Introduction
Pancreatic cancer is an aggressive malignant disease
due to the lack of an early diagnosis and treatment options, and as
such is the fourth leading cause of cancer mortality worldwide
(1). Metastasis occurs frequently
in the early stages of tumor development. In total, ∼75% of
pancreatic cancer patients are diagnosed with the unresectable form
of the disease (2). At present,
gemcitabine, a standard first-line treatment for advanced
pancreatic cancer, is extensively utilized, but offers only a
modest benefit due to acquired chemoresistance and multiple adverse
effects (3). In this context, the
development of new effective therapeutic approaches for pancreatic
cancer remains one of the most challenging goals in cancer
research.
Numerous tropical plants have interesting biological
activities with potential therapeutic applications. Traditional
uses and later scientific findings have suggested that mangosteen
(Garcinia mangostana, Linn; GML) is a potential candidate
for an anticancer agent. GML belongs to the Guttiferae
family and is termed ‘the queen of fruits’; it exerts a wide
variety of pharmacological activities including antioxidant,
antitumor and anti-inflammatory actions (4–6).
Phytochemical studies have shown that the xanthones, as
characteristic secondary metabolites of GML, are associated with
these therapeutic roles (7). Of
these, α-, β- and γ-mangostin are the most abundant
representatives, as well as the most studied. The biological
activities of α-mangostin, which have been demonstrated to include
anti-proliferative and apoptotic effects on various cancer cells,
including human leukemia and lung, colon and breast cancer
(8–11), are the most significant. Although
the anti-proliferative role of α-mangostin in malignant diseases
has been increasingly recognized, its function in pancreatic cancer
cells remains undetermined. The role of α-mangostin in inhibiting
the invasion of pancreatic cancer has been particularly
elusive.
Invasion and metastasis occurs through a complex,
multistep process, which involves the invasion of cells from
primary tumors into the circulation, migration of these cells to
distant organs, adhesion to endothelial cells and infiltration into
tissues. Matrix metalloproteinase (MMP)-2 and MMP-9 are presumed to
be associated with the progression and invasion of various types of
cancer cells and are highly expressed in pancreatic cancer.
E-cadherin expression in pancreatic cancer is significantly lower
than in normal pancreatic tissue and has been associated with lymph
node and liver metastasis (12,13).
It has been suggested that metastasis is responsible for the
majority of failures in cancer treatment and is the major cause of
mortality in patients with various cancer types. Therefore, in
addition to minimizing the growth of existing tumors, treatments
that limit the spread of cancer cells and block invasion have been
pursued to enhance the survival of cancer patients. It was
previously reported in various other cancer types that α-mangostin
inhibited invasion by modulating MMP expression (9,14). The
purpose of the present study was to demonstrate the effect of
α-mangostin in inhibiting the invasion of pancreatic cancer cell
lines. The molecular mechanisms of α-mangostin were also
investigated in human pancreatic cancer cell invasion and
metastasis.
Materials and methods
Reagents
α-mangostin (purity >98%) was acquired from
ChromaDex Inc. (Irvine, CA, USA). Dimethylsulfoxide (DMSO) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma Chemicals (St. Louis, MO, USA).
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum
(FBS) were purchased from HyClone (Logan, UT, USA). Millicell
culture plate inserts were purchased from Millipore (Bedford, MA,
USA). Matrigel and the One-Step RT-PCR kit were purchased from BD
Biosciences (Bedford, MA, USA). The antibodies against ERK,
E-cadherin, MMP-9, MMP-2 and β-actin were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). All drug solutions were
freshly prepared on the day of testing.
Cell cultures and treatments
The human pancreatic cancer cell lines, BxPC-3 and
MIAPaCa-2, were obtained from the American Type Culture Collection
(Manassas, VA, USA). The study was approved by the Ethics Committee
of Yan’an University, Yan’an, China. The cells were cultured in
DMEM containing 10% dialyzed heat-inactivated FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin in a humidified
atmosphere of 5% CO2 at 37°C. In the invasion and
migration assays, the cells were cultured in DMEM without FBS. A
100 mM solution of α-mangostin was prepared in DMSO and stored at
−20°C. For the treatment, this solution was diluted in DMEM and
added to the culture medium to the desired final concentration.
Untreated cultures received an equivalent amount of the solvent
(DMSO, 0.1%).
Proliferation assay
Cell proliferation was determined by the MTT uptake
method. BxPC-3 and MIAPaCa-2 cells were seeded onto 96-well plates
at a density of 1×104 cells per well and incubated
overnight in 10% FBS medium. The cells were then incubated with
various concentrations of α-mangostin in 0.1% DMSO. The cells that
were treated with 0.1% DMSO alone were designated as the control
group. Following incubation for 6, 12, 18, 24 and 48 h at 37°C, 15
μl MTT solution (5 mg/ml in phosphate-buffered saline; PBS)
was added to each well and the cells were incubated for 4 h at
37°C. A total of 100 μl DMSO was then added to each well at
37°C. The optical density (OD) value at 490 nm was determined with
a spectrophotometer (Bio-Rad, CA, USA). The results were calculated
as the percentages relative to the controls. The proliferation
inhibition rate = (1 − ODsample / ODcontrol)
× 100.
Cell migration assay
The cell migratory ability was evaluated with a
wound-healing assay. Pancreatic cancer cells were seeded onto
24-well plates (1.0×105 cells/500 μl) and grown
to 90% confluency. A wound line was then made between the confluent
cells using sterile plastic pipette tips, and any cellular debris
was removed by washing with PBS. The wounded monolayers were then
incubated in the absence or presence of lipopolysaccharide (LPS; 5
μg/ml) or α-mangostin (5 μM) for 24 h and images were
digitally captured. The migration area was measured using Image-Pro
Plus 5.0 (Media Cybernetics Inc., Rockville, MD, USA).
Cell invasion assay
The invasion of the pancreatic cancer cells was
investigated using Transwell chambers. The Millicell culture plate
filter inserts (8-μm pore size, with a
polyvinyl/pyrrolidone-free polycarbonate membrane) were coated with
100 μl 1:10 diluted matrigel (5 mg/ml in cold DMEM). A thin
continuous film was then formed on the top of the filter at 37°C
for 4 h. The pancreatic cancer cells were detached from the culture
flask and suspended in serum-free DMEM supplemented with 0.1%
bovine serum albumin (BSA) and/or LPS/α-mangostin. The cells were
seeded onto the upper compartments of the filter inserts. A total
of 500 μl DMEM with 10% FBS was placed into the lower
compartments as a chemoattractant. Subsequent to 24 h of
incubation, the filter inserts were removed from the plates. The
cells on the surface of the filter in the upper compartment were
removed by scraping with a cotton swab. The invasive cells on the
lower surface of the filter were fixed in methanol and stained with
the crystal violet reagent. The number of cells in five random
fields was counted under a microscope (Olympus, Tokyo, Japan)
(magnification, ×100). The data presented are the mean values
obtained from three separate chambers.
RT-PCR (reverse transcription polymerase
chain reaction)
Total RNA from the cells was isolated using TRIzol
reagent (Gibco-BRL. The first-strand cDNA was synthesized from 2
μg total RNA using the RevertAid kit (Gibco BRL, Carlsbad,
CA, USA). Quantitative normalization of the cDNA in each sample was
performed using the expression of the β-actin gene as an internal
control. The PCR primers that were used for the detection of MMP-2,
MMP-9 and E-cadherin were synthesized as follows: MMP-2 (332 bp)
forward, 5′-TGG TCC TGG TGC TCC TGG TG-3′ and reverse, 5′-GCT GCC
TGT CGG TGA GAT TGG-3′; MMP-9 (111 bp) forward, 5′-TGG TCC TGG TGC
TCC TGG TG-3′ and reverse, 5′-GCT GCC TGT CGG TGA GAT TGG-3′;
E-cadherin (126 bp) forward, 5′-CAA TGG TGT CCA TGT GAA CA-3′ and
reverse, 5′-CCT CCT ACC CTC CTG TTC G-3′; and β-actin (179 bp)
forward, 5′-ATC GTG CGT GAC ATT AAG GAG AAG-3′ and reverse, 5′-AGG
AAG AAG GCT GGA AGA GTG-3′. The PCR conditions were as follows: one
cycle of denaturing at 94°C for 3 min, followed by 35 cycles at
94°C for 30 sec, 55°C for 30 sec and 72°C for 35 sec, follwed by a
final extension at 72°C for 5 min. The PCR products were loaded
onto 1.5% agarose gels and visualized with ethidium bromide under
UV light.
Western blotting
Briefly, 5×105 cells were incubated on
ice for 30 min in 0.5 ml ice-cold whole-cell lysate buffer. Any
debris was removed by centrifugation. The protein content of the
cells was determined and the cellular lysates were separated by 10%
SDS-PAGE, then electro-transferred onto nitrocellulose membranes.
Subsequent to being blocked with 5% skimmed milk in Tris-buffered
saline with Tween 20 (TBST), the membranes were incubated with
primary antibodies at 4°C overnight, followed by 1:2,000
horseradish peroxidase (HRP)-conjugated secondary antibodies for 2
h. Immunoreactive bands were visualized using an enhanced
chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ,
USA). The western blotting signals were quantitated by
densitometric analysis using TotalLab Nonlinear Dynamics Image
analysis software (Nonlinear Dynamics, Durham, NC, USA).
Small interfering RNA (siRNA) assay
siRNA oligos were used to inhibit the expression of
ERK (ERK siRNA target sequence, 5′-UAAAGGUUAACAUCCGGUG-3′; Qiagen,
Gaithersburg, MD, USA). The BxPC-3 cells (n=2×106) were
transfected with siRNA targeted against ERK (100 nm/l) or a control
siRNA (Qiagen) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA). The cells were covered overnight prior to starvation. This
was then followed by the treatment with α-mangostin (5 μM)
for 24 h. Finally, the cells were harvested for western blotting
and an invasion assay.
Statistical analysis
Each experiment was performed at least three times.
Statistical analysis was performed using SPSS software (version
16.0; SPSS Inc. Chicago, IL, USA). The results are presented as the
mean ± standard deviation (SD). The treatment differences were
assessed using the Student’s t-test. Multiple group comparisons
were performed with a one-way analysis of variance (ANOVA) followed
by the Bonferroni post hoc test. Confirmation that the difference
was statistically significant required the rejection of the null
hypothesis, which would indicate that there was no difference
between the mean value obtained from the replicate sets when
P=0.05. Therefore, P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of α-mangostin on the
proliferation of pancreatic cancer cells
The cytotoxicity of α-mangostin was first determined
using the MTT assay. BxPC-3 and MIAPaCa-2 cells were treated with
α-mangostin at various concentrations (0, 5, 7.5, 10 or 15
μM) for 6, 12, 18, 24 or 48 h. The results demonstrated that
the proliferative abilities of the BxPC-3 and MIAPaCa-2 cells were
decreased by α-mangostin in a time- and dose-dependent manner. In
addition, treatment with α-mangostin at ≤5 μM exhibited no
cytotoxic effects on the BxPC-3 or MIAPaCa-2 cells (Fig. 1). Therefore, this concentration of
α-mangostin was used for the subsequent experiments.
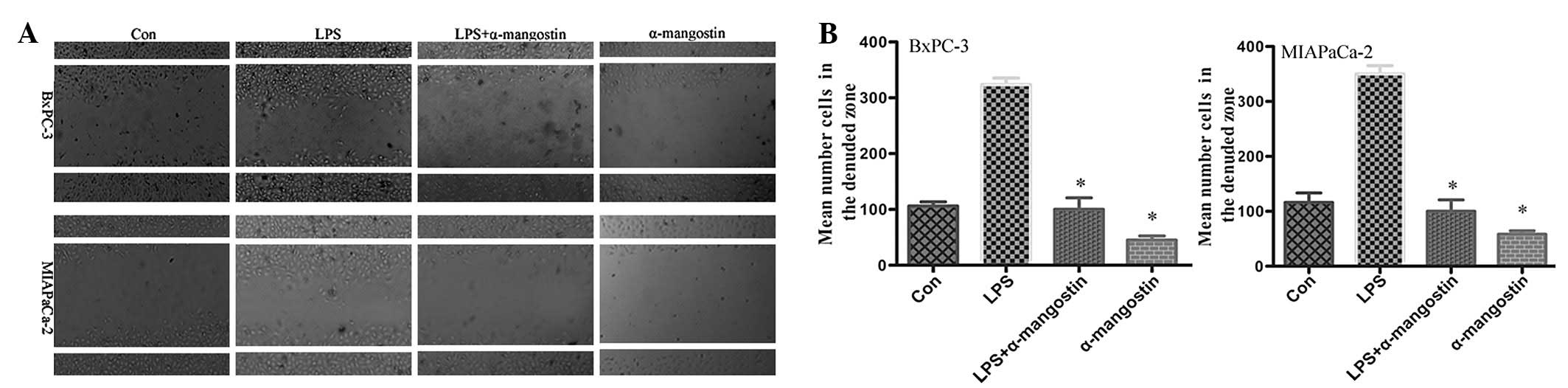
α-mangostin inhibits the migration of
pancreatic cancer cells
To evaluate the effect of α-mangostin on pancreatic
cancer cell motility, the cell culture wound-healing assay, an
established method for studying directional cell migration in
vitro, was used. The serum-starved BxPC-3 and MIAPaCa-2 cells
in the LPS (5 μg/ml) group exhibited marked cell migration
in the wound area 24 h after wounding, whereas the wounds treated
with α-mangostin (5 μM) showed delays in wound healing under
the same conditions (Fig. 2). This
result indicated that α-mangostin inhibited the migration of
pancreatic cancer cells in vitro.
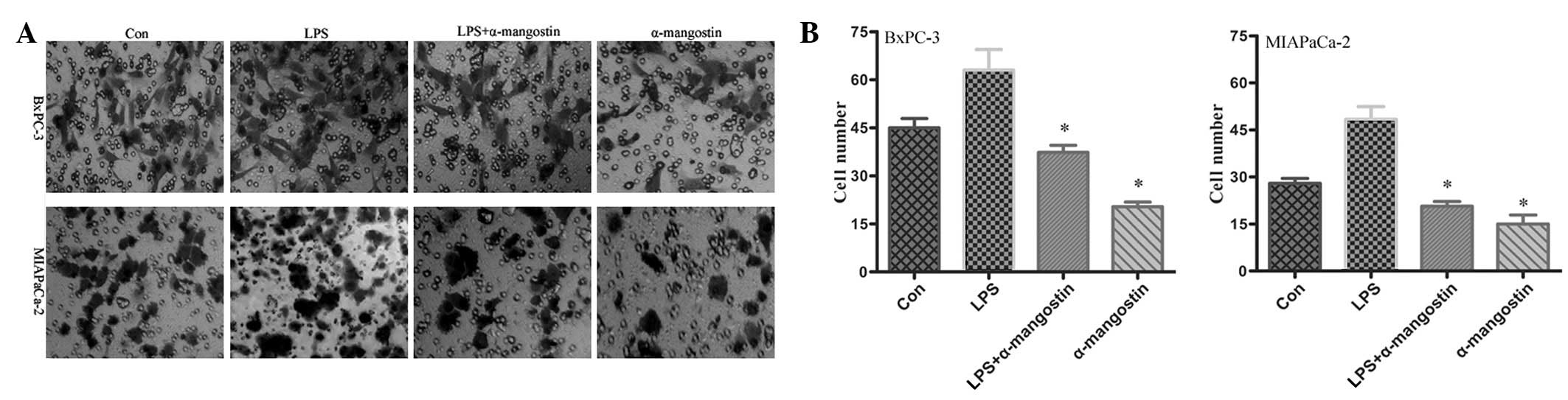
α-mangostin inhibits the invasion of
pancreatic cancer cells
To examine the potential effects on cell
invasiveness, an invasion assay was performed on the BxPC-3 and
MIAPaCa-2 cells. The motile phenotypes of the cells treated with
LPS and the combination of LPS plus α-mangostin were evaluated.
Following treatment with LPS alone, the number of invasive cells
increased significantly compared with the untreated cells. The
number of invasive cells was significantly reduced in the cells
co-treated with LPS and α-mangostin (P<0.05) (Fig. 3). These results suggested that
α-mangostin blocked the effect of LPS, which increased the
invasiveness of the pancreatic cancer cells. This observation
revealed that α-mangostin may be an effective inhibitor of the
invasion of pancreatic cancer cells.
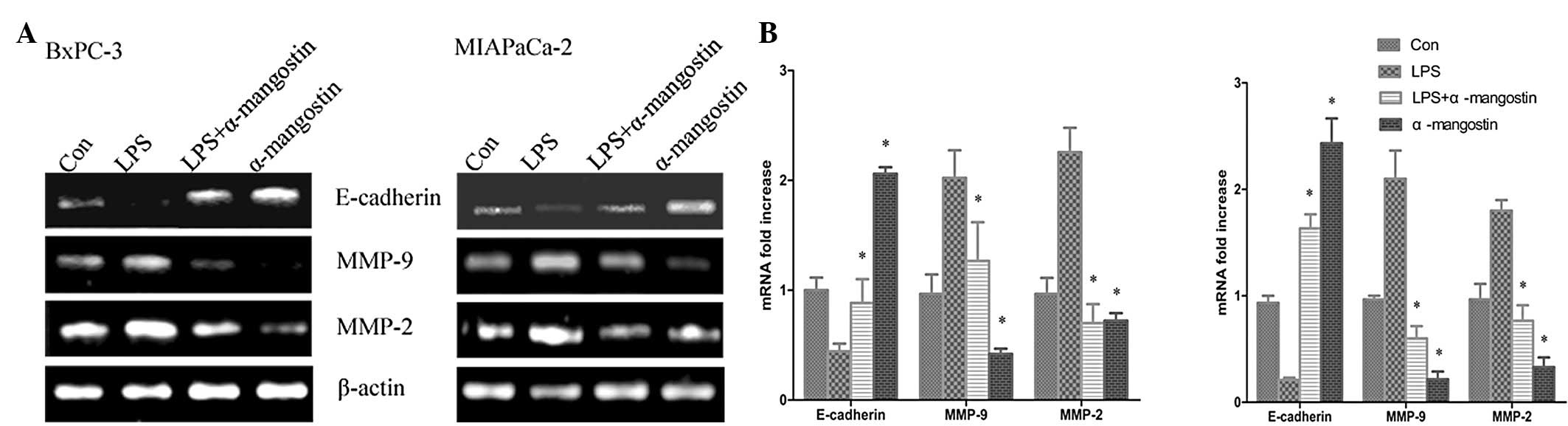
Effects of α-mangostin on the mRNA and
protein expression of MMP-2, MMP-9 and E-cadherin
A number of studies have investigated the importance
of E-cadherin in interations between the cells and the ECM, which
may inhibit cell migration and invasion. Metastasis has been shown
to be accompanied by various physiological alterations involved in
the degradation of the ECM, including the overexpression of
proteolytic enzyme activity. To further confirm the inhibitory
effects of α-mangostin on LPS-induced migration and invasion, the
mRNA and protein expression levels of MMP-9, MMP-2 and E-cadherin
were sequentially analyzed using semi-quantitative RT-PCR analysis
and western blotting, respectively. The semi-quantitative PCR
results (Fig. 4) indicated that the
mRNA levels of MMP-9 and MMP-2 were significantly increased, while
E-cadherin was significantly suppressed by LPS treatment
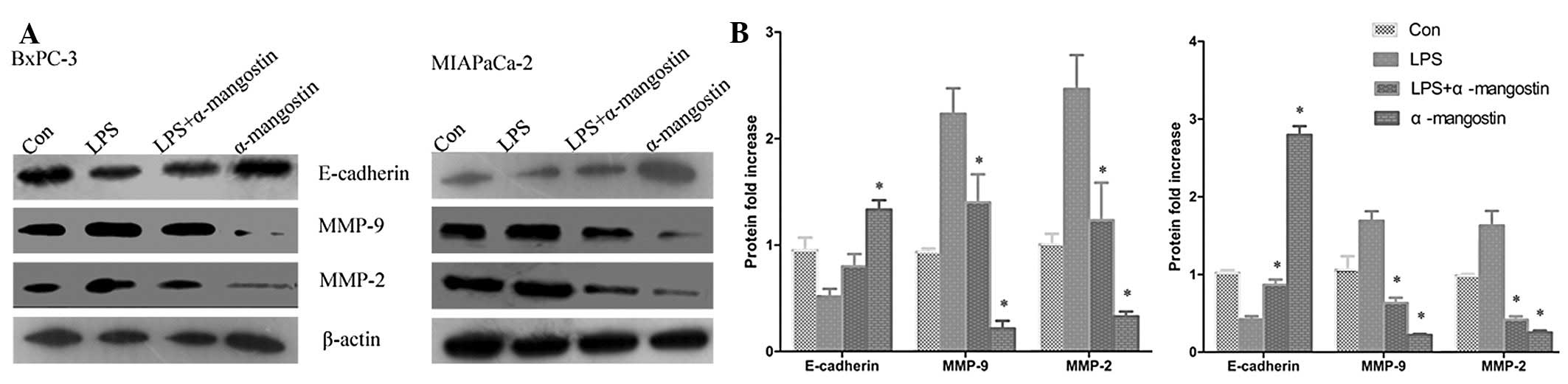
(P<0.05). The western blotting (Fig.
5) showed that the expression of the E-cadherin protein was
significantly downregulated in the LPS group compared with the
control (P<0.05), whereas MMP-9 and MMP-2 protein expression was
significantly increased (P<0.05). Notably, α-mangostin reversed
this phenomenon as induced by LPS, causing the re-induction of
E-cadherin and the inhibition of MMP-9 and MMP-2 expression
(P<0.05) (Figs. 4 and 5). These results further suggested that
α-mangostin has an inhibitory effect on cellular migration and
invasion.
ERK signaling has a key role in the
effect of α-mangostin on the expression of MMP-2, MMP-9 and
E-cadherin
Previous studies have reported that α-mangostin is
able to inhibit cancer cell invasion by suppressing the ERK1/2
signaling pathway (9). To further
investigate whether the inhibition of LPS-induced MMP-2 and MMP-9
expression by α-mangostin occurred via ERK1/2 activation, ERK1/2
phosphorylation was detected by western blotting. The results
showed that the phosphorylation of ERK1/2 was significantly
upregulated in the LPS group compared with the control (P<0.05),
whereas α-mangostin reversed the LPS-induced ERK1/2 activation
(Fig. 6A and B). To further
demonstrate that α-mangostin inhibited invasion through the ERK
signaling pathway, the BxPC-3 cells were transiently transfected
with ERK siRNA. The efficacy of the ERK siRNA for knocking down ERK
was demonstrated by western blotting. It was observed that the ERK
protein level (Fig. 6B) was barely
detectable in the ERK siRNA-transfected cells compared with the
control siRNA-transfected cells (Fig.
6C). Subsequently, the effects of α-mangostin were determined
by comparing results in the presence or absence of α-mangostin. The
protein expression levels of MMP-2, MMP-9 and E-cadherin were
evaluated by western blotting. As shown in Fig. 6E and F, sole treatment with
α-mangostin reduced the LPS-induced expression levels of MMP-2 and
MMP-9 and increased the expression of E-cadherin. The combined
treatment (ERK siRNA and α-mangostin) was able to markedly reduce
the expression levels of MMP-2 and MMP-9 compared with LPS
treatment alone (P<0.05). Additionally, invasion and migration
was reduced in the ERK siRNA group compared with the LPS group
(Fig. 6D). These data indicate that
the ERK signaling pathway may have a key role in the inhibition of
LPS-induced invasion and migration by α-mangostin in pancreatic
cancer cells.
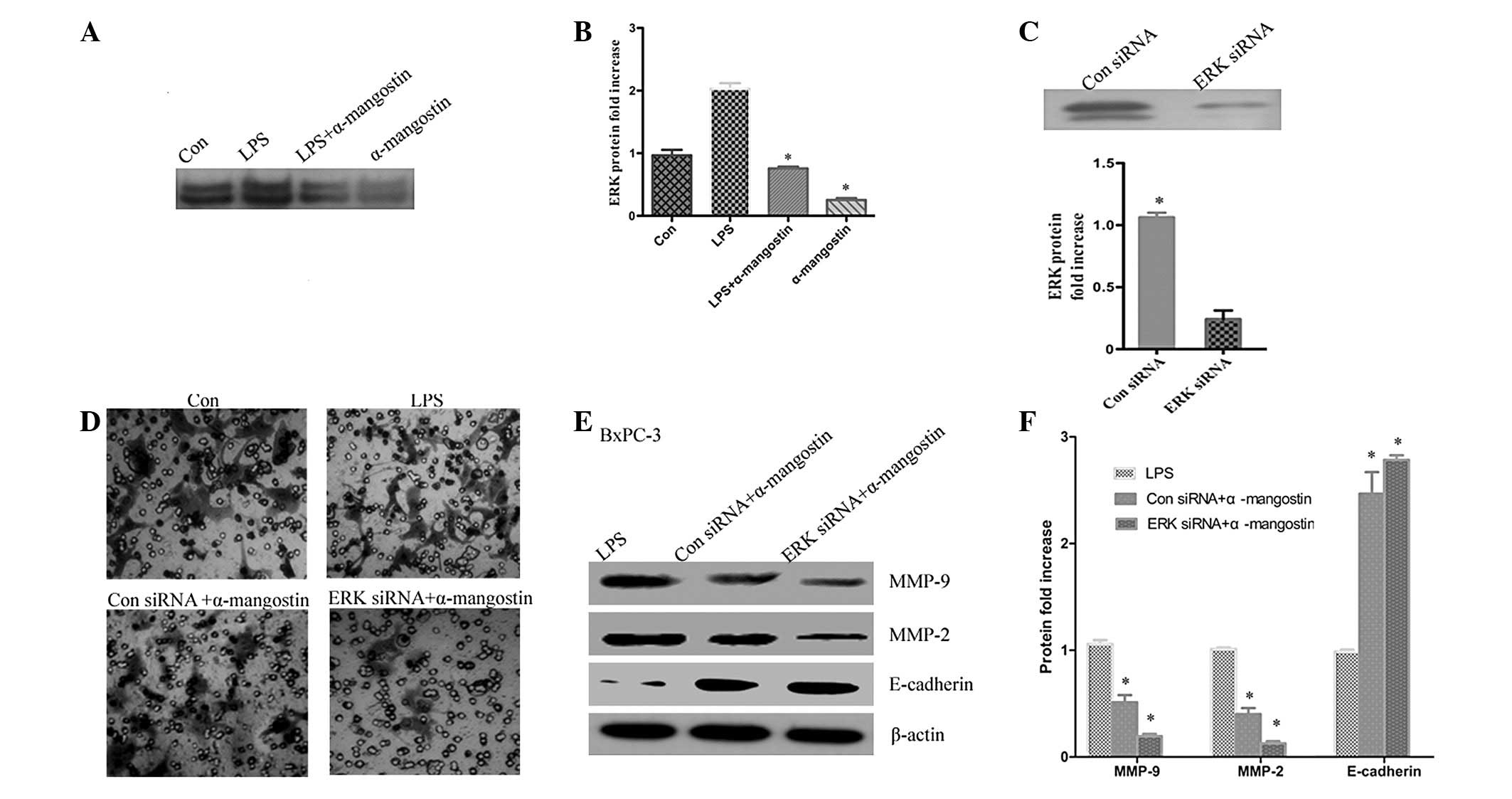 | Figure 6ERK signaling is key to the effect of
α-mangostin on the expression of MMP-2, MMP-9 and E-cadherin. (A)
α-mangostin reversed LPS-induced ERK1/2 activation. (B)
Quantification of protein levels. (C) The efficacy of ERK siRNA for
knocking down ERK protein was demonstrated by western blotting. (D)
Cells were treated with LPS (5 μg/ml), α-mangostin (5
μM) and/or ERK siRNA for 24 h, then subjected to analyses
for cell invasion. (E) BxPC-3 cells were treated with LPS (5
μg/ml) and/or α-mangostin (5 μM), with or without ERK
siRNA. After 24 h, the E-cadherin, MMP-9 and MMP-2 protein
expression levels were detected using western blotting. (F)
Quantification of protein levels. Data from at least three
independent experiments with duplicate determinations are expressed
as the mean ± SEM. *P<0.05 was considered to indicate
statistically significant results for LPS + α-mangostin group
compared with the LPS group LPS, lipopolysaccharide; MMP, matrix
metalloproteinase; siRNA, small interfering RNA; Con, control. |
Discussion
Cancer metastasis refers to the spread of cancer
cells from the primary neoplasm to distant sites and the growth of
secondary tumors at sites that are distant from the primary tumor.
Pancreatic cancer is a highly metastatic tumor. Blocking its
invasion may lead to new, effective treatments for pancreatic
cancer. Active compounds with anti-invasive and anti-metastatic
properties have defined a new catalogue of chemotherapeutic agents,
including α-mangostin, which have important roles in cancer
treatment.
In previous studies, α-mangostin was able to inhibit
the invasion of lung and breast cancer (9,14); the
invasion inhibiting effect was greater in highly invasive carcinoma
cells, but independent of its anti-proliferative action. The
present study has shown that BxPC-3 and MIAPaCa-2 cell invasion and
migration may be induced by LPS. Treatment with α-mangostin was
able to effectively inhibit the LPS-induced effect, similar to the
results of previous studies. These pancreatic cancer cells showed
reduced migratory ability and delays in wound healing following
incubation with α-mangostin. In the invasion assay, the average
number of cells invading the lower chamber in 24 h was less than
the control group following incubation with α-mangostin.
α-mangostin not only restored metastatic activation, but also
blocked the expression of MMP-9 and MMP-2 and upregulated
E-cadherin. It was also demonstrated that ERK signaling is required
for α-mangostin-mediated inhibition of the metastatic activation of
the BxPC-3 and MIAPaCa-2 cells. These findings aid in the
understanding of the mechanism by which α-mangostin acts to inhibit
cancer cell invasiveness.
Invasion and metastasis is a highly complicated
process that requires the interaction of numerous cell types,
including connective tissue and blood vessel components within
various organs (15,16). The invasion of tumor cells into
tumor-associated stroma and the subsequent metastasis are central
events in tumor progression. The metastasis of cancer cells
involves changes in cell adhesion, rearrangement of the ECM,
anoikis-suppression and reorganization of the cytoskeleton
(17). Multiple molecular
mechanisms are involved in this process. E-cadherin is a key
molecule involved in regulating adhesion between cells through the
binding of E-cadherin in the cytoplasm to catenin, which integrates
the cytoskeleton of adjacent cells. The loss of E-cadherin provides
cancer cells with an invasive ability (18). The association between MMP
expression and the invasive activity of various types of cancer has
been well documented. MMP-2 and MMP-9 enzymes are capable of
degrading type IV collagen, which is a major constituent of the
basement membrane (19,20).
α-mangostin has been demonstrated to be an
inhibitory agent for MMP-2 and MMP-9 in lung and breast cancer
(9,14). In the present study, it was observed
that MMP-9 and MMP-2 mRNA expression in two pancreatic cancer cell
lines decreased following treatment with α-mangostin, indicating
that the inhibition of pancreatic cancer cell invasion by
α-mangostin may be partly mediated by the downregulation of MMP-9
and MMP-2 expression. In addition, the effects of α-mangostin
treatment on E-cadherin expression have not been reported
previously. In the present study, E-cadherin was observed to be
expressed at low levels in the two pancreatic cancer cell lines,
but following incubation with α-mangostin, the expression of
E-cadherin was significantly increased. This may be another
mechanism by which α-mangostin inhibits the invasion of pancreatic
cancer cells. Han et al(21) reported that genistein
decreased the invasion of MIAPaCa-2 cells, upregulated the mRNA and
protein expression of E-cadherin and reversed the characteristic
morphology of epithelial-mesenchymal trans-differentiation (EMT).
Thus, α-mangostin may reverse EMT by increasing E-cadherin
expression. This is likely to require elucidation in future
investigations.
Previously, α-mangostin has been shown to suppress
the phosphorylation of ERK, leading to the inhibition of MMP-2 and
MMP-9 in pancreatic cancer cells (10). In the present study, α-mangostin was
also observed to directly inhibit ERK activity. Moreover, the
combined treatment using ERK siRNA and α-mangostin was able to
markedly reduce the expression of MMP-2 and MMP-9, while increasing
that of E-cadherin. This result suggests that α-mangostin may
target proteins upstream of ERK.
Acknowledgements
The authors would like to thank the
staff of the Biology and Genetics Laboratory of Xi’an Jiaotong
University for their technical assistance in the present study.
References
|
1
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Książkiewicz M, Markiewicz A and Zaczek
AJ: Epithelial-mesenchymal transition: a hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012.PubMed/NCBI
|
|
3
|
Brunner T: Gemcitabine in the
chemoradiotherapy for locally advanced pancreatic cancer: a
meta-analysis. Strahlenther Onkol. 188:366–367. 2012.(In
German).
|
|
4
|
Ngawhirunpat T, Opanasopi P, Sukma M, et
al: Antioxidant, free radical-scavenging activity and cytotoxicity
of different solvent extracts and their phenolic constituents from
the fruit hull of mangosteen (Garcinia mangostana). Pharm
Biol. 48:55–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martínez-Abundis E, García N, Correa F,
Hernández-Reséndiz S, Pedraza-Chaverri J and Zazueta C: Effects of
alpha-mangostin on mitochondrial energetic metabolism.
Mitochondrion. 10:151–157. 2010.PubMed/NCBI
|
|
6
|
Bumrungpert A, Kalpravidh RW, Chuang CC,
et al: Xanthones from mangosteen inhibit inflammation in human
macrophages and in human adipocytes exposed to
macrophage-conditioned media. J Nutr. 140:842–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sánchez-Pérez Y, Morales-Bárcenas R,
García-Cuellar CM, et al: The alpha-mangostin prevention on
cisplatin-induced apoptotic death in LLC-PK1 cells is associated to
an inhibition of ROS production and p53 induction. Chem Biol
Interact. 188:144–150. 2010.PubMed/NCBI
|
|
8
|
Lee YB, Ko KC, Shi MD, et al:
Alpha-Mangostin, a novel dietary xanthone, suppresses TPA-Mediated
MMP-2 and MMP-9 expressions through the ERK signaling pathway in
MCF-7 human breast adenocarcinoma cells. J Food Sci. 75:H13–H23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hung SH, Shen KH, Wu CH, Liu CL and Shih
YW: Alpha-Mangostin suppresses PC-3 human prostate carcinoma cell
metastasis by inhibiting matrix metalloproteinase-2/9 and
urokinase-plasminogen expression through the JNK signaling pathway.
J Agric Food Chem. 57:1291–1298. 2009. View Article : Google Scholar
|
|
10
|
Matsumoto K, Akao Y, Ohguchi K, et al:
Xanthones induce cell-cycle arrest and apoptosis in human colon
cancer DLD-1 cells. Bioorg Med Chem. 13:6064–6069. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X and Kang Y: Hypoxia and
hypoxia-inducible factors: master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins DF, Kimura K, Bernhardt WM, et al:
Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of
epithelial-to-mesenchymal transition. J Clin Invest. 117:3810–3820.
2007.PubMed/NCBI
|
|
13
|
Shih YW, Chien ST, Chen PS, Lee JH, Wu SH
and Yin LT: Alpha-mangostin suppresses phorbol 12-myristate
13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3
integrin/FAK/ERK and NF-kappaB signaling pathway in human lung
adenocarcinoma A549 cells. Cell Biochem Biophys. 58:31–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel P and Chen EI: Cancer stem cells,
tumor dormancy, and metastasis. Front Endocrinol (Lausanne).
3:1252012.PubMed/NCBI
|
|
15
|
Nakayama K, Nakayama N, Katagiri H and
Miyazaki K: Mechanisms of ovarian cancer metastasis: biochemical
pathways. Int J Mol Sci. 13:11705–11717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilmanns C, Steinhauer S, Großmann J,
Schmitt-Gräff A and Ruf G: Cooperate concept of metastasis:
site-specific requirement of activated differentiation and dynamic
deterioration. Cancer Metastasis Rev. 31:269–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buda A and Pignatelli M: E-cadherin and
the cytoskeletal network in colorectal cancer development and
metastasis. Cell Commun Adhes. 18:133–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kowluru RA, Zhong Q and Santos JM: Matrix
metalloproteinases in diabetic retinopathy: potential role of
MMP-9. Expert Opin Investig Drugs. 21:797–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romi F, Helgeland G and Gilhus NE: Serum
levels of matrix metalloproteinases: implications in clinical
neurology. Eur Neurol. 67:121–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han L, Zhang HW, Zhou WP, Chen GM and Guo
KJ: The effects of genistein on transforming growth
factor-β1-induced invasion and metastasis in human pancreatic
cancer cell line Panc-1 in vitro. Chin Med J (Engl). 125:2032–2040.
2012.
|