Introduction
Melanomas are malignant tumors prone to early
metastasis, which occurs with a high incidence in the Caucasian
populations (1). Almost any organ
and structure may be involved in metastatic melanoma due to its
ubiquitous spreading pattern. The most common forms of metastatic
melanoma of the spine are vertebral metastatic melanoma and
intramedullary spinal cord metastatic melanoma (2,3). Spine
metastasis of melanoma carries the potential for difficult
problems, such as pain, weakness and sensory deficit. However,
there is few stuides describing intraspinal extradural metastatic
melanoma. The present study reports a case of a delayed metastatic
intraspinal extradural melanoma of the lumbar spine in a
non-Caucasian patient. The diagnosis of the tumor was complicated
by its delayed metastasis, location and unusual appearance in
magnetic resonance imaging (MRI) scans. Written informed consent
was obtained from the patient.
Case report
A 67-year-old female patient was admitted with a
six-month history of lower back pain accompanied by a two-month
history of intermittent claudication. The patient had visited a
community hospital when the lower back pain developed and was
diagnosed with osteoporosis following assessment by radiography of
the lumbar spine. Despite treatment with medication and
physiotherapy, the patient’s symptoms did not subside. Two months
prior to this admission the patient began to experience progressive
intermittent claudication. The patient was referred to the West
China Hospital (Sichuan University, Chengdu, China) as an MRI of
the lumbar spine had been recommended three weeks prior to the
admission. A neurological examination showed no notable changes in
the function of the spinal nerve.
The patient had previously been diagnosed with a
vulvar melanoma (13 years prior to the current admittance) and had
undergone a melanoma resection at the West China Hospital. The
patient received post-surgical radiotherapy. No local melanoma
recurrence or other metastatic melanoma was reported prior to the
present admission. The patient had been treated with
anti-hypertensive drugs for 11 years, but had no other medical
history.
The current MRI revealed compression of the lumbar
spinal cord in the spinal canal from the posterior direction caused
by an extradural mass at the L3 and L4 level. The mass showed a low
signal intensity on the T2-weighted images, a mixed low signal
intensity on the T1-weighted images and slight enhancement
following a gadolinium-contrast injection (Fig. 1A). Over the subsequent week,
radiography and computed tomography (CT) of the lumbar spine were
recommended. There were no clear changes in the radiograph
(Fig. 1B). CT scans faintly
revealed the mass (Fig. 1C).
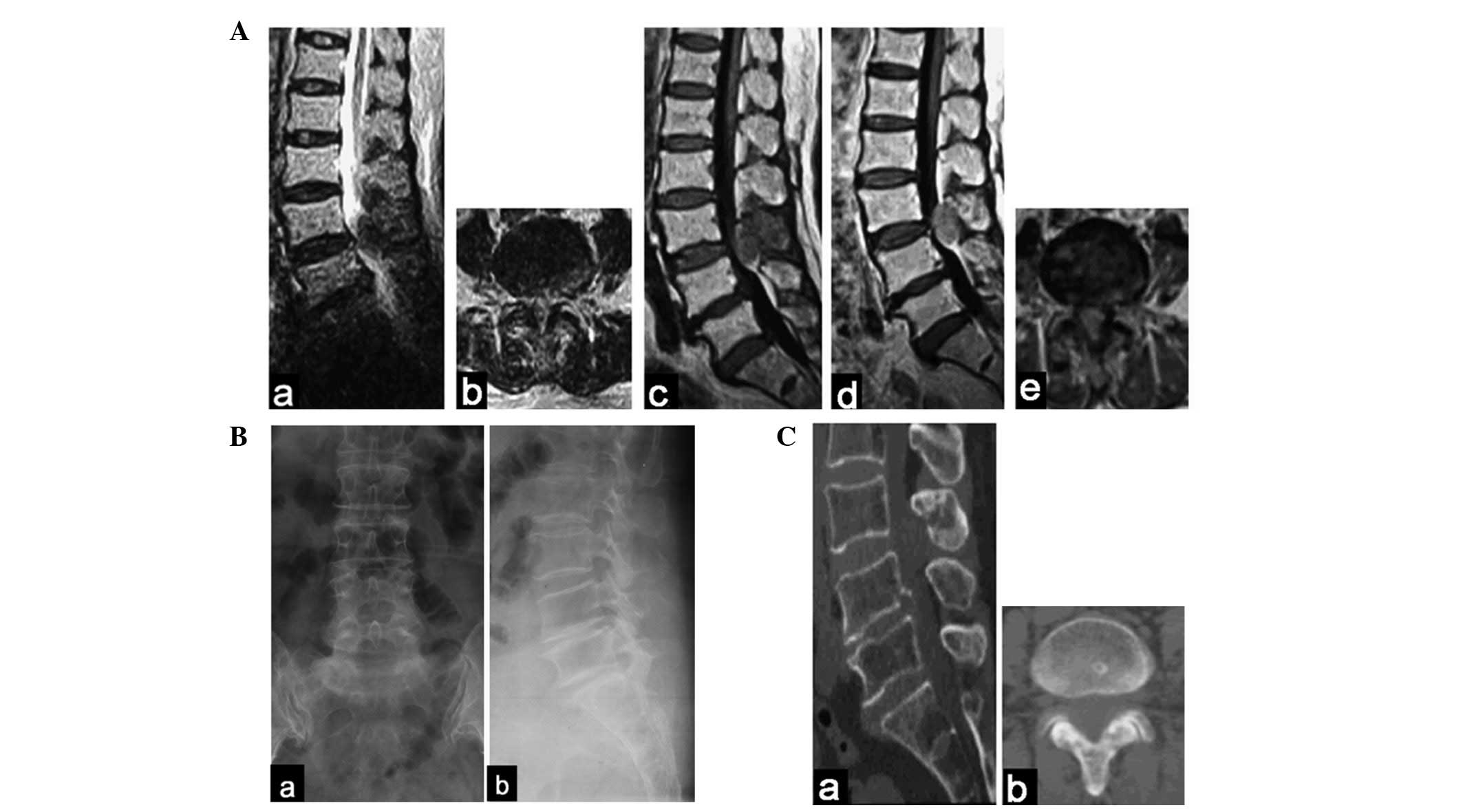 | Figure 1(A) At the L3–L4 level, MRI revealed
an extradural posterior oval mass with (a and b) low signal
intensity on T2-weighted images, (c) mixed low intensity on
T1-weighted images and (d and e) slight enhancement images. (Ba and
b) Radiography of the lumbar spine revealed no evident changes. (Ca
and b) CT scans faintly revealed the mass. MRI, magnetic resonance
imaging. Aa, T2/sagittal plane; Ab, T2/transverse plane; Ac,
T1/sagittal plane; Ad, enhanced/sagittal plane; Ae,
enhanced/transverse plane; Ba, posteroanterior; Bb, lateral; Ca,
sagittal plane; Cb, transverse plane. |
Although the patient had a medical history of vulvar
melanoma, a lumbar stenosis resulting from hypertrophy of the
ligamentum flavum was suspected instead of metastatic melanoma due
to the extended time-period (13 years) since the original vulvar
melanoma, the location of the mass and its appearance in MRI scans.
Treatment with medication was prescribed to the patient. However,
two weeks later, the intermittent claudication was significantly
aggravated so the patient was re-admitted. The laboratory data
showed normal values.
A posterior laminectomy and fusion with
instrumentation were attempted on the patient. However, during the
surgery, a dark gray solid mass was observed under the L3 vertebral
plate. The mass was dissected carefully from the surrounding
structures and gross total removal with the L3 lamina and inferior
articular process was performed. The solid mass (5.0×4.7×3.0 cm)
was located between the L3 lamina and dura and was ellipsoid in
shape. The mass appeared to be wrapped in a membrane and adhered to
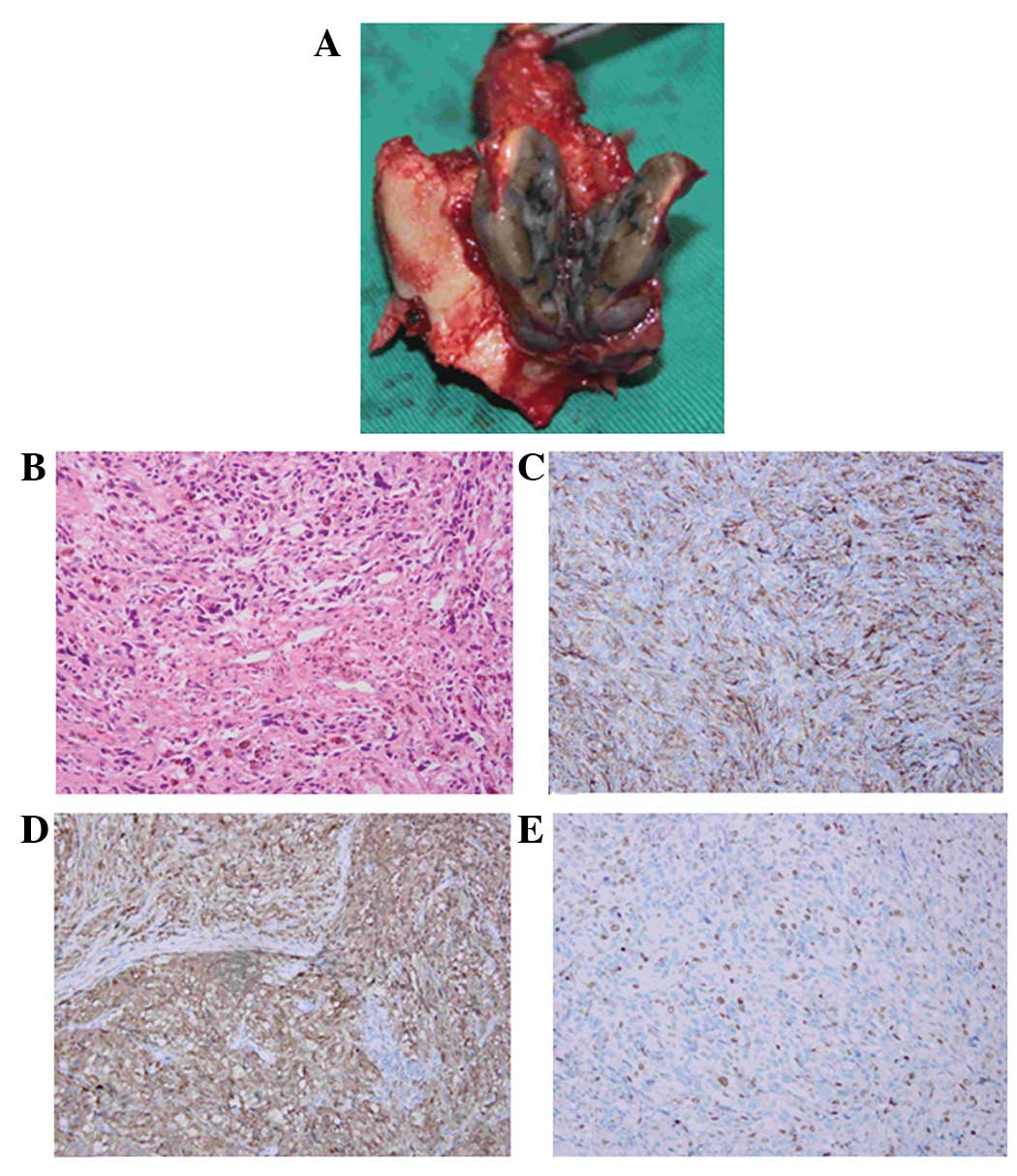
the dorsal dural surface (Fig. 2A).
Spine fixation was subsequently performed at the L2-4 level and
interbody fusion was performed at the L3-4 level (Fig. 3A).
The hematoxylin and eosin (HE) histological analysis
revealed a pigmented tumor (Fig.
2B), while the immunohistochemical analysis revealed cells
stained markedly positive for the monoclonal antibody anti-HBM-45,
which is a useful marker of melanocytic differentiation in
neoplasms (Fig. 2C). The S-100
protein stain, a marker for cells derived from the neural crest,
was positive in the neoplastic cells (Fig. 2D). Furthermore, the Ki-67 index was
positive in ∼25% of the cells. (Fig.
2E). The pathological diagnosis based on these observations was
one of malignant melanoma.
Subsequent to the surgery, the general condition of
the patient was good with no lower back pain. At two weeks
post-surgery, bone scans showed an increased radioactive uptake,
not only at L3, but also at the right sacroiliac joint. The patient
was subsequently treated with radiotherapy and immunotherapy. The
metastatic melanoma was absent in all areas, including the right
sacroiliac joint, following this comprehensive therapy. At the
final 13-month follow-up, the patient showed no evidence of
recurrence and the intermittent claudication had disappeared
(Fig. 3B).
Discussion
Melanomas occurs most commonly in Caucasian
populations and are rare in Asian populations (4). However, the incidence of melanomas is
also rising in each population (5,6).
Melanomas are prone to early distant metastasis involving almost
any organ or structure, and following the development of a distant
metastasis, the prognosis is poor (1). The most common forms of metastatic
melanoma of the spine are vertebral metastatic melanoma and
intramedullary metastatic melanoma. Gokaslan et al(2) reported 133 cases of vertebral
metastatic melanoma over a period of 11 years and Ishii et
al(3) reviewed reports of nine
cases of intramedullary spinal cord metastatic melanoma. In the
present case, the metastatic melanoma was located in the extradural
space of the spinal canal and the metastasis was detected 13 years
after the vulvar melanoma resection. For these reasons, a diagnosis
of metastatic melanoma was not initially considered.
MRI is an optional method for the diagnosis of
spinal tumors that aids in the diagnosis of spinal melanoma. In a
case of vertebral metastatic melanoma, MRI reveals an increased
signal intensity in the vertebral body, with soft-tissue extension
into the extradural space (2,7). MRI
scans of intraspinal melanomas show hypointense signals on
T2-weighted images and hyperintense signals on T1-weighted images.
Unal and Castillo (8) reported a
primary thoracic extradural spinal malignant melanoma with
T2-hypointense and T1-hyperintense MRI signals. Lee et
al(9) presented the MRI
analysis of a patient with a primary intradural extramedullary
melanoma of the cervical spinal cord, with decreased signal
intensity on T2-weighted images and increased signal intensity on
T1-weighted images. Lee et al(10) reported a case in which the MRI of
the patient revealed an enhanced mass in the intra- and extradural
space compressing the spinal cord at the left neural foramen at the
C6-7 level. However, MRI does not consistently show a homogeneous
pattern. The MRI signal of melanocytic tumors depends on the
presence of melanin, acute or chronic intratumoral hemorrhages and
fat deposits. Therefore, as in the present case, the interpretation
of the MRI pattern may easily lead to misdiagnosis (11). MRI of the lumbar spine of the
present patient showed a mass with low intensity on T2-weighted
images, mixed low signal intensity on T1-weighted images and slight
enhancement following gadolinium-contrast injection. Consequently,
lumbar stenosis resulting from hypertrophy of the ligamentum flavum
was initially suspected as it is a common disease in older
individuals.
Usually, the confirmatory diagnosis of spinal
melanoma is only made on the basis of post-surgical pathological
studies or autopsies (3,7–9,12,13).
Immunohistochemical studies are also important in a diagnosis.
Anti-melanoma antibody (HMB-45) and S-100 protein staining may aid
in the diagnosis of malignant melanoma. Furthermore,
immunohistochemistry may be used to distinguish spinal melanoma
from other types of tumors (9,14). In
the present case, staining for the translocation factor E3, smooth
muscle actin, pan-cytokeratin, desmin and collagen IV was negative,
thus aiding in the differential diagnosis.
Surgical resection of spinal melanomas is extremely
important as it leads to the regression of neurological symptoms
(13). Although the efficacies of
radiotherapy and chemotherapy remain controversial in melanoma,
radical removal of the tumor should be followed by radiotherapy due
to the malignant nature of this tumor (9,15,16).
The present patient received post-surgery radiotherapy and
immunotherapy, not only for the treatment of the extradural
melanoma of the lumbar spine, but also for the melanoma of the
right sacroiliac joint. With comprehensive treatment, patient
survival-times range between one week and 43 months following the
diagnosis of vertebral metastatic melanoma, and between eight weeks
and 18 years following the initial presentation in patients with
intramedullary metastatic melanoma (2,7,8,17,18).
Although the present patient remains alive and in a good condition
at the 13th-month follow-up, we propose that an earlier surgery
instead of the treatment with medication would have been beneficial
for the patient’s survival and health.
In summary, due to the delayed metastasis, location
and unusual appearance of the tumor in the MRI scans, a metastatic
melanoma was not initially suspected in the present case. Early
surgical removal of the tumor allows tissue diagnosis and the
selection of an appropriate comprehensive treatment, which is
critical for patient survival. The present case indicates that
caution should be used in the diagnosis of similar future
cases.
References
|
1
|
O’Day SJ, Kim CJ and Reintgen DS:
Metastatic melanoma: chemotherapy to biochemotherapy. Cancer
Control. 9:31–38. 2002.PubMed/NCBI
|
|
2
|
Gokaslan ZL, Aladag MA and Ellerhorst JA:
Melanoma metastatic to the spine: a review of 133 cases. Melanoma
Res. 10:78–80. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishii T, Terao T, Komine K and Abe T:
Intramedullary spinal cord metastases of malignant melanoma: an
autopsy case report and review of the literature. Clin Neuropathol.
29:334–340. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crombie IK: Racial differences in melanoma
incidence. Br J Cancer. 40:185–193. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
6
|
Ishihara K, Saida T, Otsuka F and Yamazaki
N; Prognosis and Statistical Investigation Committee of the
Japanese Skin Cancer Society: Statistical profiles of malignant
melanoma and other skin cancers in Japan: 2007 update. Int J Clin
Oncol. 13:33–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eck JC, Tressler MA and Triantafyllou SJ:
Delayed presentation of metastatic melanoma of the cervical spine.
Orthopedics. 31:1672008.PubMed/NCBI
|
|
8
|
Unal B and Castillo M: MRI features of a
primary thoracic epidural melanoma: a case report. Clin Imaging.
31:273–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Moon KY, Chung CK, Kim HJ, Chang
KH, Park SH and Jahng TA: Primary intradural extramedullary
melanoma of the cervical spinal cord: case report. Spine (Phila Pa
1976). 35:E303–E307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee NK, Lee BH, Hwang YJ, et al: Findings
from CT, MRI, and PET/CT of a primary malignant melanoma arising in
a spinal nerve root. Eur Spine J. 19(Suppl 2): S174–S178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farrokh D, Fransen P and Faverly D: MR
findings of a primary intramedullary malignant melanoma: case
report and literature review. AJNR Am J Neuroradiol. 22:1864–1866.
2001.PubMed/NCBI
|
|
12
|
Vij M, Jaiswal S, Jaiswal AK and Behari S:
Primary spinal melanoma of the cervical leptomeninges: report of a
case with brief review of literature. Neurol India. 58:781–783.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolasa M, Jesionek-Kupnicka D, Kordek R
and Kolasa P: Primary spinal cord melanoma - a case report. Folia
Neuropathol. 48:212–216. 2010.PubMed/NCBI
|
|
14
|
Kounin GK, Romansky KV, Traykov LD,
Shotekov PM and Stoilova DZ: Primary spinal melanoma with bilateral
papilledema. Clin Neurol Neurosurg. 107:525–527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
François P, Lioret E and Jan M: Primary
spinal melanoma: case report. Br J Neurosurg. 12:179–182. 1998.
|
|
16
|
Narayan RK, Rosner MJ, Povlishock JT,
Girevendulis A and Becker DP: Primary dural melanoma: a clinical
and morphological study. Neurosurgery. 9:710–717. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Connolly ES Jr, Winfree CJ, McCormick PC,
Cruz M and Stein BM: Intramedullary spinal cord metastasis: report
of three cases and review of the literature. Surg Neurol.
46:329–337. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishihara M, Sasayama T, Kondoh T, Tanaka
K, Kohmura E and Kudo H: Long-term survival after surgical
resection of primary spinal malignant melanoma. Neurol Med Chir
(Tokyo). 49:546–548. 2009. View Article : Google Scholar : PubMed/NCBI
|