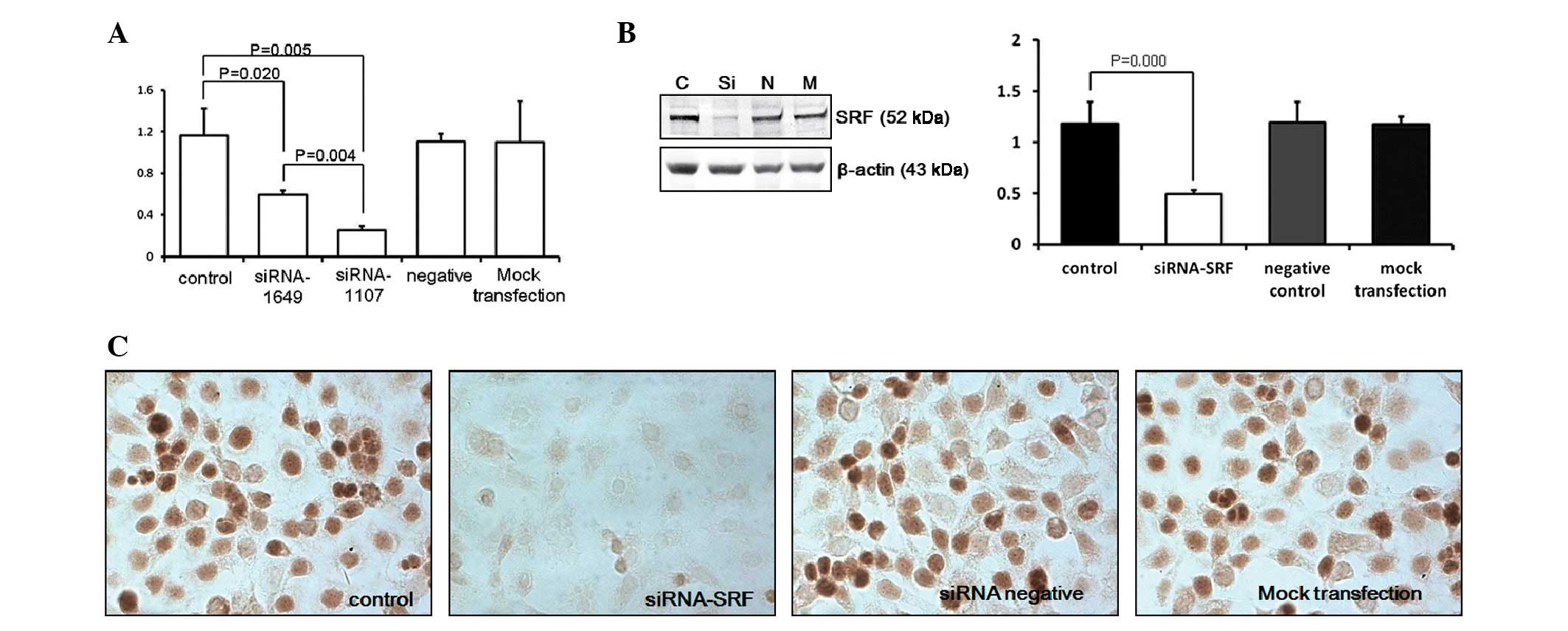
siRNA-SRF-1107 reduces SRF mRNA and
protein levels in Eca-109 cells
The ability of siRNA to reduce SRF mRNA and protein
expression was analyzed using real-time PCR and western blot
analysis, respectively. The expression levels of SRF mRNA in
Eca-109 cells transfected with SRF-siRNA were reduced to 21.55% of
those in the blank control group (P<0.01; Fig. 2A). SRF protein levels were reduced
in Eca-109 cells transfected with SRF-siRNA to 41.53% of their
levels in the blank controls (P<0.05). In addition, no
difference between the blank control, negative siRNA control and
mock transfection groups was observed (P>0.05; Fig. 2B and C).
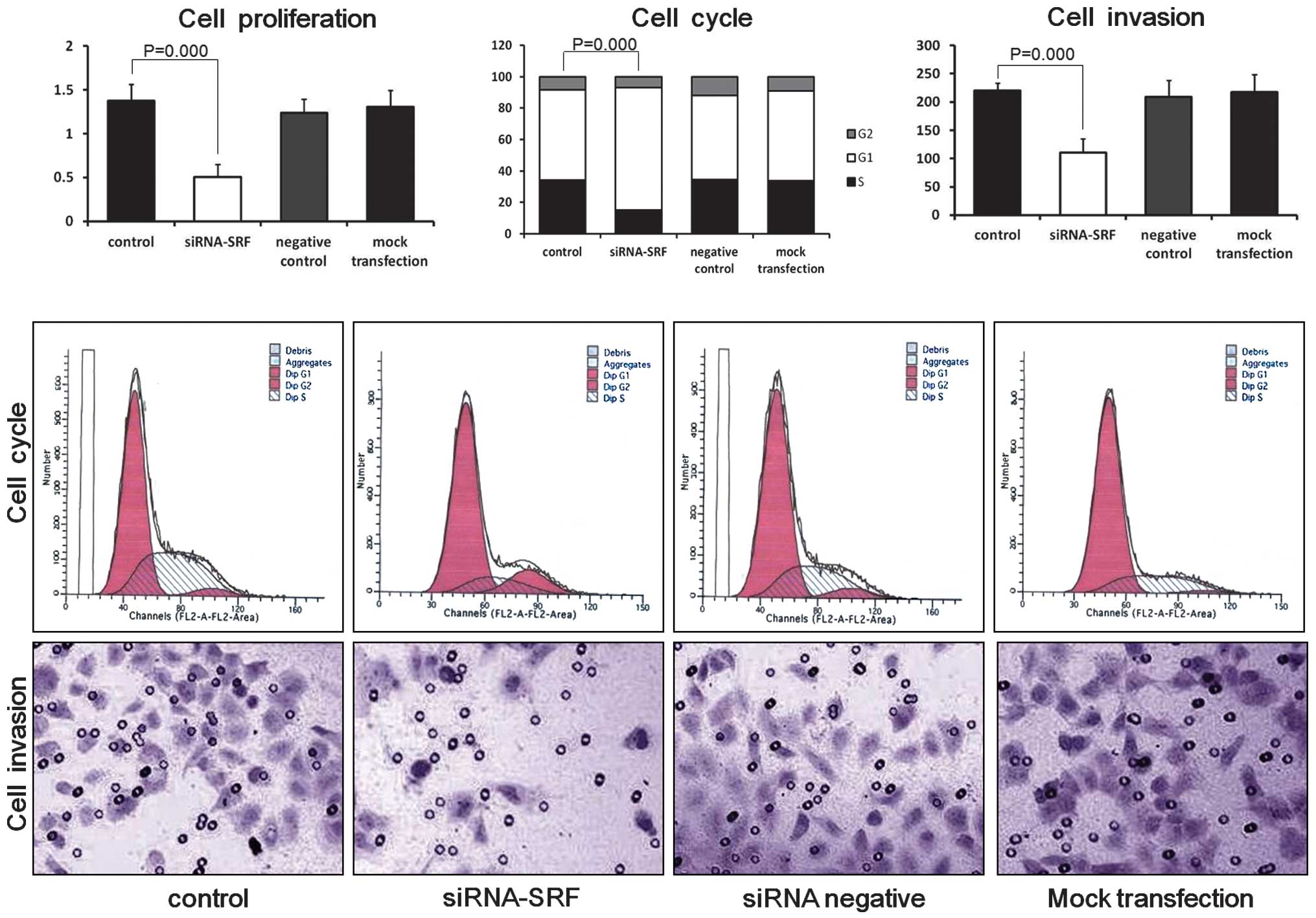
Effect of siRNA-SRF-1107 on proliferation
of Eca-109 cells
Cells in the four groups were harvested 48 h after
transfection. The proliferation rate of the Eca-109 cells was
significantly lower in the SRF-siRNA group than in the blank
control (P<0.01), negative siRNA control (P<0.01) and mock
transfection groups (P<0.01). No significant difference was
observed between the blank control (P>0.05), negative siRNA
control (P>0.05) and mock transfection groups (P>0.05;
Fig. 3). These results suggest that
the downregulation of SRF significantly inhibits the proliferation
of Eca-109 cells.
Downregulation of SRF affects cell cycle
distribution in Eca-109 cells
The effect of SRF-siRNA on the cell cycle was
evaluated by flow cytometry (Fig.
3). The four groups of cells were collected for cell cycle
analysis 48 h after transfection. The percentage of S-phase cells
in the siRNA-SRF-transfected group was lower than those for the
blank control (P<0.05), negative siRNA control (P<0.05) and
mock transfection groups (P<0.05). Therefore, SRF silencing may
arrest the cell cycle at the G1 phase in Eca-109 cells.
Effect of siRNA-SRF-1107 on invasion of
Eca-109 cells
The invasive potential of Eca-109 cells was
determined by using a Matrigel invasion assay (Fig. 3). Cells transfected with siRNA-SRF
showed decreased migration (110.50±24.84) through the Matrigel
compared with the blank control (220.17±12.94), negative siRNA
control cell (217.67±31.26) and mock transfection groups
(208.67±29.75; P<0.05). In addition, no difference between the
blank control (P>0.05), negative siRNA control cell (P>0.05)
and mock transfection groups (P>0.05) was observed. These
results suggest that the downregulation of SRF significantly
inhibits the invasive capacity of Eca-109 cells.
Effect of siRNA-SRF-1107 on E-cadherin,
β-catenin and cyclin D1 expression in Eca-109 cells
Western blot analysis revealed that siRNA-SRF
treatment of Eca-109 cells resulted in down-regulation of β-catenin
and cyclin D1 protein expression by 52.53 and 38.14%, as compared
with blank controls (Fig. 4A).
Furthermore, gene silencing by siRNA-SRF-1107 markedly upregulated
the E-cadherin expression 2.03-fold, compared with the control
group (Fig. 4B). In addition, no
difference between the blank control (P>0.05), negative siRNA
control cell (P>0.05) and mock transfection groups (P>0.05)
was observed.
Discussion
Our results showed that SRF was more highly
expressed in ESCC than normal esophageal tissue and that SRF levels
were correlated with patient clinical parameters. We subsequently
evaluated SRF function in Eca-109, an ESCC cell line. Knockdown of
SRF in Eca-109 cells inhibited cell proliferation and invasion
in vitro. These results suggest that SRF is involved in the
development and progression of ESCC.
Serum response factor has been reported to be
involved in promoting the carcinogenesis and progression of
colorectal cancer (6),
hepatocelluar carcinoma (7,8,10,11),
breast cancer (5) and thyroid
carcinoma (9). However, the role of
SRF in ESCC and its mechanism of action have not been reported. The
overexpression of SRF in cancer has increasingly been shown to
enhance invasion and migration of cancer cells, due to loss of
cell-cell adhesion (6),
acceleration of cell migration and invasion in hepatocellular
carcinoma, and acquisition of mesenchymal phenotypes due to the
expression of a mesenchymal marker (vimentin) and the activation of
immediate early genes (10,11). High SRF levels in carcinomas also
contribute to ECM degradation and progressive tumor cell migration
and invasion (8,12).
The current study characterizes SRF as a tumorigenic
enhancer that regulates β-catenin and cyclin D1. β-catenin is an
important mediator in the Wnt signaling pathway, and when
activated, is translocated into the nuclei where it stimulates the
transcription of target genes involved in cell proliferation
(13). Cyclin D1 is a major
transcriptional target of β-catenin signals that promotes G1/S
transition in the cell cycle (14).
We found that downregulation of SRF decreased β-catenin and cyclin
D1 levels and this correlated with inhibition of cell proliferation
and cell cycle arrest. The association of SRF inhibition with
decreased levels of β-catenin and cyclin D1 that we identified may
be relevant since β-catenin signaling is strongly linked to ESCC
(15–17). Furthermore, we found that SRF
upregulation in ESCC is associated with poor differentiation, deep
invasion and lymph node metastasis. Therefore, SRF may enhance the
metastatic capability of tumor cells. Consequently, SRF may be a
risk factor for ESCC metastasis. We also found that SRF gene
silencing strongly inhibits the cellular invasion that accompanies
the upregulation of E-cadherin. Consequently, inhibiting the
expression of E-cadherin blocks its activity in cell-cell adhesion,
cancer invasion and metastasis (18).
In summary, our study demonstrated that ESCC had
increased expression levels of SRF as well as altered expression
levels of E-cadherin and β-catenin. Blocking SRF expression
inhibited the proliferation and invasion of cancerous cells.
Acknowledgements
This study was performed within the
research budget of Hebei Medical University.
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
No authors listed. Esophageal cancer:
epidemiology, pathogenesis and prevention. Nat Clin Pract
Gastroenterol Hepatol. 5:517–526. 2008. View Article : Google Scholar
|
|
3
|
Mehlen P and Puisieux A: Metastasis: a
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian X, Liu Z, Niu B, et al:
E-cadherin/beta-catenin complex and the epithelial barrier. J
Biomed Biotechnol. 2011:5673052011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Q, Guo C, Li Y, Aronow BJ and Zhang J:
LMO7 mediates cell-specific activation of the Rho-myocardin-related
transcription factor-serum response factor pathway and plays an
important role in breast cancer cell migration. Mol Cell Biol.
31:3223–3240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi HN, Kim KR, Lee JH, et al: Serum
response factor enhances liver metastasis of colorectal carcinoma
via alteration of the E-cadherin/beta-catenin complex. Oncol Rep.
21:57–63. 2009.PubMed/NCBI
|
|
7
|
Farra R, Dapas B, Pozzato G, et al: Serum
response factor depletion affects the proliferation of the
hepatocellular carcinoma cells HepG2 and JHH6. Biochimie.
92:455–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KR, Bae JS, Choi HN, et al: The role
of serum response factor in hepatocellular carcinoma: an
association with matrix metalloproteinase. Oncol Rep. 26:1567–1572.
2011.PubMed/NCBI
|
|
9
|
Kim HJ, Kim KR, Park HS, et al: The
expression and role of serum response factor in papillary carcinoma
of the thyroid. Int J Oncol. 35:49–55. 2009.PubMed/NCBI
|
|
10
|
Kwon CY, Kim KR, Choi HN, et al: The role
of serum response factor in hepatocellular carcinoma: implications
for disease progression. Int J Oncol. 37:837–844. 2010.PubMed/NCBI
|
|
11
|
Park MY, Kim KR, Park HS, et al:
Expression of the serum response factor in hepatocellular
carcinoma: implications for epithelial-mesenchymal transition. Int
J Oncol. 31:1309–1315. 2007.PubMed/NCBI
|
|
12
|
Zhe X, Yang Y and Schuger L: Imbalanced
plasminogen system in lymphangioleiomyomatosis: potential role of
serum response factor. Am J Respir Cell Mol Biol. 32:28–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rimerman RA, Gellert-Randleman A and Diehl
JA: Wnt1 and MEK1 cooperate to promote cyclin D1 accumulation and
cellular transformation. J Biol Chem. 275:14736–14742. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shtutman M, Zhurinsky J, Simcha I, et al:
The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway.
Proc Natl Acad Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pradeep A, Sharma C, Sathyanarayana P, et
al: Gastrin-mediated activation of cyclin D1 transcription involves
beta-catenin and CREB pathways in gastric cancer cells. Oncogene.
23:3689–3699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song BJ, Park YJ, Kim HS, Kim CN and Chang
SH: Expression of beta-catenin and E-cadherin in early gastric
cancer: correlation with clinicopathologic parameters. Korean J
Gastroenterol. 43:82–89. 2004.(In Korean).
|
|
17
|
Jiang H, Xia J, Kang J, Ding Y and Wu W:
Short hairpin RNA targeting beta-catenin suppresses cell
proliferation and induces apoptosis in human gastric carcinoma
cells. Scand J Gastroenterol. 44:1452–1462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo KJ, Hu Y, Wen J and Fu JH: CyclinD1,
p53, E-cadherin, and VEGF discordant expression in paired regional
metastatic lymph nodes of esophageal squamous cell carcinoma: a
tissue array analysis. J Surg Oncol. 104:236–243. 2011. View Article : Google Scholar : PubMed/NCBI
|