Introduction
Erythropoietin (Epo) is a 30-kDa glycoprotein that
functions as an important cytokine in erythrocytes. Epo is usually
produced by stromal cells of the adult kidney cortex or fetal liver
and then released into the blood, with its production initially
induced by hypoxia or hypotension (1–5). In
the bone marrow, Epo binds to the erythropoietin receptor (EpoR)
expressed in erythroid progenitor cells or undifferentiated
erythroblasts, which induces signal transduction mechanisms that
protect the undifferentiated erythrocytes from apoptosis and
promote their proliferation and differentiation.
Previous studies have demonstrated that Epo and EpoR
are not only produced or expressed in the kidneys, but also in
other cells and tissues, including macrophages (6), vascular endothelial cells (7), neurons (8), myoblasts (9), the uterus, ovaries (10) and mammary glands (11), and in various malignant tumors,
including angioblastomas (12),
meningiomas (13), uterine or
ovarian cancer (14) and breast
cancer (15). The secretion of Epo
due to hypoxia is upregulated by the induction of Epo mRNA
via hypoxia-inducible factor (HIF)-1 signaling (16).
HIF-1 contains two subunits, HIF-1α and β. The
expression of HIF-1α is altered by various conditions. HIF-1α is
ubiquitinated and proteolyzed in the proteasome under normoxic
conditions. With hypoxia, however, it is stabilized to bind with
HIF-1β to form a heterodimer, which is internalized into the
nucleus and binds with a hypoxia responsive element to induce Epo
transcription. HIF-1β is constitutively expressed.
HIF-1 contributes to hypoxia adaptation at the
tissue or cellular level. At the tissue level, it upregulates the
expression levels of Epo and vascular endothelial growth factor
(VEGF), which promote hematopoiesis and angiogenesis. At the
cellular level, HIF-1 regulates cellular metabolism by the
upregulation of glycolytic enzymes and glucose transporters, which
shifts the energy metabolism to oxygen-independent glycolysis.
Dysfunction in these pathways may be associated with the onset or
progression of cancer (17), e.g.,
the progression of tumor angiogenesis by VEGF overexpression may be
correlated with tumor infiltration or metastasis (18). Numerous anti-angiogenesis therapies
against HIF-1 or VEGF have been developed and tested (19).
Epo binds to EpoR, resulting in the activation of
the janus kinase-signal transducer and activator of transcription
(JAK-STAT) signal transduction pathway (20). JAK2 phosphorylates STAT5, which is
internalized into the nucleus to induce the transcription of
specific genes (21). The
proliferation signaling pathways, including the mitogen-activated
protein kinase (MAPK) and phosphatidylinositol 3-kinase-Ak-thymoma
(PI3K-AKT) pathways, which are involved in tumor proliferation, are
also activated (22). A previous
study demonstrated that EpoR was expressed in kidney tissues
removed by nephrectomy or in renal cell carcinoma (RCC) cell lines,
and that the proliferation rate of these cells increased with the
addition of Epo in a dose-dependent manner (23). Furthermore, RCC patients with high
expression levels of EpoR in the kidney tissues and high serum Epo
(s-Epo) concentrations (>30 mU/ml) had better 5-year survival
rates than those with low EpoR levels and s-Epo concentrations
(24).
In the present study, five RCC cell lines were
characterized and two cell lines in which Epo and EpoR were highly
expressed were identified. The comparative study of these two cell
lines revealed that the induction of Epo may accelerate cellular
proliferation in either a HIF-1α-dependent or -independent manner.
The possibility of using Epo as a promising target for anti-RCC
drugs was also addressed.
Material and methods
Materials
The monoclonal anti-HIF-1α antibody was purchased
from Invitrogen (Carlsbad, CA, USA) and the anti-Epo antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The siRNAs against HIF-1α or Epo were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The 769P (renal cell adenocarcinoma), 786O (renal
cell adenocarcinoma) and Ku19/20 cells (RCC) were maintained in
RPMI-1640. The SKRC44 cells (RCC) were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) and the Caki-1 cells (RCC) were
grown in minimal essential medium (MEM) in a humidified atmosphere
containing 5% CO2 in the air (normoxia) (25). Unless stated otherwise, the media
were supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 μg/ml streptomycin. The study was
approved by the Ethics Committee of Osaka Medical College,
Takatsuki, Osaka, Japan (569-0801).
Real-time PCR
Total RNA was prepared from each of the five cell
lines using TRIzol reagent (Invitrogen), according to the
manufacturer’s instructions. Real-time PCR analyses were performed
using a Thermal Cycler Dice Real Time System (Takara Bio, Shiga,
Japan). The cDNA was synthesized using Superscript III reverse
transcriptase (Invitrogen), according to the manufacturer’s
instructions. Each reaction was performed in a total volume of 25
μl, with 1× SYBR Premix Ex Taq polymerase, 200 nM primers, 2
μl of a 1:10 dilution of the cDNA and RNase-free water. The
thermal cycling conditions for the real-time PCR were; 10 sec at
95°C to activate the SYBR Ex Taq polymerase, followed by 40 cycles
of denaturation for 5 sec at 95°C and annealing/extension for 20
sec at 60°C. The mean number of cycles required to reach the
threshold of fluorescence detection was calculated for each sample
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
expression was quantified for normalization of the amount of cDNA
in each sample. The specificity of the amplified product was
monitored using its melting curve. The primers used in the present
study are as follows: Epo, forward primer,
5′-CCCTGTTGGTCAACTCTTCC-3′ and reverse primer,
5′-GTGTACAGCTTCAGCTTTCC-3′; EpoR, forward primer,
5′-GCACCGAGTGTGTGCTGAGCAA-3′ and reverse primer,
5′-GGTCAGCAGCACCAGGATGAC-3′; GAPDH, forward primer,
5′-ATTGCCCTCAACGACCACTT-3′ and reverse primer,
5′-AGGTCCACCACCCTGTTGCT-3′ (26,27)
Cell proliferation assay [water-soluble
tetrazolium salt-1 (WST-1) assay]
Cell proliferation under normoxic or hypoxic
conditions was measured using Cell Counting Kit-8 (Wako Pure
Chemicals, Osaka, Japan). Briefly, the cells were seeded at a
density of 1×104 per well in 96-well plates and allowed
to attach overnight in appropriate serum-containing media. The
cells were rendered quiescent by starvation in serum-free media for
24 h. FBS was used as a positive control at 10% of the total media
volume. Various Epo doses (0.1, 0.33, 1, 3.3 and 10 ng/ml) were
then added and incubation was continued for a further 24 h
(28). The cellular proliferation
was measured by adding a 1/10 volume of WST-1. Following a 1-h
incubation with WST-1, the absorbance at 450 nm was recorded using
a plate reader (Bio-Rad, Hercules, CA, USA).
HIF-1α induction
For HIF-1α induction, the cells were incubated with
125 μM of cobalt chloride (CoCl2) or were
cultured in a hypoxia chamber (Stemcell Technologies, Vancouver,
Canada), which was adjusted to 5% O2 and 5%
CO2 for the period of interest.
Western blot analysis
The cells were harvested in lysis buffer containing
20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM
ethylenediaminetetraacetic acid (EDTA), 1% (w/v) Nonidet P-40, 10%
(w/v) glycerol, 5 mM sodium pyrophosphate, 10 mM sodium fluoride, 1
mM sodium orthovanadate, 10 mM β-glycerophosphate, 1 mM
phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 5
μg/ml leupeptin, and 1 mM dithiothreitol. In total, five
micrograms of the cellular proteins (50 μg for Epo
detection) were electrophoresed and blotted onto a polyvinylidene
difluouride (PVDF) membrane (FluoroTrans, Pall, Pensacola, FL,
USA). Subsequent to blocking with 5% skimmed milk in Tris-buffered
saline (TBS; 25 mM Tris, 0.15 M NaCl, pH 7.4) containing 0.1%
Tween-20 (TBS-T) for 1 h, the membrane was incubated with a 1:1000
dilution of each antibody for a further hour. The membrane was
washed three times with TBS-T for 10 min each, then the second
antibody was added at a dilution of 1:2500. Following another three
washes with TBS-T for 10 min each, the membrane was developed with
Immobilon Western developer (Merck Millipore, Billerica, MA, USA)
according to the manufacturer’s instructions. The image was
captured using a ChemiDoc imaging system (Bio-Rad).
Knock-down of HIF-1α or Epo using siRNA
in Caki-1 and SKRC44 cells
The siRNA was transfected into Caki-1 and SKRC44
cells using Lipofectamine 2000 (Invitrogen), following the
manufacturer’s instructions. Briefly, 50 pmol siRNA in 100
μl Opti-MEM was mixed with 3 μl of Lipofectamine 2000
diluted in 100 μl Opti-MEM. The mixture was allowed to stand
for 20 min and then was added to the cultured cells to incubate
overnight in 1 ml antibiotic-free medium in a 12-well plate. After
48 h, the cells were utilized for the cell proliferation assays and
in the determination of the HIF-1α and Epo mRNA
expression levels.
Statistical analysis
For comparisons between the two groups, the paired
Student’s t-test was used to compare the significance of the
differences between data. When more than two groups were compared,
a one-way analysis of variance (ANOVA) was used followed by
multiple comparisons using Dunnett’s test. P<0.05 was considered
to indicate a significant difference. All data are expressed as the
mean ± standard deviation (SD).
Results
Characterization of RCC cell lines
The mRNA expression levels of Epo and its
receptor, EpoR, were evaluated in five RCC cell lines; 769p,
786O, Ku19/20, Caki-1 and SKRC44. Real-time PCR confirmed that the
Epo expression was significantly higher in the 786O,
Ku19/20, Caki-1 and SKRC44 cells, compared to the 769P cells. In
contrast, the Epo-R expression was significantly higher in
the Caki-1 and SKRC44 cells, but was unchanged in the 786O and
Ku19/20 cells (Fig. 1). Next,
following the addition of the Epo protein, cellular proliferation
was analyzed using the WST-1 cell proliferation assay. Of note was
the fact that the SKRC44 and Caki-1 cells grew more rapidly in the
presence of Epo, while the proliferation of the other cells
remained unchanged by the addition of Epo (Fig. 2).
Effects of CoCl2 treatment or
hypoxia on the induction of HIF-1α protein expression in Caki-1 and
SKRC44 cells
Since it was recently reported that RCC patients
with high expression levels of Epo and EpoR have a poor prognosis
(24), we selected Caki-1 and
SKRC44 cells, the two cell lines in which Epo and EpoR are highly
expressed, for further characterization of the mechanisms
responsible for this prognosis. As Epo expression is regulated by
HIF-1α, the expression level of the HIF-1α protein was evaluated in
each cell line. To induce HIF-1α, CoCl2, which is known
to stabilize HIF-1α, was first exploited by interfering with its
prolyl hydroxylation. HIF-1α was induced by CoCl2
treatment in the Caki-1 cells in a time-dependent manner. In
contrast, the HIF-1α protein expression was not increased in the
SKRC44 cells, even following 12-h incubations with CoCl2
(Fig. 3A).
 | Figure 3Cobalt chloride (CoCl2)-
or hypoxia-induced hypoxia inducible factor (HIF)-1α expression in
Caki-1 and SKRC44 cells (A) For HIF-1α induction, the cells were
incubated with 125 μM CoCl2. HIF-1α and β-actin
protein levels were detected by western blot analysis of whole-cell
extracts, as described in the Materials and methods, and were
measured at 0, 3 and 12 h subsequent to incubation. (B) Caki-1 and
SKRC44 cells were exposed to hypoxia for 8 h and measured at 0, 1,
2, 4, 6 and 8 h. Hypoxia induces Hif-1α expression in Caki-1 cells,
but not in SKRC44 cells. HIF-1α and β-actin protein levels were
detected by western blot analysis of whole-cell extracts, as
described in the Materials and methods. IB, immunoblot. |
To further confirm the HIF-1α expression patterns in
the Caki-1 and SKRC44 cells, the cells were cultured under hypoxic
conditions for 0, 1, 2, 4, 6 and 8 h. HIF-1α protein was induced
under hypoxic conditions, as well as with CoCl2
treatment, in the Caki-1 cells, but not in the SKRC44 cells
(Fig. 3B). As shown in Fig. 1B, the Epo mRNA was highly
expressed in the Caki-1 and SKRC44 cells. Despite the high
expression level of Epo, HIF-1α protein expression was not
induced following CoCl2 treatment or with hypoxic
conditions in the SKRC44 cells.
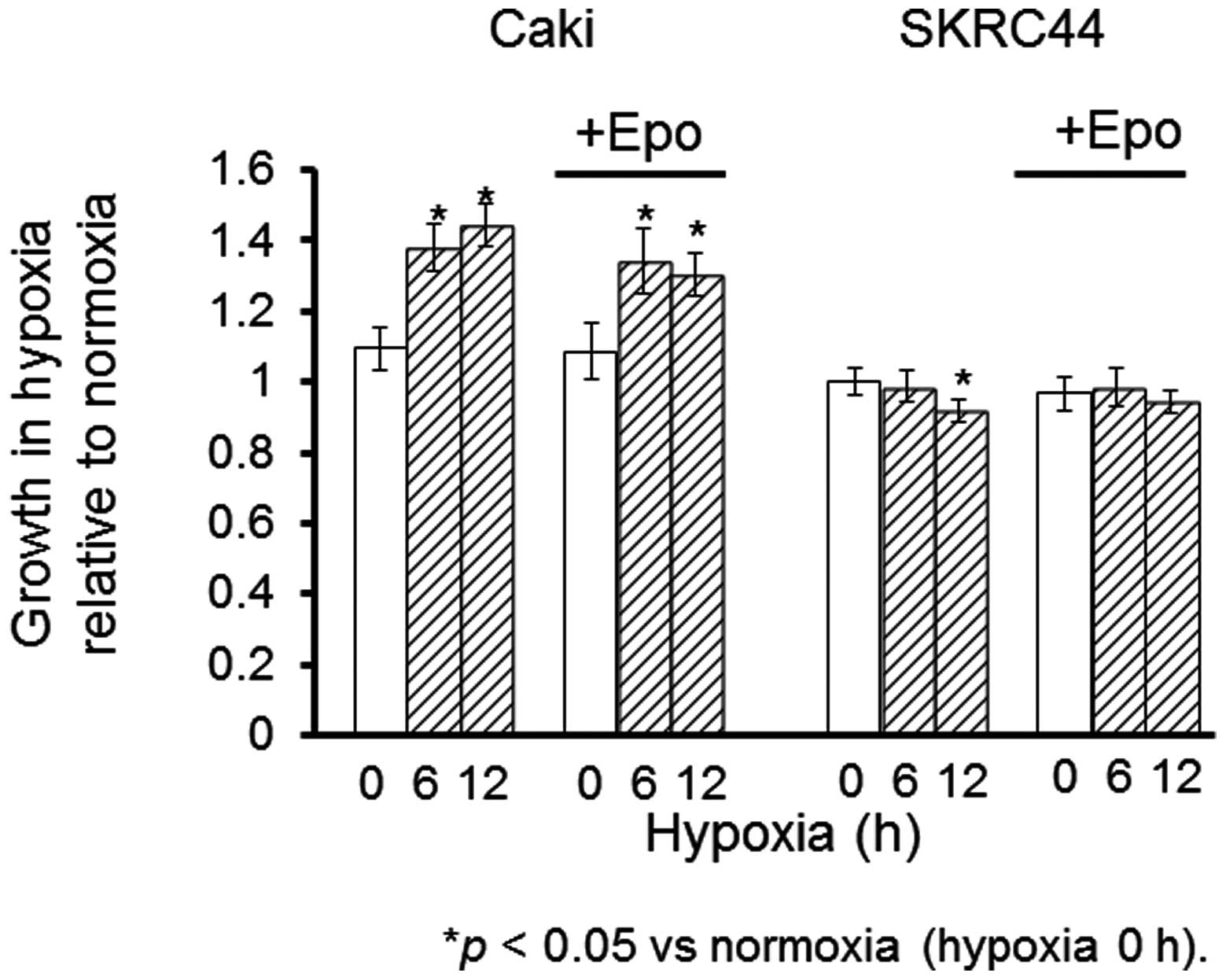
Effects of hypoxia on cell proliferation
in Caki-1 and SKRC44 cells
Tthe proliferation rates were measured in the Caki-1
and SKRC44 cells under hypoxic conditions for 6 or 12 h with or
without exogenous Epo (0.1 ng/ml). Epo stimulated the proliferation
of the Caki-1 cells under normoxia; this increase was maintained
under hypoxia. In contrast, the proliferation rate of the SKRC44
cells was not changed by the addition of Epo under hypoxia.
Instead, this rate was significantly decreased under hypoxia for 12
h relative to the rate at normoxia. Exogenous Epo reversed the
suppression of proliferation in the SKRC44 cells under hypoxic
conditions (Fig. 4).
Effects of Epo or HIF-1α silencing on the
proliferation of SKRC44 and Caki-1 cells
The proliferation rate was significantly increased
under hypoxia in the SKRC44 and Caki-1 cells. To elucidate whether
HIF-1α or Epo was involved in this increased proliferation rate
under normoxia or hypoxia in the cells, HIF-1α or Epo were knocked
down using siRNA. It is well-known that HIF-1α induces the
expression of various molecules associated with cell proliferation,
including Epo. Epo expression was significantly decreased with the
use of siRNA against Epo, but not with siRNA against HIF-1α in the
SKRC44 cells, while the Epo expression was significantly decreased
with the siRNAs against Epo and HIF-1α in the Caki-1 cells (data
not shown).
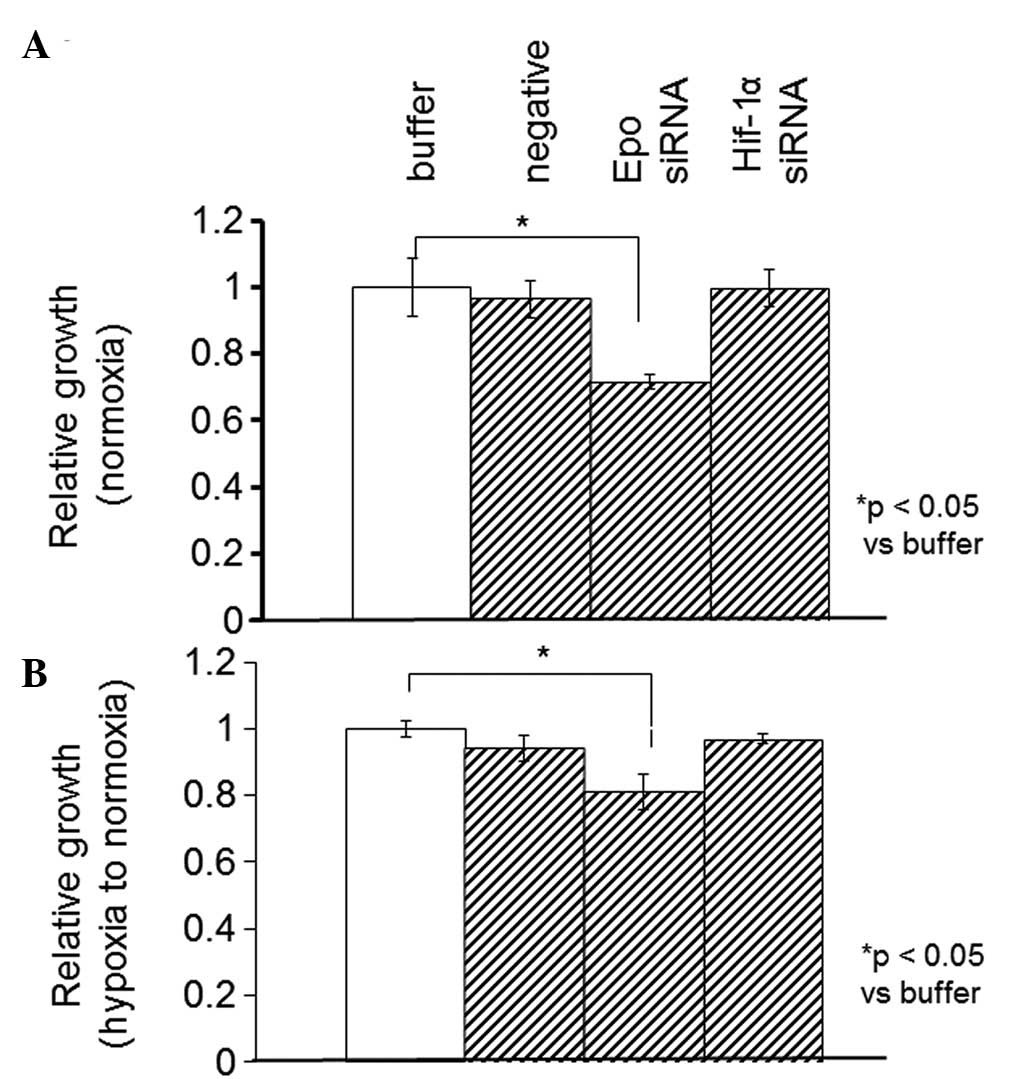
Using the WST-1 assay, the proliferation rate was
measured in the SKRC44 or Caki-1 cells 12 h after the addition of
the control buffer (10 mM Tris, 20 mM NaCl and 1 mM EDTA; pH 8.0),
negative siRNA (Mission siRNA Universal Negative Controls,
Sigma-Aldrich) or siRNAs against Epo or HIF-1α under normoxia or
hypoxia. In the SKRC44 cells, the proliferation rate when using the
siRNA against Epo was significantly decreased compared with the
rate following the addition of the control buffer under both
normoxic and hypoxic conditions. The proliferation rate was
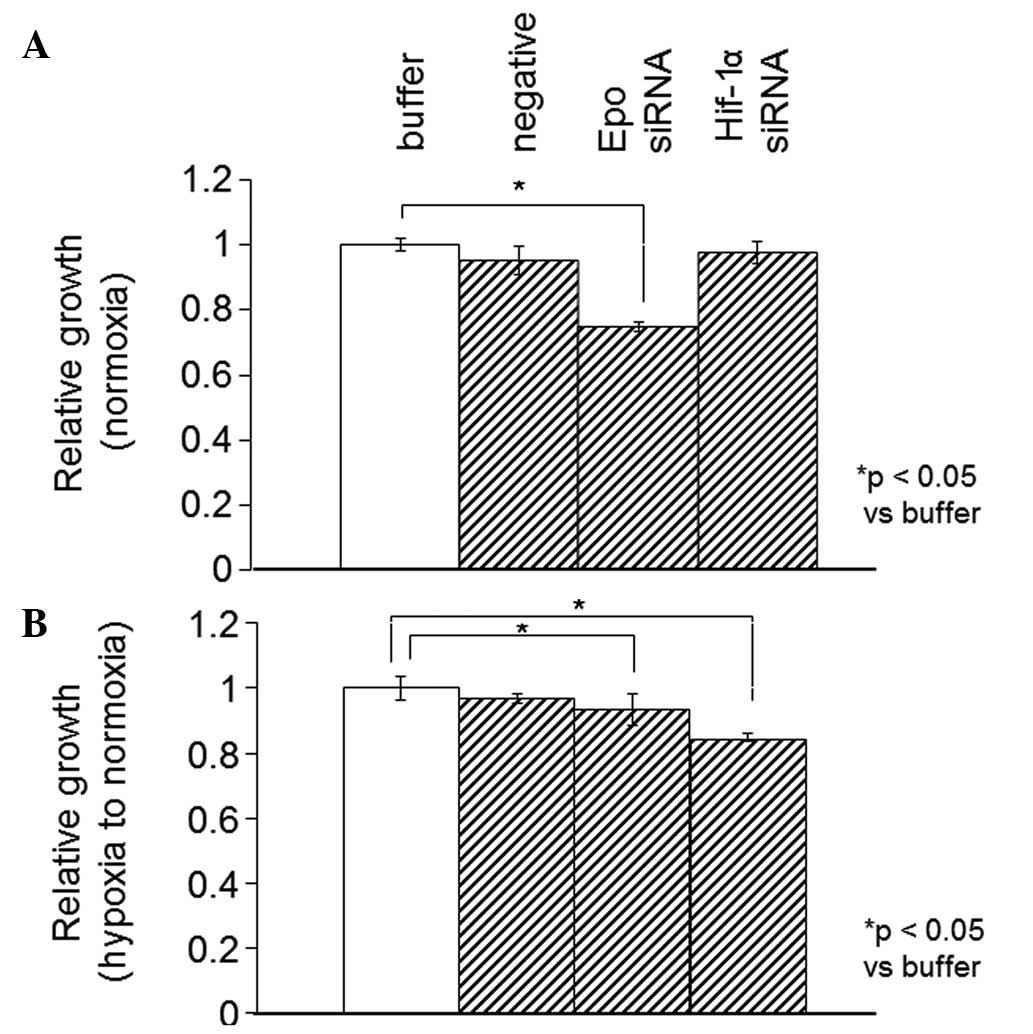
unchanged by the siRNA against HIF-1α (Fig. 5). In the Caki-1 cells, the Epo
expression level was significantly decreased by the siRNAs against
Epo and HIF-1α (data not shown). The WST-1 proliferation assay was
also performed 12 h subsequent to the same treatments, as described
previously for the SKRC44 cells under normoxia or for hypoxia in
the Caki-1 cells. In the Caki-1 cells, the proliferation rate when
using the siRNA against Epo was significantly decreased under
normoxia and hypoxia. Under hypoxia, however, this rate was
significantly decreased with the siRNAs against Epo and HIF-1α
(Fig. 6).
Discussion
It has been previously demonstrated that EpoR, the
receptor through which Epo stimulates mitogenesis, is expressed in
human RCC tissue and cell lines (23). The co-expression of Epo and EpoR has
been detected in numerous renal cysts, providing further evidence
that renal cysts are potential precursors to RCC. In conjunction
with von Hippel-Lindau (VHL) gene deficiency, the co-expression of
Epo and EpoR in renal cysts and tumors may reflect developmental
arrest in immature mesenchymal cells. Such arrest may lead to
autocrine stimulation, cellular proliferation and renal tumor
development (26).
In the present study, Epo and EpoR were each
identified to be highly expressed in Caki-1 and SKRC44 cells, and
the proliferation rate in these two cell lines was observed to be
increased in the presence of Epo.
The expression of Epo in RCC and renal cysts may
result from VHL gene deficiency through the HIF-1 pathway (27,28).
EpoR expression normally occurs during the angioblast stage of
embryonic development as part of the response to hypoxia. During
normal development, cellular EpoR expression is transient (29). Continuous high expression of Epo and
EpoR in primitive mesenchymal cells may lead to cellular
proliferation via autocrine stimulation and become a critical
pathogenic step in tumor formation (30). Therefore, the co-expression of Epo
with EpoR in VHL-associated RCC and renal cysts suggests that a
precursor cell of renal lesions is a developmentally arrested,
pluripotent embryonic cell derived from nephrogenous mesenchyme
(26).
HIF-1α is a well-known transcriptional inducer of
survival proteins, such as Epo (31). Epo, a downstream protein of HIF-1α,
has been observed to counteract the hypoxia-induced apoptosis of
breast cancer cells (32). This
association was demonstrated by the correlations between the
degrees of immunohistochemical expression of HIF-1 and Epo in
previous studies (33).
To examine whether HIF-1α is involved in the
expression of Epo, the HIF-1α expression levels in the Caki-1 and
SKRC44 cells were determined by western blot analyses. HIF-1α
protein expression was increased under hypoxic conditions in the
Caki-1 cells, but not in the SKRC44 cells (Fig. 3); although the expression level of
Epo and EpoR in normoxic conditions was higher in the SKRC44 cells
compared with the Caki-1 cells (Fig.
1). These observations indicate that Epo and EpoR expression
may not be induced by HIF-1α in SKRC44 cells. As Caki-1 and SKRC44
cells are each derived from clear cell carcinomas, the high Epo and
EpoR expression levels may be either dependent or independent of
HIF-1α in clear cell carcinoma cell lines.
The overexpression of HIF-1α has been reported in
numerous human cancers, including colon, brain, breast, gastric,
lung, skin, ovarian, prostate, renal and pancreatic carcinoma, and
is associated with a poor prognosis and failure of tumor treatment
(34). In tumor cells, HIF-1α may
also be regulated by other genetic factors, including oncogenes
(Ras and PI3-K) or the loss of tumor suppressors
[VHL or phosphatase and tensin homolog (PTEN)] even
under aerobic conditions. Therefore, the inhibition of HIF-1α may
represent an attractive strategy with synergistic potential when
used with other therapies (35,36).
We next measured the proliferation rates in the
Caki-1 and SKRC44 cells under hypoxia with or without exogenous Epo
(Fig. 4). The proliferation rate
was significantly increased under hypoxia in the Caki-1 cells in
which HIF-1α was inducible, while it was decreased in the SKRC44
cells in which HIF-1α expression was not increased. To elucidate
the involvement of HIF-1α in increasing the proliferation of the
Caki-1 and SKRC44 cells under hypoxic conditions, HIF-1α was
knocked out using siRNA. The proliferation rate of the Caki-1 cells
was significantly decreased by the siRNA against HIF-1α, although
it remained unchanged in the SKRC44 cells (Figs. 5 and 6). These results indicated that HIF-1α may
be partially implicated in the progression of RCC.
Epo expression levels are regulated by pVHL, which
has been shown to control the stability of HIF-1 through
ubiquitination and proteasomal degradation mechanisms (37,38).
Upregulated HIF-1α stimulates Epo expression in tumors, indicating
that the VHL-HIF-Epo pathway may be significant in controlling the
proliferation of RCC cells (39).
The present study demonstrated that Epo expression was independent
of this pathway; therefore, blocking this pathway alone may not be
sufficient to inhibit Epo expression. Thus, siRNA against Epo was
transfected into the Caki-1 and SKRC44 cells. The proliferation of
the two cell lines was significantly decreased by the siRNA against
Epo (Figs. 5 and 6). This observation indicated that Epo may
be implicated in the progression of RCC. Since Caki-1 and SKRC44
cells are RCC-derived cells, HIF-dependent and-independent Epo
expression may control the proliferation of RCC cells. Regardless
of the mechanism by which HIF-1α is expressed, the RCC progression
rate under normoxia and hypoxia could be reduced by modulating the
expression level of Epo.
Recently, a number of molecular target based drugs
have been developed for the treatment of RCC. Tyrosine kinase
inhibitors, including sunitinib and sorafenib, are representative
of such agents that inhibit the activity of VEGF-mediated signal
transduction in cancer cells. Everolimus, an inhibitor of the
mammalian target of rapamycin (mTOR), has been shown to be
effective for the treatment of advanced RCC following treatment
failure with the first-line drugs sunitinib or sorafenib.
Everolimus reduces cellular proliferation, angiogenesis and glucose
uptake via the inhibition of HIF-1α expression, which upregulates
VEGF-mediated signal transduction in cancer cells (40,41).
In the present study, we demonstrated that the
induction of Epo in a HIF-1-dependent and -independent manner
increases the cellular proliferation rate in RCC cell lines.
Notably, it has been reported that the proliferation rate of the
RCC cells with HIF-1-independent Epo overexpression was not fully
reduced by everolimus. Elucidation of the mechanism of
HIF-1-independent Epo induction in RCC may lead to the
identification of a new molecular target candidate for RCC therapy,
particularly in clear cell carcinoma. Although further study is
required to identify the involvement of the HIF-Epo pathway in RCC
pathogenesis, the pathway may be a new molecular therapeutic target
candidate for the treatment of RCC, particularly in advanced
stages.
Abbreviations:
|
Epo
|
erythropoietin;
|
|
EpoR
|
erythropoietin receptor;
|
|
RCC
|
renal cell carcinoma;
|
|
HIF-1α
|
hypoxia-inducible factor-1α;
|
|
VHL
|
von Hippel-Lindau
|
Acknowledgements
This study was supported, in part, by
a grant-in-aid for Scientific Research (C; No. 17590249) from the
Japan Society for the Promotion of Science (M.A).
References
|
1
|
Koury ST, Bondurant MC, Koury MJ and
Semenza GL: Localization of cells producing erythropoietin in
murine liver by in situ hybridization. Blood. 77:2497–2503.
1991.PubMed/NCBI
|
|
2
|
Krantz SB: Erythropoietin. Blood.
77:419–434. 1991.PubMed/NCBI
|
|
3
|
Youssoufian H, Longmore G, Neumann D, et
al: Structure, function, and activation of the erythropoietin
receptor. Blood. 81:2223–2236. 1993.PubMed/NCBI
|
|
4
|
Sasaki R, Masuda S and Nagao M:
Erythropoietin: multiple physiological functions and regulation of
biosynthesis. Biosci Biotechnol Biochem. 64:1775–1793. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winkelmann JC: The human erythropoietin
receptor. Int J Cell Cloning. 10:254–261. 1992. View Article : Google Scholar
|
|
6
|
Vogt C, Pentz S and Rich IN: A role for
the macrophage in normal hemopoiesis: III. In vitro and in vivo
erythropoietin gene expression in macrophages detected by in situ
hybridization. Exp Hematol. 17:391–397. 1989.PubMed/NCBI
|
|
7
|
Anagnostou A, Liu Z, Steiner M, et al:
Erythropoietin receptor mRNA expression in human endothelial cells.
Proc Natl Acad Sci USA. 91:3974–3978. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masuda S, Nagao M, Takahata K, et al:
Functional erythropoietin receptor of the cells with neural
characteristics. Comparison with receptor properties of erythroid
cells. J Biol Chem. 268:11208–11216. 1993.
|
|
9
|
Ogilvie M, Yu X, Nicolas-Metral V, et al:
Erythropoietin stimulates proliferation and interferes with
differentiation of myoblasts. J Biol Chem. 275:39754–39761. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasuda Y, Masuda S, Chikuma M, et al:
Estrogen-dependent production of erythropoietin in uterus and its
implication in uterine angiogenesis. J Biol Chem. 273:25381–25387.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Juul SE, Zhao Y, Dame JB, et al: Origin
and fate of erythropoietin in human milk. Pediatr Res. 48:660–667.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trimble M, Caro J, Talalla A and Brain M:
Secondary erythrocytosis due to a cerebellar hemangioblastoma:
demonstration of erythropoietin mRNA in the tumor. Blood.
78:599–601. 1991.PubMed/NCBI
|
|
13
|
Bruneval P, Sassy C, Mayeux P, et al:
Erythropoietin synthesis by tumor cells in a case of meningioma
associated with erythrocytosis. Blood. 81:1593–1597.
1993.PubMed/NCBI
|
|
14
|
Yasuda Y, Musha T, Tanaka H, et al:
Inhibition of erythropoietin signalling destroys xenografts of
ovarian and uterine cancers in nude mice. Br J Cancer. 84:836–843.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Acs G, Zhang PJ, Rebbeck TR, et al:
Immunohistochemical expression of erythropoietin and erythropoietin
receptor in breast carcinoma. Cancer. 95:969–981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Hypoxia-inducible factor 1 and
the molecular physiology of oxygen homeostasis. J Lab Clin Med.
131:207–214. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semenza GL: Hypoxia and cancer. Cancer
Metastasis Rev. 26:223–224. 2007. View Article : Google Scholar
|
|
18
|
Du R, Lu KV, Petritsch C, et al: HIF1alpha
induces the recruitment of bone marrow-derived vascular modulatory
cells to regulate tumor angiogenesis and invasion. Cancer Cell.
13:206–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Powis G and Kirkpatrick L: Hypoxia
inducible factor-1alpha as a cancer drug target. Mol Cancer Ther.
3:647–654. 2004.PubMed/NCBI
|
|
20
|
Ihle JN, Witthuhn BA, Quelle FW, et al:
Signaling by the cytokine receptor superfamily: JAKs and STATs.
Trends Biochem Sci. 19:222–227. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mulcahy L: The erythropoietin receptor.
Semin Oncol. 28(2 Suppl 8): 19–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leyland-Jones B: Trastuzumab: hopes and
realities. Lancet Oncol. 3:137–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Westenfelder C and Baranowski RL:
Erythropoietin stimulates proliferation of human renal carcinoma
cells. Kidney Int. 58:647–657. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito K, Yoshii H, Asano T, et al: Impact of
increased erythropoietin receptor expression and elevated serum
erythropoietin levels on clinicopathological features and prognosis
in renal cell carcinoma. Exp Ther Med. 3:937–944. 2012.
|
|
25
|
Laugsch M, Metzen E, Svensson T, et al:
Lack of functional erythropoietin receptors of cancer cell lines.
Int J Cancer. 122:1005–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YS, Vortmeyer AO, Lubensky IA, et al:
Coexpression of erythropoietin and erythropoietin receptor in von
Hippel-Lindau disease-associated renal cysts and renal cell
carcinoma. Clin Cancer Res. 11:1059–1064. 2005.PubMed/NCBI
|
|
27
|
Maxwell PH and Ratcliffe PJ: Oxygen
sensors and angiogenesis. Semin Cell Dev Biol. 13:29–37. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaelin WG Jr: Molecular basis of the VHL
hereditary cancer syndrome. Nat Rev Cancer. 2:673–682. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee R, Kertesz N, Joseph SB, et al:
Erythropoietin (Epo) and EpoR expression and 2 waves of
erythropoiesis. Blood. 98:1408–1415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mitjavila MT, Le Couedic JP, Casadevall N,
et al: Autocrine stimulation by erythropoietin and autonomous
growth of human erythroid leukemic cells in vitro. J Clin Invest.
88:789–797. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang GL and Semenza GL: General
involvement of hypoxia-inducible factor 1 in transcriptional
response to hypoxia. Proc Natl Acad Sci USA. 90:4304–4308. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Acs G, Chen M, Xu X, et al: Autocrine
erythropoietin signaling inhibits hypoxia-induced apoptosis in
human breast carcinoma cells. Cancer Lett. 214:243–251. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wincewicz A, Koda M, Sulkowska M, et al:
STAT3 and hypoxia induced proteins - HIF-1alpha, EPO and EPOR in
relation with Bax and Bcl-xL in nodal metastases of ductal breast
cancers. Folia Histochem Cytobiol. 47:425–430. 2009.PubMed/NCBI
|
|
34
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
35
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McCarty MF: Barroso-Aranda J and Contreras
F: Practical strategies for suppressing hypoxia-inducible factor
activity in cancer therapy. Med Hypotheses. 74:789–797. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Turner KJ, Moore JW, Jones A, et al:
Expression of hypoxia-inducible factors in human renal cancer:
relationship to angiogenesis and to the von Hippel-Lindau gene
mutation. Cancer Res. 62:2957–2961. 2002.PubMed/NCBI
|
|
38
|
Ohh M, Park CW, Ivan M, et al:
Ubiquitination of hypoxia-inducible factor requires direct binding
to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol.
2:423–427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong K, Zhang N, Zhang K and Na Y: The
relationship of erythropoietin overexpression with von
Hippel-Lindau tumour suppressor gene mutations between
hypoxia-inducible factor-1α and -2α in sporadic clear cell renal
carcinoma. Int J Mol Med. 26:907–912. 2010.PubMed/NCBI
|
|
40
|
Motzer RJ, Escudier B, Oudard S, et al:
Efficacy of everolimus in advanced renal cell carcinoma: a
double-blind, randomized, placebo-controlled phase III trial.
Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miyazaki M, Yasuda M, Fujita M, et al:
Therapeutic strategy targeting the mTOR–HIF-1α–VEGF pathway in
ovarian clear cell adenocarcinoma. Path Int. 59:19–27. 2009.
|