Introduction
The term stereotactic body radiosurgery (SBRS)
implies the delivery of a focused single dose of radiation therapy
(1). This technique has been used
in the treatment of various types of cancer in different anatomic
sites, including primary or metastatic lung tumors (2–4),
primary or secondary liver tumors (5–7),
pancreatic tumors (8),
gynecological cancer recurrences (9) and bone metastases (10).
With the delivery of a very high dose single
fraction of radiation therapy, SBRS requires steep dose gradients,
usually obtained by dynamic techniques or non-coplanar fixed
fields. SBRS also requires high precision in the treatment delivery
process. Therefore, it requires a short fraction length to reduce
the risk of intra-fraction set-up deviations or organ motion.
Volumetric modulated arc therapy (VMAT) is a novel
radiotherapy technique. VMAT differs both from standard
intensity-modulated radiation therapy (IMRT) and three-dimensional
conformal radiotherapy (3D-CRT), which operate in static
conditions, and is characterized by dose delivery by dynamic arcs
(11). During VMAT, the delivery of
radiation occurs with a rotational movement of the linear
accelerator (LINAC) gantry while a continuous variation of the
beam’s profile and intensity is obtained. VMAT requires a
sophisticated technique for complex treatment planning. As VMAT has
evolved from IMAT, VMAT has the advantage of high-dose conformity
and improved sparing of healthy tissues. Therefore, VMAT may be
theoretically useful for dose escalation and improved tumor control
probability. In addition, the duration of dose delivery is very
short, allowing the advantages of IMRT (high conformity index) to
be combined in a reduced treatment time. The consequences are
represented by a higher operating efficiency of each treatment
unit, enhanced patient comfort and reduced risk of intrafraction
deviations both in terms of set-up errors or organ motion.
For these reasons, VMAT is a potentially ideal
technique for SBRS. However, it is not yet clear whether
administration of very high doses in single fraction delivery with
such a complex technique is possible. Additionally, the true
capacity of VMAT to respect dose-volume constraints even in the
case of high doses per fraction is uncertain. To the best of our
knowledge, no data on VMAT-SBRS have been published.
Based on this background, a feasibility study
regarding SBRS based on the VMAT technique (DESTROY-2 protocol) has
been planned. The purpose of this analysis is to report the
preliminary results of this study.
Materials and methods
Study characteristics
This trial was conceived as a prospective dose
escalation study. All patients consecutively observed at our
Radiotherapy Unit (Catholic University, Campobasso, Italy) and
matching the inclusion criteria were enrolled. The trial was
approved by the Catholic University Institutional Review Board. A
preliminary evaluation of technical feasibility was planned
following the enrollment of the first 20 patients. Written informed
patient consent was obtained from the patients.
Study objectives
The primary study end point was the definition of
maximum tolerated dose (MTD) of SBRS with VMAT. The secondary
objectives of the study were: i) feasibility evaluation in terms of
dose-volume constraints; ii) analysis of the correlation between
dosimetric and toxicity data; iii) analysis of the clinical
response and iv) evaluation of local control.
Radiosurgery dose escalation
Each enrolled subject was included in a study arm
according to the tumor site and disease stage, as demonstrated in
Table I. Patients were sequentially
assigned to a specific dose level as detailed in Table II. VMAT dose escalation was based
primarily on the acute and subacute toxicity, as late toxicity is
capable of occurring months or years later. Acute-subacute
toxicities were defined as those that occurred within 6 months of
receiving treatment. Toxicities registered ≥6 months post-radiation
were defined as late toxicities. Dose-limiting toxicities (DLTs)
were defined as any treatment-related non hematological adverse
effects rated as ≥grade 3 or any hematological toxicity rated as
≥grade 4, by the National Cancer Institute Common Terminology
Criteria for Adverse Events v.4.03 (12). If the DLT was not observed in the
three patients at a given dose level, the trial proceeded to the
next dose level, provided that 6 months of follow-up had occurred
following the VMAT for the third patient of the cohort. If a DLT
occurred in one of the three patients at a given dose level,
treatment of up to three additional patients at this dose level was
required. If the DLT occurred in more than one patient of the three
patients’ cohort, dose escalation was halted, and the dose level
below that was considered to be the MTD. If a DLT occurred in two
or more patients of the expanded six-patient cohort, dose
escalation was terminated, and the dose level below that was
considered to be the MTD. If a DLT occurred in less than two
patients of the expanded six-patient cohort, the trial proceeded to
the subsequent dose level. Different total VMAT doses were selected
on initiation of the study as the highest dose levels to be
evaluated, and were dependent on the study arm. Late toxicities
were continuously monitored regardless of whether patients had
documented disease progression.
 | Table IInclusion criteria. |
Table I
Inclusion criteria.
| Study arm | Criteria |
|---|
| Lung | Primary or
secondary lung tumors |
| Number of lesions:
1–5 |
| Largest diameter
<5 cm |
| Surgical treatment
not indicated |
| No prior RT at the
same site |
| No chemotherapy 14
days before and after SBRS |
| Absence of
bronchopulmonary |
| Infections in
active phase |
| Liver | Primary or
secondary liver tumors |
| Number of lesions
≤3 (four if two lesions <3 cm and close together) |
| Largest diameter
<6 cm (5 cm for 1 lesion, 4 cm for 2 lesions, and 3 cm for 3
lesions) |
| Distance >6 mm
from the gastrointestinal tract |
| Surgical treatment
not indicated |
| No previous RT to
the liver |
| No chemotherapy 14
days before and after SBRS |
| Absence of active
liver infections |
| Bone | Bone
metastases |
| Number of lesions:
1–5 |
| Largest diameter of
the single lesion <6 cm |
| Other | Advanced primary
tumor or local recurrence or distant metastasis |
| Surgical treatment
not indicated |
| Excluded from other
arms of the study |
 | Table IIDose levels (Gy) planned and reached
(underlined) in the different arms of the study. |
Table II
Dose levels (Gy) planned and reached
(underlined) in the different arms of the study.
| Level | Lung | Liver | Bone | Advanced |
|---|
| 1 | 26 | 26 | 12 | 16 |
| 2 | 28 | 28 | 14 | 18 |
| 3 | 30 | 30 | 16 | 20 |
| 4 | 32 | 32 | 18 | 22 |
| 5 | 34 | | 20 | 24 |
| 6 | | | 22 | |
| 7 | | | 24 | |
Inclusion criteria
The following inclusion criteria were used:
histological diagnosis of solid tumor (with the exception of
germinal tumors) with the site and tumor stage as demonstrated in
Table I; age, >18 years; ECOG
performance status, 0–3; adequate bone marrow function, which
included neutrophil count, >1500 μl; platelets,
>100,000/ml; hemoglobin, >9 g/dl. Additionally, for patients
receiving irradiation to the kidney (lumbar/abdominal area) the
inclusion criterion was creatinine, <1.8 mg/dl; while the
criteria for patients receiving irradiation to the liver were total
bilirubin, <3 mg/dl; lactate dehydrogenase, <3-fold the
normal value; aspartate aminotransferase, <3-fold the normal
value; alanine aminotransferase, <3-fold the normal value and
alkaline phosphatase, <3-fold the normal value. Previous
treatment with surgery and/or chemotherapy and/or radiotherapy was
permitted.
Exclusion criteria
The following exclusion criteria were employed:
ECOG, >3; the presence of medical conditions which
contraindicate radiation therapy, such as connective system
disorders, severe uncompensated heart disease (in case of heart
irradiation), acute diverticulitis, ulcerative colitis and pelvic
inflammatory disease (in case of irradiation of the pelvis);
comorbidities that in the opinion of the referring physician may
constitute a risk to clinical trial participation.
End points and statistical analysis
Toxicity was evaluated by the Common Toxicity
Criteria for Adverse Events (CTCAE) scale, version 4.03 (12). The presence of focal liver reaction
was evaluated as outlined by Herfarth et al(13). The survival curves were calculated
with the Kaplan-Meier method (14).
Statistical analysis was performed using SYSTAT software, version
11.0 (SPSS, Inc.; Chicago, IL, USA).
Patient set-up
Patient immobilization was performed with a
stereotactic body frame (SBF; Elekta; Crawley, UK), which is an
immobilization device used to define a stereotactic system of
coordinates for the target position as opposed to anatomical
landmarks such as bony structures or skin markers. This device was
described in detail by Lax et al(15) and clinical results have been
published by Blomgren et al(16). The SBF is a U-shaped rigid plastic
frame, within which different sized vacuum pillows allow a
reproducible immobilization for the repositioning of each patient.
Patient repositioning is supported by a laser system directly
attached to the body frame at defined longitudinal positions.
Alignment of the stereotactic coordinate system of the SBF to the
isocenter of the computed tomography (CT) machine or the treatment
unit is performed by a stereotactic arc with scales in the
anterior-posterior and lateral directions. The longitudinal
stereotactic coordinate is found on a scale along the body frame
sidewalls and is simply read on each CT-slice using a system of
straight and oblique copper pieces, which function as fiducials.
Moreover, to reduce the respiration mobility of targets close to
the diaphragm, a compressor, attached to the SBF by a rigid arc,
may be mechanically pressed into the patient’s epigastrium to
decrease the respiration motion.
CT simulation
To evaluate the reproducibility of the set-up, three
CT scan evaluations were performed on three different days, with
the aim of verifying that the set-up deviation was <3 mm. In
order to evaluate the organ motion produced by the respiratory
movements, target displacement was measured. During free breathing,
30 axial CT scans were performed on the same slice. In the case of
a displacement >5 mm, the abdominal compressor of the SBF was
applied and the CT scan for organ motion assessment was repeated.
The final CT simulation, for the acquisition of axial images
necessary for stereotactic localization and plan calculations, was
produced with a spiral technique. Subsequently, 3-mm scans were
acquired with a 3-mm interval between scans in the target region.
For the remainder of the SBF, 10-mm slices were acquired and the
interval between scans was 10 mm. In treating abdominal or pelvic
targets, patients received 2 cc of oral Gastrografin, diluted in
0.5 l of water 30 min prior to CT scan acquisition. In case of
mediastinal, abdominal or pelvic target volumes, intravenous
infusion of an iodinated contrast medium was also used.
Volumes of interest
The clinical target volume (CTV) was defined as the
gross tumor volume (GTV) in case of metastases and primary lung
tumors. A 5-mm margin was added to the GTV to define the CTV in
primary tumors of the liver. The planning target volume (PTV) was
individually defined for each patient based on the internal margin
(IM) and the set-up margin (SM) assessment. The IM was defined
based on respiratory excursions in 3D. The SM was set at 3 mm
according to the ROSEL study (17).
The OARs considered included: i) The thorax: the spinal cord,
lungs, esophagus, heart, brachial plexus, peripheral nerves, large
vessels, trachea and ribs; ii) The abdomen: the spinal cord, liver,
stomach, small bowel, colon and kidneys; iii) The pelvis: the
sacral plexus, small bowel, colon, rectum, anal canal, bladder,
femoral heads and penile bulb.
Prescription
A uniform method for the selection of the
prescription isodose surface (IDS) was adopted. According to the
ROSEL study (17), for each plan
the IDS was selected as the greatest IDS fulfilling the two
following criteria: 95% of the PTV volume reached 100% of the
prescription dose and 99% of the PTV reached ≥90% of the
prescription dose. The aim was to increase the dose heterogeneity
so as to intensify the dose within the GTV. The maximum dose within
the PTV should not exceed 140% of the prescribed dose. Careful
attention was paid to ensure the maximum dose always remained
within the GTV.
Treatment planning
VMAT plans were generated using the
ERGO++ treatment planning system (TPS), version 1.7.3
(Elekta). This is an anatomy-based TPS that supplies a simplified
approach to creating VMAT plans, by predefining a series of
aperture shapes using Boolean operations in conjunction with the
beam’s eye view of the target and OARs. In the current study, all
plans were generated with a single-arc rotation except for patient
number 16, who was treated for two liver lesions and therefore
required two arcs. The dose calculation was performed using the
pencil beam algorithm with inhomogeneity correction and a dose grid
resolution of 2 mm. VMAT plans were exported to the record and
verify (R&V) system Mosaiq v. 1.6 (Impac Software; Elekta) by
DICOM-RT for later irradiation. Table
III lists the dose-volume constraints used (5,18,19).
 | Table IIIDose-volume constraints. |
Table III
Dose-volume constraints.
| Organ | Dose (Gy) or volume
(% or cc) | Reference |
|---|
| Ribs | Dmax=30 | NCCN v.2.2010
(18) |
|
Heart/pericardium | Dmax=22 | NCCN v.2.2010
(18) |
| Skin | Dmax=26 | NCCN v.2.2010
(18) |
| Esophagus | Dmax=15.4 | NCCN v.2.2010
(18) |
| Liver | V12Gy
<30% | |
| V7Gy
<50% | Herfarth KK, 2001
(5) |
| Great vessels
(mediastinum) | Dmax=37 | NCCN v.2.2010
(18) |
| Bowel (small
bowel/colon) | Dmax=12 | Herfarth KK, 2001
(5) |
| Spinal cord | Dmax=14 | NCCN v.2.2010
(18) |
| Brachial
plexus | Dmax=17.5 | NCCN v.2.2010
(18) |
| Sacral plexus | Dmax=18 | Timmerman RD, 2008
(19) |
| Lungs |
V7.4Gy=1000 cc | Timmerman RD, 2008
(19) |
| Kidneys |
V8.4Gy=800 cc (cortical
area) | |
|
V10.6Gy=2/3 volume (ilo) | Timmerman RD, 2008
(19) |
| Stomach | Dmax=12.4 | NCCN v.2.2010
(18) |
| Trachea/large
bronchus | Dmax=20.2 | NCCN v.2.2010
(18) |
Quality assurance
Set-up deviation and organ motion assessments were
performed as previously described. For quality assurance through
treatment planning and delivery, two independent checks (IC1 and 2)
were performed by medical and physics staff, as previously
described (20).
Supportive therapy
Supportive therapy was prescribed according to the
irradiated site. In the case of irradiation of two anatomic sites,
such as the chest and the abdomen, supportive care was provided for
both sites. In patients receiving irradiation to the chest,
prescriptions included betamethasone 0.5 mg orally, 3 times daily
for 1 month, followed by a gradual reduction, associated with
gastric protection (H2-inhibitors). Patients receiving abdominal
irradiation were prescribed metoclopramide 10 mg orally, 3 times a
day, for ≤1 week following radiation therapy and rabeprazole 40 mg
orally, once daily for 12 months (in case of irradiation of the
stomach and/or the duodenum only). In addition, patients receiving
irradiation to the upper abdomen were prescribed dexamethasone 12
mg intravenously (IV) 1 fl immediately prior to radiosurgery and 6
h after treatment, while 3 mg granisetron was administered
immediately prior to radiosurgery by IV slow infusion.
Evaluation of response and follow-up
The tumor response assessment was performed 8–12
weeks after treatment. Morphological imaging modalities were
employed (CT with contrast medium and/or MRI with or without
contrast) in all patients. Using this method, the tumor response
was based on the response evaluation criteria in solid tumors
(RECIST) criteria (21). If
feasible, the response was also assessed with functional imaging,
which included (18F)-fluorodeoxyglucose (FDG)-PET or
choline PET for prostate cancer. In this study, the European
Organisation for Research and Treatment of Cancer (EORTC) criteria
were used (22). Specifically, the
PET-based response was assessed according to criteria including
progressive metabolic disease (PMD), stable metabolic disease
(SMD), a partial metabolic response (PMR) and a complete metabolic
response (CMR). PMD involved an increase in the tumor
(18F)-FDG standardized uptake value (SUV) of >25%
within the tumor region defined on the baseline scan, a visible
increase in the extent of tumor (18F)-FDG uptake of
>20% in the longest dimension, or the appearance of novel tumor
(18F)-FDG uptake in metastatic lesions. SMD comprised an
increase in the tumor (18F)-FDG SUV of <25% or a
decrease of <15%, and no visible increase in the extent of the
(18F)-FDG tumor uptake (i.e., not >20% in the longest
dimension). A partial metabolic response required a reduction of
>25% in the tumor (18F)-FDG SUV. A reduction in the
extent of the tumor (18F)-FDG uptake was not a
pre-requesite for a PMR, whereas a CMR negated a complete
resolution of the (18F)-FDG uptake within the tumor
volume, in order that it was indistinguishable from the surrounding
normal tissue. The follow-up was performed according to the scheme
detailed in Table IV.
 | Table IVFollow-up. |
Table IV
Follow-up.
| Study arm | First
follow-up | Subsequent
follow-up |
|---|
| Lung | | Chest CT and PET-CT
at 3 months and every 6 months thereafter |
| Liver | 2 weeks after SBRS
to evaluate acute toxicity | Abdominal CT and
PET-CT at 3 months and every 6 months thereafter; focal hepatic
reaction evaluation |
| Bone | | Bone CT and PET-CT
or bone-scan at 3 months and every 6 months thereafter (anticipated
if symptoms) |
| Advanced | | Body CT and PET-CT
at 3 months and every 6 months thereafter (anticipated if
symptoms) |
Quality of life (QoL) evaluation
The cancer linear analog scale (CLAS) score was used
to evaluate the impact of SBRS on the patient’s quality of life
(CLAS1), energy level (CLAS2) and ability to undertake daily
activities (CLAS3), both prior to and 3–4 weeks after radiotherapy.
Patients scored their perceptions of these symptoms by placing a
mark on a 100-mm line (23).
Results
Patient characteristics
The preliminary analysis was based on the first 20
enrolled patients who had a total of 25 lesions (Table V). The median PTV size was 37.8 cc
(range, 0.9–202.4). The prescribed dose ranged from 12–26 Gy to the
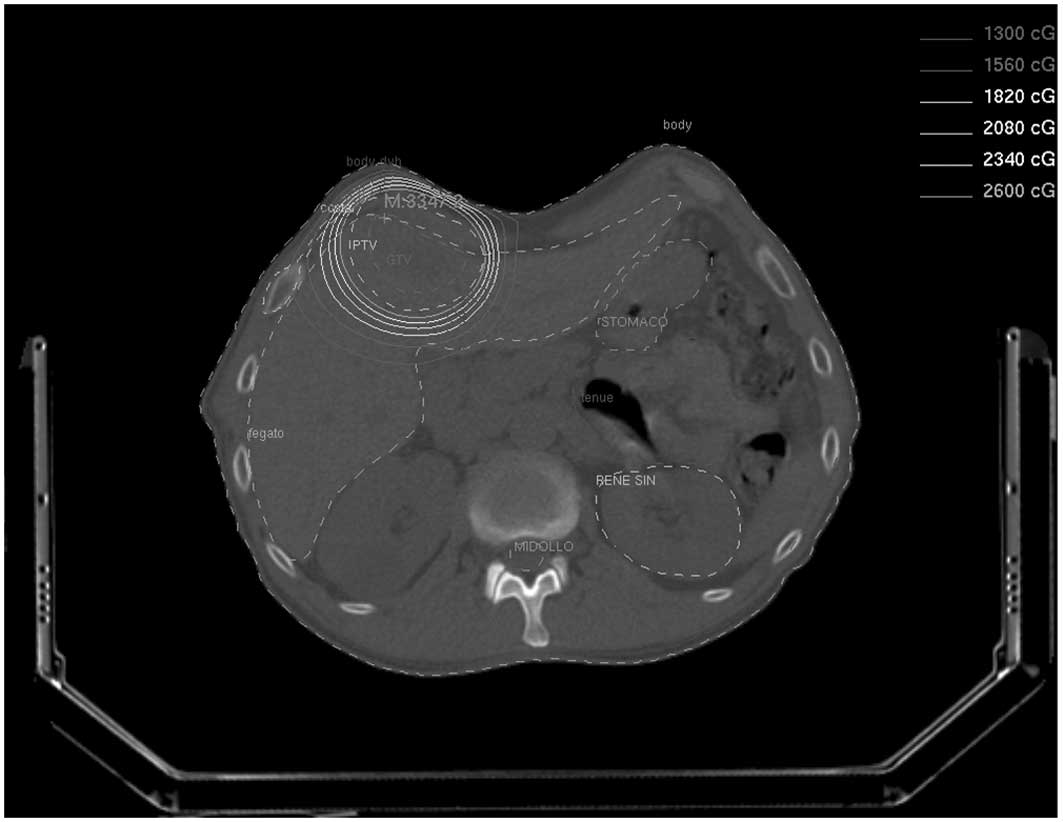
PTV (Fig.1).
 | Table VPatients characteristics and
results. |
Table V
Patients characteristics and
results.
| Patient | Gender | Age (years) | Study arm | Tumor | PTV (cc) | Prescribed dose
(Gy) | Acute toxicity
(CTCAE 4.03) | PET response | CT or MRI
response | Local failure (0,
no; 1, yes) | Local failure
(months) |
|---|
| 1 | F | 47 | Bone | Breast: bone
metastasis | 6.2 | 12 | 0 | | NC | 0 | 16 |
| | | | Breast: bone
metastasis | 11.7 | 12 | 0 | | NC | 0 | 16 |
| 2 | M | 65 | Liver | Nasopharynx: single
liver metastasis | 85.4 | 26 | Skin
hyper-pigmentation G1 | CR | CR | 0 | 15 |
| 3 | M | 82 | Advanced | Prostate: pelvic
nodal metastasis | 137.5 | 16 | 0 | CRa | CR | 0 | 13 |
| | | | Prostate pelvic
nodal metastasis | 133.8 | 16 | 0 | PRa | PR | 1 | 13 |
| 4 | M | 59 | Bone | Prostate: bone
metastasis | 8.1 | 12 | 0 | CRa | CR | 0 | 13 |
| 5 | F | 81 | Advanced | Vagina: nodule on
the right wall | 75.1 | 16 | Vaginal
inflammation and pain G1 | PR | NC | 1 | 8 |
| 6 | F | 64 | Lung | Colon: single lung
metastasis | 22.3 | 26 | 0 | | NC | 0 | 8 |
| 7 | M | 67 | Bone | Prostate: bone
metastasis | 0.9 | 14 | 0 | CRa | NC | 0 | 12 |
| 8 | M | 72 | Bone | Prostate: bone
metastasis | 8.4 | 14 | 0 | CRa | NC | 0 | 6 |
| 9 | M | 82 | Advanced | Prostate: pelvic
nodal metastasis | 37.8 | 16 | 0 | NCa | NC | 0 | 8 |
| | | | Prostate: pelvic
nodal metastasis | 12.5 | 16 | 0 | CRa | CR | 0 | 8 |
| 10 | F | 63 | Lung | Cervix: single lung
metastasis | 6.3 | 26 | Pneumonitis G1,
esophagitis G1 | PR | NC | 0 | 4 |
| 11 | F | 63 | Lung | Colon: single lung
metastasis | 50.9 | 26 | esophagitis G1 | CR | CR | 0 | 8 |
| 12 | M | 66 | Bone | Prostate: bone
metastasis | 10.2 | 14 | 0 | NCa | NC | 0 | 9 |
| 13 | F | 70 | Advanced | Breast: liver
metastasis | 69.4 | 16 | 0 | | PR | 0 | 9 |
| | | | Breast: liver
metastasis | 14.6 | 16 | 0 | | PR | 0 | 9 |
| 14 | M | 56 | Bone | Lung: bone
metastasis | 78.8 | 16 | 0 | PR | NC | 0 | 7 |
| 15 | M | 72 | Lung | NSCLC: primary
tumor | 43.3 | 26 | 0 | CR | CR | 0 | 7 |
| 16 | M | 67 | Liver | Nasopharynx: liver
metastases | 111.8 | 26 | 0 | PR | NC | 1 | 5 |
| | | | Nasopharynx: liver
metastases | 52.6 | 26 | 0 | PR | NC | 0 | 7 |
| 17 | F | 86 | Advanced | Colon: abdominal
nodal metastasis | 95.6 | 16 | 0 | CR | NC | 0 | 7 |
| 18 | M | 80 | Bone | Prostate: bone
metastasis | 202.4 | 16 | 0 | | PR | 0 | 4 |
| 19 | F | 67 | Advanced | Cervix: single
nodal metastasis | 15 | 18 | 0 | PR | PR | 0 | 5 |
| 20 | F | 49 | Liver | Colon: liver
metastasis | 26.8 | 26 | 0 | | CR | 0 | 4 |
Technical issues
The dose-volume constraints for OARs were observed
in all patients using a single-arc technique. Only one patient, who
was treated for two liver lesions, required a two-arc technique. To
administer the prescribed doses, 1401.9–3246.2 monitor units
(median, 2157.75) were employed with a median beam-on time of 6 min
and 6 sec (range, 4 min and 0 sec to 9 min and 13 sec). In all
patients, the treatment was performed without interruption or any
other technical issues.
Acute toxicity and response
All patients were evaluable for acute toxicity.
Twenty per cent of patients experienced grade 1 acute toxicity. No
patients demonstrated acute toxicity > grade 1. Twenty five
lesions were evaluable for clinical response by morphological
imaging. In the irradiated site, the tumor responses included 7
lesions with a complete response (CR; 28%), 5 with a partial
response (PR; 20%) and 13 with stable disease (SD; 52%). Moreover,
18 lesions were evaluable for a clinical response by functional
imaging as follows: 9 lesions with a CR (50%), 7 with a PR (39%)
and 2 with SD (11%) (Fig. 2). No
difference in the CLAS score was observed prior to SBRS compared
with at the first follow-up (data not shown).
Late toxicity and outcome
With a median follow-up time of 12 months (range,
8–20), no patients presented with late toxicity. Overall, 3
patients experienced local disease progression. One-year actuarial
progression-free survival in the irradiated site was 88%, while 13
patients (65%) demonstrated progressive disease in sites different
from the irradiated one.
Discussion
We describe our initial experience with radiosurgery
by VMAT. Large radiation doses were delivered to the 20 patients in
this study, and the constraints of the OARs were observed and a
simple single-arc technique was implemented (in 19/20 patients) in
<10 min. Acute toxicity was exclusively grade 1 (CTCAE 4.03).
Considering the 25 lesions, a morphological response rate of 48%
(95% CI, 24.2–70.2) and a functional response rate of 89% (95% CI,
58.6–98.7) were demonstrated.
There are few studies in the literature regarding
the use of stereotactic VMAT. The majority of these are dosimetric
studies concerned with spine (24,25),
lung (24,26–28),
brain (29,30) and adrenal metastases (31). All of these studies have
demonstrated an increased efficiency of VMAT in terms of treatment
time, with respect to 3D-conformal or IMRT techniques. A number of
these have also described improved conformity compared with 3D
techniques (26–28) and a similar (30) or higher (27,28)
conformity compared with standard IMRT techniques. Clinical studies
are less numerous and are concerned with the spine (32–34),
arteriovenous malformations (35)
and abdominal targets (36). These
preliminary studies have mainly documented the technical
feasibility of stereotactic VMAT, and all the authors have employed
this technique in fractionated treatments. To the best of our
knowledge, the present series represents the first clinical study
on radiosurgery using VMAT.
In terms of feasibility, we stress that the
dose-volume constraints were met in all patients in the current
study. The use of relatively small doses, in this first phase of
the study, likely facilitated this result. In addition, the use of
high doses was tolerated at least in terms of acute toxicity.
Moreover, the analysis of QoL- and fatigue-related indicators prior
to and following radiosurgery demonstrated that SBRS was not
associated with any detrimental effects. The low number of patients
and the short follow-up time mean that is is not possible to assess
the local control and late toxicity. However, the high index of an
immediate response, particularly if assessed with functional
imaging, and the absence of relevant toxicity should be noted.
From a practical perspective, introducing VMAT for
radio-surgery resulted in a marked reduction in the treatment time.
In our previous experience with stereotactic radiation therapy
based on non-coplanar fixed fields, a time of 45 min was reserved
for each treatment. In the present study concerning VMAT, a machine
time of only 20 min per treatment was reserved. Considering the
promising results in terms of the feasibility and the preliminary
clinical results, the study should continue with the recruitment of
additional patients to the subsequent dose levels.
References
|
1
|
Potters L, Steinberg M, Rose C, et al:
American Society for Therapeutic Radiology and Oncology and
American College of Radiology practice guidelines for the
performance of stereo-tactic body radiation therapy. Int J Radiat
Oncol Biol Phys. 60:1026–1032. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagata Y, Matsuo Y, Takayama K, et al:
Current status of stereotactic radiotherapy for lung cancer. Int J
Clin Oncol. 12:3–7. 2007. View Article : Google Scholar
|
|
3
|
Nagata Y, Negoro Y, Aoki T, et al:
Clinical outcomes of 3D conformal hypofractionated single high-dose
radiotherapy for one or two lung tumors using a stereotactic body
frame. Int J Radiat Oncol Biol Phys. 52:1041–1046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakagawa K, Aoki Y, Tago M, Terahara A and
Ohtomo K: Megavoltage CT-assisted stereotactic radiosurgery for
thoracic tumors: original reseach in the treatment of thoracic
neoplasms. Int J Radiat Oncol Biol Phys. 48:449–457. 2000.
View Article : Google Scholar
|
|
5
|
Herfarth KK, Debus J, Lohr F, et al:
Stereotactic single-dose radiation therapy of liver tumors: results
of a phase I/II trial. J Clin Oncol. 19:164–170. 2001.PubMed/NCBI
|
|
6
|
Wulf J, Guckenberger M, Haedinger U, et
al: Stereotactic radiotherapy of primary liver cancer and hepatic
metastases. Acta Oncol. 45:838–847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kavanagh BD, McGarry RC and Timmerman RD:
Extracranial radiosurgery (stereotactic body radiation therapy) for
oligometastases. Semin Radiat Oncol. 16:77–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koong AC, Christofferson E, Le QT, et al:
Phase II study to assess the efficacy of conventionally
fractionated radiotherapy followed by a stereotactic radiosurgery
boost in patients with locally advanced pancreatic cancer. Int J
Radiat Oncol Biol Phys. 63:320–323. 2005. View Article : Google Scholar
|
|
9
|
Deodato F, Macchia G, Grimaldi L, et al:
Stereotactic radiotherapy in recurrent gynecological cancer: a case
series. Oncol Rep. 22:415–419. 2009.PubMed/NCBI
|
|
10
|
Gerszten PC, Ozhasoglu C, Burton SA, et
al: CyberKnife frameless stereotactic radiosurgery for spinal
lesions: clinical experience in 125 cases. Neurosurgery. 55:89–98.
2004.PubMed/NCBI
|
|
11
|
Song Y, Zhang P, Wang P, et al: The
development of a novel radiation treatment modality-volumetric
modulated arc therapy. Conf Proc IEEE Eng Med Biol Soc.
2009:3401–3404. 2009.PubMed/NCBI
|
|
12
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events 4.03 (CTCAE 4.03).
Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAccessed
February 22, 2012.
|
|
13
|
Herfarth KK, Hof H, Bahner ML, et al:
Assessment of focal liver reaction by multiphasic CT after
stereotactic single-dose radiotherapy of liver tumors. Int J Radiat
Oncol Biol Phys. 57:444–451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaplan EL and Meier P: Non-parametric
estimation from incomplete observations. J Am Statist Assoc.
53:457–481. 1985. View Article : Google Scholar
|
|
15
|
Lax I, Blomgren H, Näslund I and Svanström
R: Stereotactic radiotherapy of malignancies in the abdomen.
Methodological aspects Acta Oncol. 33:677–683. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blomgren H, Lax I, Näslund I and Svanström
R: Stereotactic high dose fraction radiation therapy of
extracranial tumors using an accelerator. Clinical experience of
the first thirty-one patients. Acta Oncol. 34:861–870. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hurkmans CW, Cuijpers JP, Lagerwaard FJ,
et al: Recommendations for implementing stereotactic radiotherapy
in peripheral stage IA non-small cell lung cancer: report from the
Quality Assurance Working Party of the randomised phase III ROSEL
study. Radiat Oncol. 4:12009. View Article : Google Scholar
|
|
18
|
National Comprehensive Cancer Network
(NCCN): Practice Guidelines in Oncology 2.2010-Non Small Cell Lung
Cancer.
|
|
19
|
Timmerman RD: An overview of
hypofractionation and introduction to this issue of seminars in
radiation oncology. Semin Radiat Oncol. 18:215–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morganti AG, Deodato F, Zizzari S, et al:
Complexity index (COMIX) and not type of treatment predicts
undetected errors in radiotherapy planning and delivery. Radiother
Oncol. 89:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Young H, Baum R, Cremerius U, et al:
Measurement of clinical and subclinical response using
[18F]-fluorodeoxiglucose and Positron Emission Tomography: review
and 1999 EORTC recommendations. European Organisation for Research
and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer.
35:1773–1782. 1999.
|
|
23
|
Sutherland HJ, Walker P and Till JE: The
development of a method for determining oncology patients’
emotional distress using linear analogue scales. Cancer Nurs.
11:303–308. 1988.
|
|
24
|
Matuszak MM, Yan D, Grills I and Martinez
A: Clinical applications of volumetric modulated arc therapy. Int J
Radiat Oncol Biol Phys. 77:608–616. 2010.PubMed/NCBI
|
|
25
|
Kuijper IT, Dahele M, Senan S and Verbakel
WF: Volumetric modulated arc therapy versus conventional intensity
modulated radiation therapy for stereotactic spine radiotherapy: a
planning study and early clinical data. Radiother Oncol.
94:224–228. 2010. View Article : Google Scholar
|
|
26
|
McGrath SD, Matuszak MM, Yan D, Kestin LL,
Martinez AA and Grills IS: Volumetric modulated arc therapy for
delivery of hypofractionated stereotactic lung radiotherapy: A
dosimetric and treatment efficiency analysis. Radiother Oncol.
95:153–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holt A, van Vliet-Vroegindeweij C, Mans A,
Belderbos JS and Damen EM: Volumetric-modulated arc therapy for
stereotactic body radiotherapy of lung tumors: a comparison with
intensity-modulated radiotherapy techniques. Int J Radiat Oncol
Biol Phys. 81:1560–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ong CL, Verbakel WF, Cuijpers JP, Slotman
BJ, Lagerwaard FJ and Senan S: Stereotactic radiotherapy for
peripheral lung tumors: a comparison of volumetric modulated arc
therapy with 3 other delivery techniques. Radiother Oncol.
97:437–442. 2010.PubMed/NCBI
|
|
29
|
Ma Y, Li M, Yin Y, et al: Hypofractionated
stereotactic radiotherapy for brain metastases: a dosimetric and
treatment efficiency comparison between volumetric modulated arc
therapy and intensity modulated radiotherapy. Technol Cancer Res
Treat. 9:499–507. 2010.
|
|
30
|
Mayo CS, Ding L, Addesa A, Kadish S,
Fitzgerald TJ and Moser R: Initial experience with volumetric IMRT
(RapidArc) for intracranial stereotactic radiosurgery. Int J Radiat
Oncol Biol Phys. 78:1457–1466. 2010.PubMed/NCBI
|
|
31
|
Scorsetti M, Mancosu P, Navarria P, et al:
Stereotactic body radiation therapy (SBRT) for adrenal metastases:
a feasibility study of advanced techniques with modulated photons
and protons. Strahlenther Onkol. 187:238–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palma DA, van Sörnsen de Koste J, Verbakel
WF, Vincent A and Senan S: Lung Density Changes After Stereotactic
Radiotherapy: A Quantitative Analysis in 50 Patients. Int J Radiat
Oncol Biol Phys. 81:974–978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palma DA, Senan S, Haasbeek CJ, Verbakel
WF, Vincent A and Lagerwaard F: Radiological and clinical
pneumonitis after stereotactic lung radiotherapy: a matched
analysis of three-dimensional conformal and volumetric-modulated
arc therapy techniques. Int J Radiat Oncol Biol Phys. 80:506–513.
2011.
|
|
34
|
Ong CL, Palma D, Verbakel WF, Slotman BJ
and Senan S: Treatment of large stage I–II lung tumors using
stereotactic body radiotherapy (SBRT): planning considerations and
early toxicity. Radiother Oncol. 97:431–436. 2010.
|
|
35
|
Subramanian S, Srinivas C, Ramalingam K,
et al: Volumetric modulated arc-based hypofractionated stereotactic
radiotherapy for the treatment of selected intracranial
arteriovenous malformations: dosimetric report and early clinical
experience. Int J Radiat Oncol Biol Phys. 82:1278–1284. 2012.
View Article : Google Scholar
|
|
36
|
Scorsetti M, Bignardi M, Alongi F, et al:
Stereotactic body radiation therapy for abdominal targets using
volumetric intensity modulated arc therapy with RapidArc:
feasibility and clinical preliminary results. Acta Oncol.
50:528–538. 2011.
|