Introduction
Granulocytic sarcoma (GS), also referred to as
myeloid sarcoma or chloroma, is a rare malignant tumor caused by
the extramedullary proliferation of myeloblasts or immature myeloid
cells (1–3). GS usually occurs concomitantly with or
following the diagnosis of acute myeloid leukemia (AML) (2). GS may also be a symptom of a
myeloproliferative disorder or leukemic transformation in
myelodysplastic syndrome (4).
Isolated GS has occasionally been reported to initially present in
the skin, bone, pancreas, conjunctiva, gastrointestine, cervix,
vagina and mediastinum. However, isolated spinal GS, particularly
with the involvement of the central nervous system (CNS), is
extremely rare.
The present study describes a case of isolated
spinal subdural GS and a further diagnosis of CNS leukemia (CNSL)
which was successfully treated with surgery, intensive chemotherapy
and intrathecal injection.
Case report
A previously healthy 34-year-old female exhibited a
5-month history of progressive anesthesia and weakness in the left
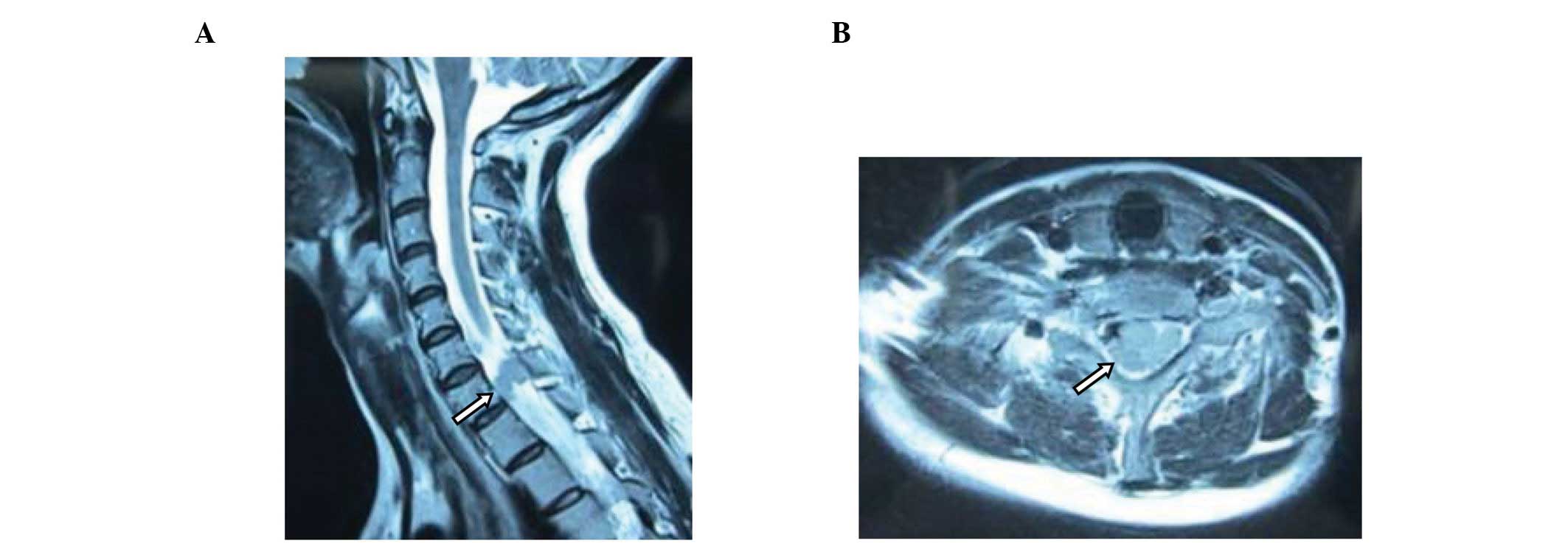
hand fingers. In March 2012, magnetic resonance imaging (MRI)
showed that the neck and thoracic portions of the spine were
involved. Soft tissue masses were observed in the spinal canal
distributed along the course of the nerve root, at the C6-T1 level
(Fig. 1). Blood tests showed a
white blood cell count (WBC) of 6.39×109/l, hemoglobin
count of 119 g/l and platelet count of 200×109/l. The
patient immediately underwent surgical intervention with the
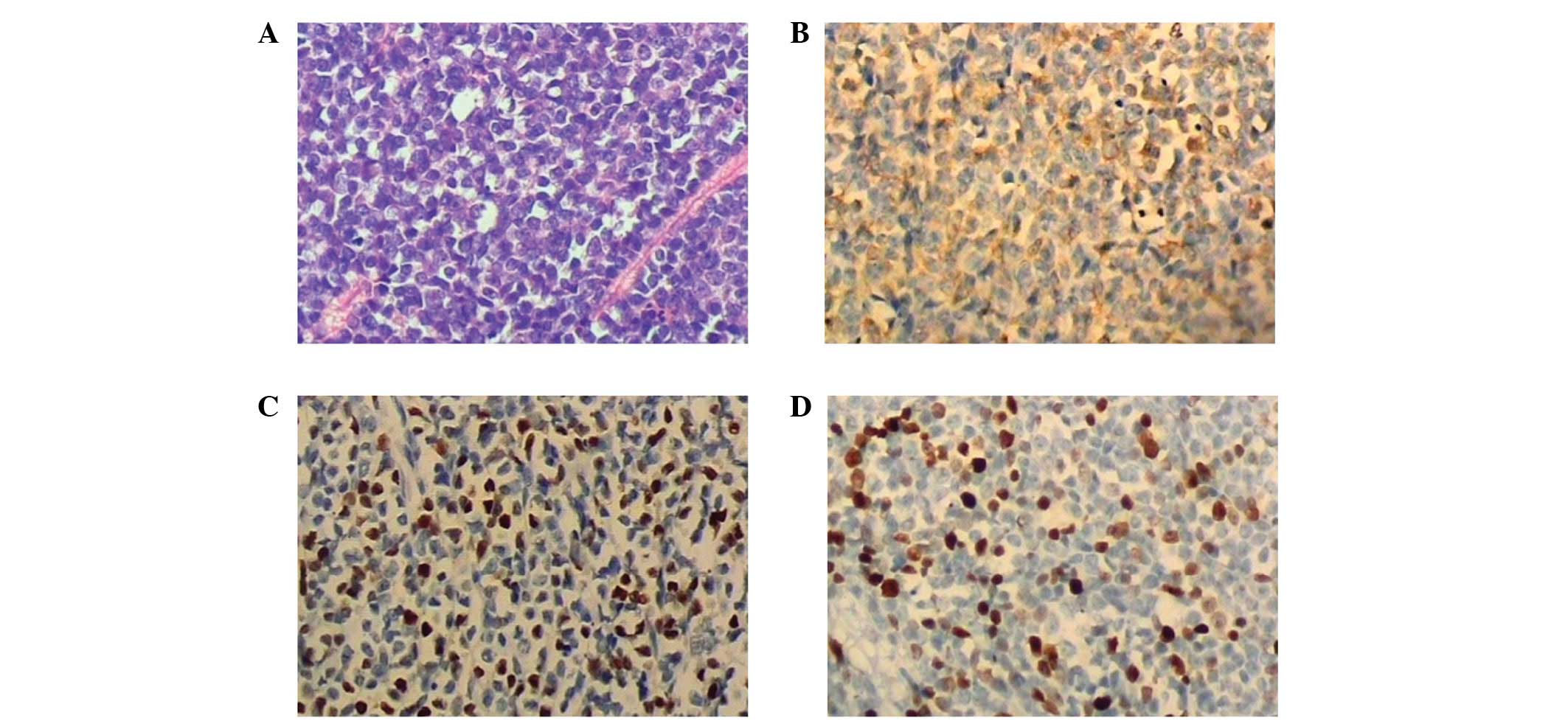
resolution of the neurological symptoms. The pathological
evaluation of the vertebral canal mass showed homogenous malignant
infiltration containing round nuclei, dispersed chromatin and
ill-defined eosinophilic cytoplasm (Fig. 2A). Immunohistochemical study showed
the vertebral canal mass to be positive for myeloperoxidase (MPO)
(Fig. 2B), partly positive for
terminal transferase (TdT) (Fig.
2C), positive for Ki67 (35%, Fig.
2D) and negative for CD20, CD79a, CD138, CD15, CD3 and CD5.
Bone marrow aspiration revealed a normal result. Based on these
findings, the final histological diagnosis was isolated GS. The
patient developed numbness and pain in the right lower limb two
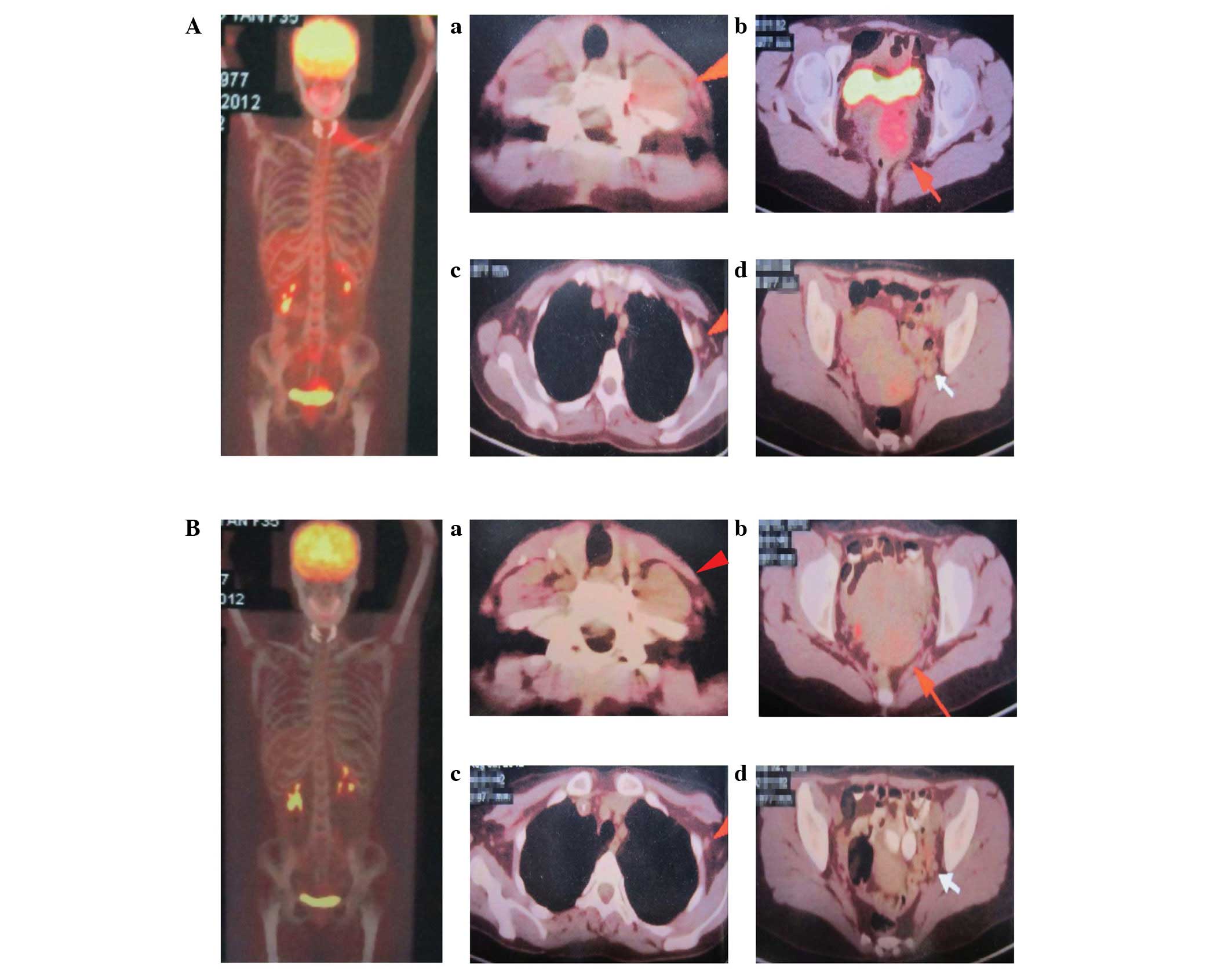
months later. Fluorodeoxyglucose (FDG)-positron emission tomography
(PET) showed FDG uptake in the left trapezius muscle with a maximal
standardized uptake value (SUV) of 2.4. The proliferation of
hypermetabolic lesions was also observed in the cervix uteri, iliac
bone, lymphadenectasis of the pelvic wall and left axillary fossa
with maximal SUVs of 4.2, 3.0, 1.5 and 1.3, respectively (Fig. 3A). Laboratory studies revealed a
hemoglobin level of 113 g/l, platelet level of 295×109/l
and WBC level of 9.06×109/l. A bone marrow biopsy
yielded a normocellular specimen. A cytogenetic study of the bone
marrow cells revealed a normal karyotype. A lumbar puncture was
performed and revealed elevated opening pressure (>140 mm
H2O). Biochemical analysis of the cerebrospinal fluid
(CSF) showed that the CSF WBC was 220×106/l and protein
was 1.19 g/l. Cytological examination of the CSF revealed a
predominance of myeloid cells, including myeloblasts. The final
histological diagnosis was CNSL.
Systemic induction chemotherapy was started
following diagnosis and consisted of daunorubicin [90 mg/day
intravenous (i.v.) on days 1, 2 and 3] and cytarabine (200 mg/day
continuous i.v. on days 1–7) for 1 course, followed by pirarubicin
(30 mg on day 1, 30 mg on day 2 and 40 mg on day 3) and Ara-C (200
mg/day continuous i.v. on days 1–7). During the chemotherapy, the
patient also received 6 intrathecal injections containing 15 mg
MTX, 50 mg Ara-C and 10 mg DXM each time. At follow-up 2 months
later, the CSF WBC had disappeared and protein was 0.24 g/l.
Cytological examination of the CSF did not reveal any clear myeloid
tumor cells.
A visual representation of the disease site and
metabolic remission was achieved by FDG-PET. The maximal SUV of the
FDG uptake in the left trapezius muscle was 1.2, much lower than
pre-treatment value. The maximal SUV decreased from 4.2 to 2.1 in
the cervix uteri, while FDG uptake disappeared in the iliac bone,
lymphadenectasis of the left axillary fossa and pelvic wall
(Fig. 3B). Bone marrow examination
revealed a normocellular specimen. At present, a further cycle of
chemotherapy in addition to the first course is being
administered.
Discussion
GS is a localized tumor formed by primitive myeloid
cells at an extramedullary site. GS was first described by Burns in
1811 and named chloroma in 1853 due to the infrequent greenish
appearance observed as a result of myeloperoxydase granules in the
malignant myeloid cells (5,6). GS may involve any organ system,
including the skin, bone, soft tissues and lymph nodes. Spinal GS
is extremely rare. It has been reported that the prevalence of GS
in the spine is 1.0% among all patients with myeloid leukemia
(7). GS in the absence of
clinically detectable leukemia is not common and only a few cases
of GS in patients without leukemia have been observed with spinal
involvement (8,9). Among these, CNS involvement has been
reported in 19% of non-leukemic GS patients (10).
Pathologically, the variable morphology may be
misleading in GS. The correct diagnosis is sometimes challenging
and is obtained in only ∼50% of non-leukemic patients due to the
histological and radiological similarities to malignant lymphoma
(11). The definitive diagnosis of
GS requires positive immunostaining for at least 1 of the myeloid
associated antigens (CD68, MPO, CD43, CD45, CD117, CD99, CD33, CD34
and CD13), as well as negative staining for the lymphoid lineages
CD3 and CD20 (2,12). Bone marrow sampling is also
necessary for the diagnosis of GS to assess the absence of AML. In
the present case, immunohistochemical studies showed positivity for
MPO and Ki67 and partly positive results for TdT, but negative
results for CD20, CD79a, CD138, CD15, CD5 and CD3, indicating GS.
The immunohistochemical findings were compatible with a monoblastic
or myelomonoblastic variant of myeloid sarcoma. In addition, bone
marrow aspiration showed a normal result, indicating no involvement
of the bone marrow.
An early and precise diagnosis of spinal GS with MRI
evaluation facilitates appropriate treatment with further therapy
(7). However, MRI is unable to
evaluate the metabolism. FDG-PET is reported to be more sensitive
for the detection of malignant tumors with increased glucose
metabolism (13). In the present
case, FDG-PET was used to estimate the malignancy of the tumor and
the treatment efficacy. It was observed that FDG-PET successfully
identified the active lesion and demonstrated the malignancy. A
decrease in FDG uptake was observed 2 months after treatment. The
follow-up FDG-PET suggested that adequate treatment contributed to
the reduction in the cellularity of the tumor.
The prognosis of patients with GS depends on the
initial context in which it occurs. Out of all isolated GS
patients, 66–88% develop AML within 9–11 months of diagnosis
(3,14). In the present case, the patient
developed CNSL 2 months after the diagnosis of GS. The optimal
treatment for GS has not been fully established, partially due to
the variety of clinical presentations. Chemotherapy, radiation
therapy, bone marrow transplantation, surgical resection or a
combination of approaches are employed in various cases. Surgery is
generally reserved for patients with acute spinal cord compression
or neurological symptoms. However, surgery is not always required
and may worsen the prognosis due to the delayed administration of
induction chemotherapy. Treating GS in the same manner as AML, even
in the absence of clinically detectable leukemia has been
previously reported (8).
Combination treatment with radiotherapy and chemotherapy resulted
in improved survival (3,10). However, isolated CNS GS and
meningeal myeloid leukemia may be successfully treated without
radiotherapy (16).
In accordance with the previously mentioned studies,
the present patient was successfully treated using surgery and
intensive anti-leukemic chemotherapy accompanied by intrathecal
injections. The present case highlighted the importance of a
correct diagnosis. Pre-therapeutic examinations should be the basis
for the diagnosis of a mass with an atypical clinical presentation.
Notably, treating GS in the same manner as AML may benefit patients
with isolated spinal GS.
References
|
1
|
Balleari E, Panarello S, Capello E, et al:
Granulocytic sarcoma: an unusual cause of spinal cord compression.
Int J Clin Oncol. 12:234–237. 2007. View Article : Google Scholar
|
|
2
|
Swerlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 4th edition. IARC
press; Lyon, France: 2008
|
|
3
|
Neiman RS, Barcos M, Berard C, et al:
Granulocytic sarcoma: a clinicopathologic study of 61 biopsy cases.
Cancer. 48:1426–1437. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Byrd JC, Edenfield WJ, Shields DJ and
Dawson NA: Extramedullary myeloid cell tumors in acute non
lymphocytic leukemia: a clinical review. J Clin Oncol.
13:1800–1816. 1995.PubMed/NCBI
|
|
5
|
Burns A: Observations on the Surgical
Anatomy of the Head and Neck. 2nd edition. Wardlaw and Cunninghame;
Glasgow, Scotland: pp. 386–387. 1824
|
|
6
|
King A: A case of chloroma. Monthly J Med.
17:971853.
|
|
7
|
Seok JH, Park J, Kim SK, Choi JE and Kim
CC: Granulocytic sarcoma of the spine: MRI and clinical review. AJR
Am J Roentgenol. 194:485–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antic D, Verstovsek S, Elezovic I, et al:
Spinal epidural granulocytic sarcoma in non-leukemic patient. Int J
Hematol. 89:95–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serefhanoglu S, Goker H, Aksu S, et al:
Spinal myeloid sarcoma in two non-leukemic patients. Intern Med.
49:2493–2497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsimberidou AM, Kantarjian HM, Estey E, et
al: Outcome in patients with nonleukemic granulocytic sarcoma
treated with chemotherapy with or without radiotherapy. Leukemia.
17:1100–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Williams MP, Olliff JF and Rowley MR: CT
and MR findings in parameningeal leukaemic masses. J Comput Assist
Tomogr. 14:736–742. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Audouin J, Comperat E, Le Tourneau A, et
al: Myeloid sarcoma: clinical and morphologic criteria useful for
diagnosis. Int J Surg Pathol. 11:271–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Go KG, Pruim J, Que TH, Vaalburg W and
Haaxma-Reiche H: Evaluation of dissemination studies with FDG
whole-body positron emission tomography in patients with suspected
metastatic tumours of brain and spine. Acta Neurochir (Wien).
142:627–631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Imrie KR, Kovacs MJ, Selby D, et al:
Isolated chloroma: the effect of early antileukemic therapy. Ann
Intern Med. 123:351–353. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JM, Song HN, Kang Y, et al: Isolated
mediastinal myeloid sarcoma successfully treated with
chemoradiotherapy followed by unrelated allogeneic stem cell
transplantation. Intern Med. 50:3003–3007. 2011. View Article : Google Scholar
|
|
16
|
Stepensky P, Revel-Vilk S, Yehuda-Gafni O,
Mali B, Resnick IB and Weintraub M: Isolated central nervous system
granulocytic sarcoma and meningeal myeloid leukemia: successful
treatment without radiotherapy. Isr Med Assoc J. 11:569–570.
2009.
|