Introduction
Endometriosis is a benign hormone-dependent
condition, occurring at various degrees of the disease in 5–15% of
females. The pathogenesis of endometriosis is yet to be completely
understood, although studies have shown that it may cause
retrograde implantation of menstrual tissue, peritoneal metaplasia
and lymphatic and venous spread (1–3). These
features have mixed traits of benign disease and malignancy.
Patients with a long history of endometriosis have a higher risk of
developing ovarian cancer (4,5). The
incidence of malignant transformations ranges between 0.7 and 1.0%
in patients with endometriosis (6,7).
Sampson (8) first defined the
following criteria for diagnosing malignant transformation of
endometriosis: i) there should be a clear example of endometriosis
in proximity to the tumor; ii) no other primary site of the tumor
may be found; and iii) the histological appearance should be
consistent with an endometrial origin (8). Scott completed Sampson’s criteria by
adding the demonstration of a transition between endometriosis and
malignant epithelium (9).
The present study describes a case of malignancy
arising from residual endometriosis in a female patient with a
family history of ovarian and colon cancer following hysterectomy
and bilateral salpingo-oophorectomy. The study was approved by the
Ethics Committee of Beijing Chaoyang Hospital, Beijing, China.
Written informed consent was obtained from the patient.
Case report
A 42-year-old female patient, para 1, was admitted
on August 30, 2011, to the Department of Obstetrics and Gynecology
at Beijing Chaoyang hospital (Beijing, China) due to a recent-onset
pelvic cyst persisting for five months. The patient had a five-year
history of ovary endometriosis and had undergone right-side
laparoscopic salpingo-oophorectomy and left ovarian cystectomy for
bilateral ovary endometriosis in November 2006. A histological
examination showed benign endometriosis in the right ovary and
salpinx (Fig. 1A) and a luteal cyst
was identified in the left ovary. After the surgery, the patient
received gonadotropin-releasing hormone (GnRH) agonist treatment
(goserelin, 3.6 mg every 4 weeks for 6 months) and was followed up
using serial cancer antigen 125 (CA125). The patient returned to
the hospital three years later complaining of having hysteromyoma
for 18 months with menorrhagia for 12 months. Laparoscopic surgery
was performed on March 18, 2009, and an 8-cm intramural myoma and
2-cm cystic mass were identified in the left ovary. The left ovary
and salpinx were markedly adhered to the sigmoid colon. Laparoscopy
with hystrorectomy and left salpingo-oophorectomy were performed.
The histology of the left ovarian cyst exhibited endometriosis
(Fig. 1B). Hormone replacement
therapy was not selected following surgery. The patient was
followed up using CA125 level and pelvic ultrasonography which
remained normal five months after surgery. The following hormone
levels were tested after the surgery: serum estrogen, follicle
stimulating hormone (FSH) and luteinzing hormone (LH; estrogen 2
127.14 pg/ml, FSH 7.44 IU/l, LH 5.86 IU/l). The serum CA125 level
was elevated to 119.8 U/ml (normal <35 U/ml). A
contrast-enhanced computed tomography (CT) scan was then performed
to aid in identifying the reasons for the abnormal test results.
The scan showed a mixed-density pelvic lobulated mass measuring
4.2×3.3×3.8 cm on the left side with an irregular surface and
exhibiting moderately nonhomegeneous arterial enhancement (Fig. 2). The patient then underwent
laparoscopy, which identified a 6×7-cm mass adhering to the left
pelvic wall and colon. Subsequently, the adhesion was released and
the mass was resected for a frozen biopsy, which revealed
adenocarcinoma of the residual left ovary. Laparoscopic staging
surgery was then performed, including excision of the tumor mass,
omentectomy, appendectomy and pelvic/paraaortic lymphadenectomy.
Histological examination following surgery showed that part of the
residual normal ovary tissue had moderately differentiated into
endometrioid adenocarcinoma directly arising from the residual
endometriosis site, without pelvic and paraaortic lymph node
involvement. A transition region between endometriosis and
endometrioid adenocarcinoma was also observed (Fig. 3). The patient was diagnosed with
ovarian endometrioid adenocarcinoma at FIGO stage IIC. The
postoperative chemotherapy consisted of 165 mg/m2
paclitaxel and AUC 5 of carboplatin.
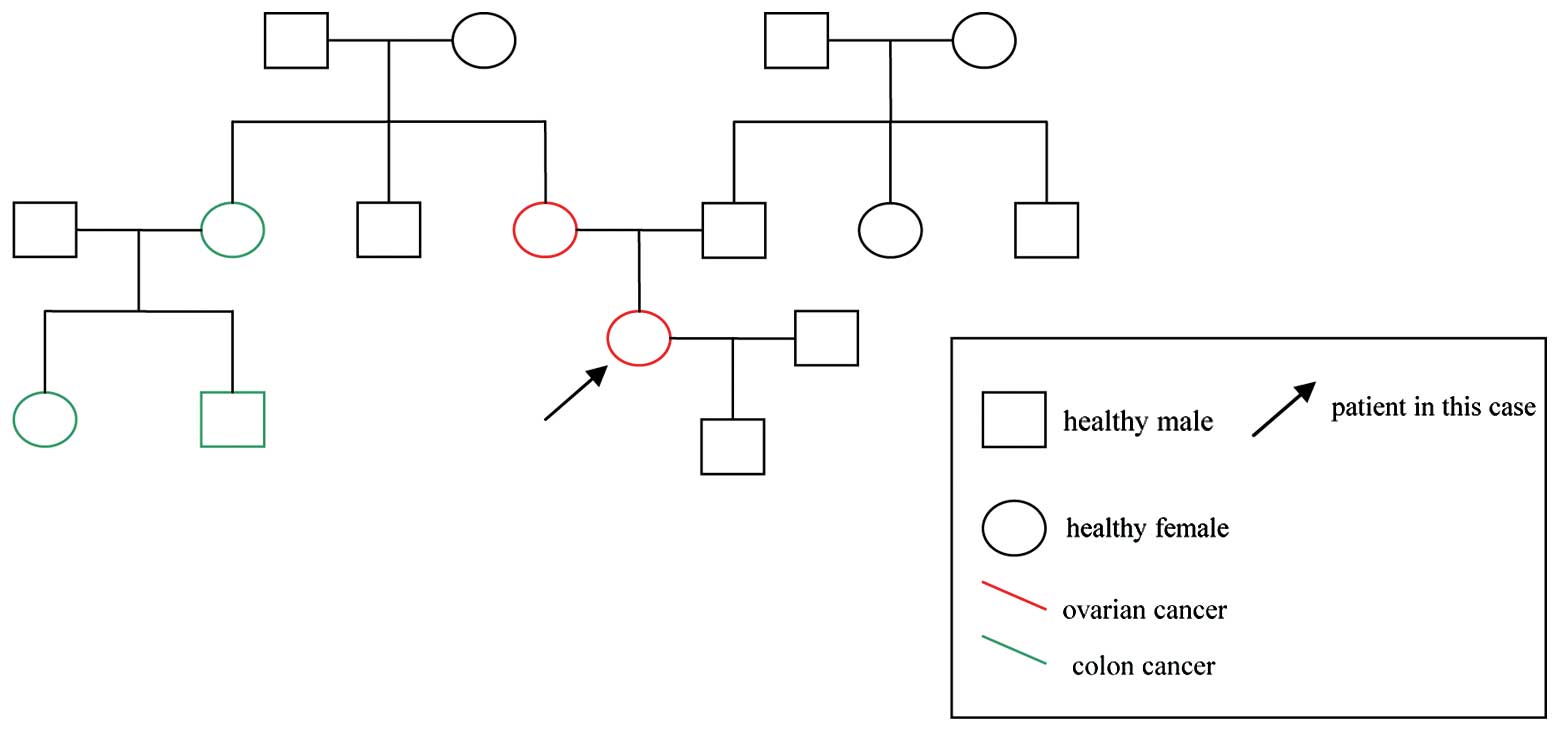
The patient’s family history for three generations
is presented in Fig. 4. Three cases
of colon cancer and one case of ovarian cancer were identified on
the maternal side of the family. No cases were identified on the
paternal side.
Discussion
It has been reported that endometrial lesions in the
ovary have the potential for malignancy (10–12).
In the present case, endometrioid adenocarcinoma was shown to arise
from endometriosis and the transition between endometriosis and
endometrioid carcinoma was confirmed and met the criteria of
Sampson and Scott (8,9). This case was a female patient with a
family history of ovarian and colon cancer who underwent a
malignant transformation two years after pelvic clearance surgery,
hysterectomy and bilateral salpingo-oophorectomy.
In the present case, five family members were
diagnosed with cancer, two with ovarian cancer and three with colon
cancer, as shown in Fig. 4. The
results of these cases fulfill the Amsterdam II criteria and the
Bethesda guidelines (Diagnosis criteria for HNPCC) (13). A diagnosis of HNPCC should be
considered (Table I). Women with
HNPCC have an increased risk of gynaecological cancer (14). Among women with HNPCC, 20–60% may
develop endometrial cancer compared with 3% of the general
population. Ovarian cancer occurs in 10–20% women with HNPCC
(15). Additionally, according to
Matalliotakis et al, there is a relative risk for women with
endometriosis and a positive family history of ovarian and colon
cancer including first- and second-degree relatives (16). The study indicated that HNPCC may be
associated with gynaecological cancer. However, little evidence has
been reported on the association between the malignant
transformation of endometriosis and HNPCC. HNPCC is an autosomal
dominantly inherited cancer disorder (Fig. 4) and has been demonstrated to be
caused by the inherited mutation of genes such as hMSH2, hMLH1,
PMS1, PMS2 and hMSH6 (17,18). The HNPCC gene mutations continue to
develop and accumulate within neoplastic but not normal tissue
(19). However, associated studies
have suggested that the malignant transformation of endometriosis
may be induced by loss of heterozygosity (LOH) events on certain
chromosomes such as the PTEN gene situated on chromosome 10q23.3
(20,21). Whether certain special HNPCC gene
mutations are involved in the malignant transformation of
endometriosis remains unknown. In the present case, the malignant
transformation of endometriosis may have arisen from the incomplete
excision of the left ovary in the patient’s second surgery. The
left ovary was identified as being markedly adhered to the colon
during the patient’s second surgery. A meticulous excision was
difficult to perform (22,23), which may have resulted in a trace
amount of residual left ovary. The blood supply of the residual
ovary tissue may account for the formation of collateral
circulation (24). According to the
levels of female hormones, the residual ovary was able to maintain
normal endocrine function (23,24).
It has been reported that hyperestrogenism is closely associated
with the malignant transformation of endometriosis (4,25). In
previously reported cases, women who underwent pelvic clearance
surgery and later underwent estrogen replacement therapy had a
markedly higher risk of malignant extra-gonadal transformation
(4). Hormonal factors may be
crucial in the origin of endometriosis and the development of
malignant transformation (4,20,26).
As discussed previously, the incomplete excision of the left ovary
may have resulted in normal levels of serum estrogen. Whether the
normal levels of female hormones contribute to the malignant
transformation of ovary endometriosis has yet to be proved.
 | Table IClinical criteria for HNPCC. |
Table I
Clinical criteria for HNPCC.
| Name | Criteria |
|---|
| Amsterdam | At least three
relatives with CRC; all the following criteria should be present:
-
One should be the first-degree relative of the other
two
-
At least two successive generations should be
affected
-
At least one CRC should be diagnosed before the age
of 50
-
Familial adenomatous polyposis should be
excluded
|
| Amsterdam II | At least three
relatives with an HNPCC-associated cancer (CRC, cancer of the
endometrium, small bowel, ureter or renal pelvis); all the
following criteria should be present:
-
One should be the first-degree relative of the other
two
-
At least two successive generations should be
affected
-
At least one CRC should be diagnosed before the age
of 50
-
Familial adenomatous polyposis should be
excluded
|
| Bethesda
(modified) |
-
Individuals with cancer in families that fulfil the
Amsterdam criteria
-
Individuals with two HNPCC-related cancers,
including synchronous or metachronous CRCs or associated
extra-colonic cancers
-
Individuals with CRC and a first-degree relative
with CRC and/or HNPCC-related extracolonic cancer and/or colorectal
adenoma; one of the cancers diagnosed at <50 years and the
adenoma diagnosed at <40 years
-
Individuals with CRC or endometrial cancer diagnosed
at <50 years
-
Individuals with right-sided CRC with an
undifferentiated pattern (solid/cribriform) on histopathology
diagnosed at <50 years
-
Individuals with signet-ring-cell-type CRC diagnosed
at <50 years
-
Individuals with adenomas diagnosed at <40
years
|
As mentioned previously, we suggest that an accurate
family history should also be obtained from women with
endometriosis. As for women with HNPCC, hysterectomy and bilateral
salpingo-oophorectomy should be considered as the patient’s first
surgical treatment. Further studies of malignant
endometriosis-associated gene detection in HNPCC should also be
performed.
Acknowledgements
The authors would like to thank Mulan
Jin from the Beijing Chaoyang Hospital Department of Pathology for
providing technical assistance.
References
|
1
|
Olive DL and Schwartz LB: Endometriosis. N
Engl J Med. 328:1759–1769. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hacker NF, Gambone JF and Hobel CJ: Hacker
and Moore’s Essentials of Obstetrics and Gynecology. 5th edition.
Saunders; Philadelphia: pp. 298–304. 2010
|
|
3
|
Czernobilsky B: Endometriosis. Fox H and
Wells M: Haines and Taylor Obstetrical and Gynaecological
Pathology. 4th edition. Churchill Livingstone; Edinburgh: pp.
1043–1062. 1995
|
|
4
|
Benoit L, Arnould L, Cheynel N, Diane B,
Causeret S, Machado A, Collin F, Fraisse J and Cuisenier J:
Malignant extraovarian endometriosis: a review. Eur J Surg Oncol.
32:6–11. 2006. View Article : Google Scholar
|
|
5
|
Munksgaard PS and Blaakaer J: The
association between endometriosis and gynecological cancers and
breast cancer: a review of epidemiological data. Gynecol Oncol.
123:157–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishida M, Watanabe K, Sato N and Ichikawa
Y: Malignant transformation of ovarian endometriosis. Gynecol
Obstet Invest. 50(Suppl 1): 18–25. 2000. View Article : Google Scholar
|
|
7
|
Corner GW, Hu CY and Hertig AT: Ovarian
carcinoma arising in endometriosis. Am J Obstet Gynecol.
59:760–774. 1950.
|
|
8
|
Sampson JA: Endometrial carcinoma of the
ovary, arising in endometrial tissue in that organ. Arch Surg.
10:1–72. 1925. View Article : Google Scholar
|
|
9
|
Scott RB: Malignant changes in
endometriosis. Obstet Gynecol. 2:283–289. 1953.
|
|
10
|
Brinton LA, Gridley G, Persson I, Baron J
and Bergqvist A: Cancer risk after a hospital discharge diagnosis
of endometriosis. Am J Obstet Gynecol. 176:572–579. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aris A: Endometriosis-associated ovarian
cancer: a ten-year cohort study of women living in the Estrie
Region of Quebec, Canada. J Ovarian Res. 19. 3:22010.PubMed/NCBI
|
|
12
|
Xu B, Hamada S, Kusuki L, Itoh R and
Kitawaki J: Possible involvement of loss of heterozygosity in
malignant transformation of ovarian endometriosis. Gynecol Oncol.
120:239–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vasen HF, Mecklin JP, Khan PM and Lynch
HT: The International Collaborative Group on Hereditary
Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum.
34:424–425. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma A, James M, Donaldson A and Fox R:
Hereditary non-polyposis colorectal cancer syndrome: combined risk
of gastrointestinal and gynaecological cancer. BJOG. 108:671–677.
2001.PubMed/NCBI
|
|
15
|
Allen BA and Terdiman JP: Hereditary
polyposis syndromes and hereditary non-polyposis colorectal cancer.
Best Pract Res Clin Gastroenterol. 17:237–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matalliotakis IM, Cakmak H, Krasonikolakis
GD, Dermitzaki D, Fragouli Y, Vlastos G and Arici A: Endometriosis
related to family history of malignancies in the Yale series. Surg
Oncol. 19:33–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peltomäki P: Mutations and epimutations in
the origin of cancer. Exp Cell Res. 318:299–310. 2012.
|
|
18
|
Vasen HF, Watson P, Mecklin JP and Lynch
HT: New clinical criteria for hereditary nonpolyposis colorectal
cancer (HNPCC, Lynch syndrome) proposed by the International
Collaborative Group on HNPCC. Gastroenterology. 116:1453–1456.
1999. View Article : Google Scholar
|
|
19
|
Jass JR, Stewart SM, Stewart J and Lane
MR: Hereditary non-polyposis colorectal cancer - morphologies,
genes and mutations. Mutat Res. 310:125–133. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato N, Tsunoda H, Nishida M, Morishita Y,
Takimoto Y, Kubo T and Noguchi M: Loss of heterozygosity on 10q23.3
and mutation of the tumor suppressor gene PTEN in benign
endometrial cyst of the ovary: possible sequence progression from
benign endometrial cyst to endometrioid carcinoma and clear cell
carcinoma of the ovary. Cancer Res. 60:7052–7056. 2000.
|
|
21
|
Xu B, Hamada S, Kusuki I, Itoh R and
Kitawaki J: Possible involvement of loss of heterozygosity in
malignant transformation of ovarian endometriosis. Gynecol Oncol.
120:239–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Minelli L, Ceccaroni M, Ruffo G, Bruni F,
Pomini P, Pontrelli G, Rolla M and Scioscia M: Laparoscopic
conservative surgery for stage IV symptomatic endometriosis:
short-term surgical complications. Fertil Steril. 94:1218–1222.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fedele L, Bianchi S, Zanconato G,
Bergamini V, Berlanda N and Carmignani L: Long-term follow-up after
conservative surgery for bladder endometriosis. Fertil Steril.
83:1729–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Achard JM, Fournier A, Mazouz H, Caride
VJ, Penar PL and Fernandez LA: Protection against ischemia: a
physiological function of the renin-angiotensin system. Biochem
Pharmacol. 62:261–271. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nezhat F, Datta MS, Hanson V, Pejovic T
and Nezhat C and Nezhat C: The relationship of endometriosis and
ovarian malignancy: a review. Fertil Steril. 90:1559–1570. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Somigliana E, Vigano P, Parazzini F,
Stoppelli S, Giambattista E and Vercellini P: Association between
endometriosis and cancer: a comprehensive review and a critical
analysis of clinical and epidemiological evidence. Gynecol Oncol.
101:331–341. 2006. View Article : Google Scholar : PubMed/NCBI
|