Introduction
The term peripheral T-cell lymphoma (PTCL) does not
refer to the site of involvement, but to the immunophenotype of
these tumors that derive from post-thymic (or mature) T cells at
various steps of differentiation (1,2). Since
natural killer (NK) cells are closely associated with T cells, and
share certain immunophenotypic and functional properties, mature
T-cell and NK-cell lymphomas are usually classed together (1,2). PTCL
comprises of a heterogeneous group of hematological tumors that the
World Health Organization (WHO) classification subdivides into
specified and unspecified (U) (3).
These tumors constitute ∼12% of all lymphoid neoplasms (4,5) and
their incidence varies between countries and races, being higher in
Japan and other Eastern areas for epidemiological reasons,
including the presence of human T-lymphotropic virus 1 (HTLV-1)
infections (6–8). These neoplasms often present at an
advanced stage in middle-aged/elderly patients at diagnosis
(9–11) and most commonly have an aggressive
clinical course. Patients succumb rapidly despite prompt therapies.
Relapse is common and the prognosis is poor. The rarity of these
tumors means that additional studies are required to improve our
understanding of their biology.
The reversion-inducing cysteine-rich protein with
Kazal motifs (RECK) gene was originally isolated using cDNA
expression cloning designed to locate transformation suppressor
genes against activated ras oncogenes (12). The RECK gene is widely
expressed in numerous normal tissues and non-neoplastic cell lines,
but its expression is low or undetectable in oncogene-transformed
fibroblasts or tumor-derived cell lines (13,14).
The RECK gene encodes a membrane-anchored glycoprotein that
is a negative regulator of the matrix metalloproteinases (MMPs),
including MMP-2, MMP-9 and MT1-MMP. The restoration of RECK
expression in malignant cells reduces pro-matrix MMP-9 secretion
and suppresses the ability to invade and metastasize, suggesting a
role for RECK in the regulation of MMPs and tumor
invasiveness (13). Numerous
studies have reported that a lower expression of RECK is
associated with a worse prognosis in a variety of cancers (15–19),
but little is known with regard to the significance of RECK
in PTCL.
Therefore, in the present study, the expression of
RECK was analyzed in patients with PTCL and these data were
compared with the clinical and pathological features to determine
whether the expression of RECK is a predictor of the
clinical behavior of PTCL.
Materials and methods
Patients and tumor samples
A total of 82 patients with PTCLs who were diagnosed
between 2006 and 2010 at the Department of Pathology, Tongji
Hospital (Wuhan, Hubei, China), were included in the present study.
Approval for the study was obtained from the Medical Ethics
Committee of the Tongji Hospital. The specimens and clinical data
were collected subsequent to obtaining informed consent in
accordance with the Declaration of Helsinki. Samples were obtained
at the initial presentation of the patients, then fixed in formalin
and embedded in paraffin. The tumor specimens that were analyzed
were from biopsies performed prior to chemotherapy. The paraffin
blocks were evaluated again to select a representative area. All
cases were reviewed carefully and the pathological diagnosis of
PTCL was made according to the WHO criteria for the classification
of malignant lymphoma following precise immunohistochemical
evaluation (20). The clinical
features of age, gender, tumor stage, performance status, serum
concentration of lactate dehydrogenase (LDH), extranodal
lymphomatous involvement, the presence or absence of B symptoms and
the International Prognostic Index (IPI) (21), treatment and follow-up were
analyzed. The recorded sites of extranodal lymphomatous involvement
included the gastrointestinal tract, liver, spleen, lungs, central
nervous system and bone marrow (21).
Among the 82 selected patients, there were 55 males
(67.1%) and 27 females (32.9%), with an average age of 42.2 years
(range, 7–77 years). The histological subtypes were PTCL-not
otherwise specified (PTCL-NOS; 28 cases), angioimmunoblastic T-cell
lymphoma (22 cases), extranodal NK/T-cell lymphoma, nasal type (16
cases) and intestinal NK/T-cell lymphoma (16 cases). The tumor
stages were classified as localized disease (stage 1 or 2) in 21
patients and advanced disease (stage 3 or 4) in 61 patients. There
were 42 patients with extranodal lymphomatous involvement (Table I).
 | Table IClinicopathological characteristics
for RECK expression. |
Table I
Clinicopathological characteristics
for RECK expression.
| RECK
expression |
|---|
|
|---|
| Variables | Negative | Positive | P-value |
|---|
| Patient Number | 52 | 30 | |
| Age (years) | | | |
| >60 | 12 | 11 | NS |
| <60 | 40 | 19 | |
| Gender | | | |
| Male | 37 | 18 | NS |
| Female | 15 | 12 | |
| Histology | | | |
| PTCL,
unspecified | 21 | 7 | NS |
| Angioimmunoblastic
T-cell lymphoma | 7 | 15 | |
| Extranodal
NK/T-cell lymphoma, nasal | 13 | 3 | |
| Intestinal
NK/T-cell lymphoma | 11 | 5 | |
| Clinical stage | | | |
| 1, 2 | 9 | 12 | NS |
| 3, 4 | 43 | 18 | |
| Extranodal
involvement | | | |
| No | 14 | 26 | 0.012a |
| Yes | 38 | 4 | |
| B symptoms | | | |
| No | 31 | 19 | NS |
| Yes | 21 | 11 | |
| Performance
status | | | |
| 0, 1 | 42 | 18 | NS |
| 2–4 | 10 | 12 | |
| LDH | | | |
| Normal | 8 | 13 | NS |
| Elevated | 44 | 17 | |
| IPI | | | |
| 0–2 | 23 | 8 | NS |
| 3–5 | 29 | 22 | |
The follow-up duration was defined from the date of
the initial presentation to the date of mortality or last
follow-up. The median follow-up period was 24.1 months (range, 0–48
months). During the follow-up, 38 patients (46.3%) were
continuously disease-free, 40 patients (48.8%) succumbed to the
disease and four patients succumbed during chemotherapy.
Immunohistochemistry
RECK expression was determined using the
streptavidin-peroxidase immunohistochemistry method. Serial
4-μm sections were cut from formalin-fixed,
paraffin-embedded blocks and placed on polylysine-coated slides.
The serial sections were deparaffinized in three changes of xylene,
rehydrated in descending concentrations of ethanol and washed three
times for 5 min each with double-distilled water. Following
rehydration, the sections were placed in 0.01 M sodium citrate
buffer (pH 6.0) for 10 min at 105°C. Subsequent to being cooled to
room temperature for 30 min, the specimens were incubated for 30
min at room temperature in 0.3% hydrogen peroxide in methanol to
inactivate endogenous peroxidase activity. The sections were then
incubated for 30 min at 37°C with phosphate-buffered saline (PBS;
pH 7.4) containing 5% bovine serum albumin (BSA; Merck, Darmstadt,
Germany), followed by overnight incubation at 4°C with
anti-RECK mouse monoclonal antibody (Clone 28; BD
Transduction Laboratories, San Diego, CA, USA) diluted 1:200 in PBS
containing 1% BSA. The sections were washed three times for 5 min
in PBS with Tween-20 and incubated for 1 h with biotinylated
anti-mouse IgG secondary antibodies (Abcam, Cambridge, UK) diluted
1:300 in PBS containing 1% BSA. Subsequent to rinsing, the immune
complexes were visualized using the standard
avidin-biotin-peroxidase complex (ABC) method and the sections were
then counterstained with Mayer’s hematoxylin and mounted. Positive
controls guaranteed the persistent quality of the staining
procedure. Negative control slides in the absence of primary
antibody were included for each staining.
The expression of RECK was independently
evaluated by two investigators without knowledge of the patients
clinicopathological features. The data from the two investigators
were averaged. An evaluation of the RECK staining reaction
was performed in accordance with the immunoreactive score (IRS):
IRS = SI (staining intensity) × PP (percentage of positive cells)
(22). The proportional scoring
categories of the positively-stained cells were as follows: i) 0,
no positivity; ii) 1+, ≤10% positive tumor cells; iii) 2+, 11–50%
positive tumor cells; iv) 3+, 50–80% positive tumor cells; and v)
4+, >80% positive tumor cells. The intensity of the RECK
immunostaining was scored as follows: i) no staining, 0; ii) weak,
1+; iii) moderate, 2+; and iv) intense, 3+. For tumors that showed
heterogeneous staining, the main pattern was taken into account for
scoring. The IRS was calculated in ≥10 areas at ×400 magnification
and ≥1,000 tumor cells were evaluated for each section. Tumor
slices scoring at least three points were defined as positive,
otherwise they were defined as negative (22).
Statistical analysis
The clinicopathological characteristics were
compared with the expression levels of RECK using the
χ2 test. The Kaplan-Meier method was used to analyze the
post-operative survival rate, and the survival differences were
analyzed using the log-rank test on the basis of the status of the
RECK expression. Any factor affecting the prognosis in a
univariate analysis was then estimated in a multivariate analysis
using Cox’s proportional hazard model with a forward conditional
stepwise procedure to determine whether the factor was acting
independently. All calculations were performed using SPSS version
13.0 (SPSS, Inc., Chicago, IL, USA) software and P<0.05 was
considered to indicate a statistically significant difference.
Results
Correlation of RECK protein expression
with clinicopathological patient features
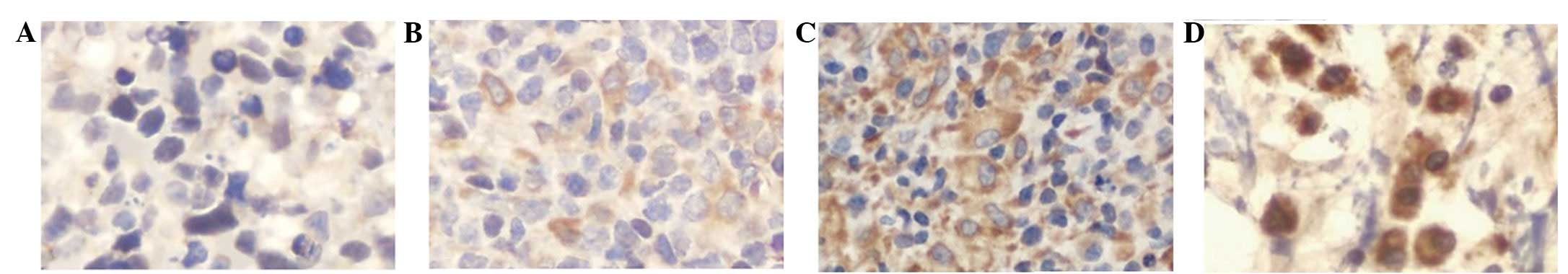
RECK staining was mainly observed in the
cytoplasm and plasma membrane of the tumor cells, often in a
granular pattern (Fig. 1). On the
basis of multiplying the intensity and proportion scores of
RECK in the tumors, 30 patients (36.6%) were classified as
RECK-positive and 52 (63.4%) as RECK-negative. No
correlation was revealed between the RECK status and the
age, gender, subtype or tumor stage (Table I). RECK expression was
inversely correlated with extranodal lymphomatous involvement
(P=0.012; Fig. 2).
Correlation of RECK protein expression
with prognostic factors
The univariate analyses of the prognostic factors
demonstrated that the RECK expression, response to
chemotherapy, IPI and tumor stage were significant prognostic
factors. No correlations were evident between the prognosis and the
age, gender or subtype. The 3-year survival rate of the
RECK-positive patients was 65.5%, which was significantly
higher than that of the RECK-negative patients (20.3%;
P=0.046; Fig. 3). A multivariate
analysis confirmed that RECK-positive protein expression was
an independent and significant factor for predicting a favorable
prognosis.
Discussion
PTCLs are a biologically diverse and uncommon group
of malignant tumors that share an poor prognosis. The cause of the
poor survival outcome of patients with PTCL compared with patients
with aggressive B-cell lymphomas remains largely unexplained.
Possible reasons include the rarity of these disorders and their
biological heterogeneity, which make them difficult to study.
Moreover, the inclusion of only small proportions of PTCL patients
in studies investigating therapies for aggressive B-cell lymphoma
has not aided these therapeutic strategies. For the majority of
PTCL subtypes, a poor outcome with a 5-year overall survival of
∼30% is reported in the predominance of studies (9,10,23–27).
Previous studies have shown that the parameters that may be
independent prognostic factors for survival in PTCL, excluding
anaplastic large cell lymphoma (ALCL), include age >60 years,
disseminated stage, LDH level higher than normal, performance
status and a higher IPI and tumor score (10). However, the superiority of these
parameters has not been well documented in subsequent studies
(28). It may be necessary to
investigate the molecular markers predicting the response to
chemotherapy, the overall prognosis and the likelihood of
extranodal lymphomatous involvement at diagnosis. This may also
provide targets for the development of new therapeutic agents.
RECK, a novel MMP inhibitor, was first
identified by Takahashi et al in the NIH3T3 cell line
transfected with the v-Ki-Ras gene (12). The RECK gene encodes a 110
kDa membrane-anchored glycoprotein that contains serine protease
inhibitor-like domains and multiple epidermal growth factor-like
repeats (12). Initially,
RECK was believed to be a novel transformation suppressor
gene, but it was later identified that RECK was able to
inhibit the secretion and activity of three MMPs; MMP-2, MMP-9 and
MTl-MMP. Numerous oncogenes, including ras, fos and
myc, downregulate the expression of RECK, indicating
that RECK may be a negatively-regulated target of oncogenes
(29). A previous study reported
that RECK is associated with angiogenesis and its
appropriate expression inhibits the development of blood vessels
(13). Studies have indicated that
there is a positive correlation between RECK expression in
tumors and the survival outcome of patients with other types of
tumors, including hepatocellular carcinoma (15), pancreatic cancer (14), breast cancer (16) and non-small cell lung cancer
(17). The correlation may
therefore be a common feature among numerous tumors and this
appears consistent with the previous findings showing that the
prognosis for patients that were positive for the expression of
RECK was better than for those who were negative.
In the present study, RECK expression in
biopsy specimens and its prognostic significance in patients with
PTCL was investigated. It was demonstrated that reduced RECK
expression was a significant factor for predicting a poor
prognosis. The clinicopathological analysis revealed that the
expression of RECK was not notably correlated with the age,
gender, pathological classification or tumor stage of the patients,
but that it was associated with extranodal lymphomatous involvement
and prognosis in PTCL. The results suggested that positive
RECK expression was significantly associated with a lower
proportion of extranodal lymphomatous involvement and a longer
overall survival, although further studies are required in order to
confirm this conclusion. In addition, clinicopathological factors
were revealed, including RECK expression and response to
chemotherapy, which may be useful prognostic determinants of
favorable overall survival in patients with PTCL.
Numerous studies have suggested that the expression
of RECK is associated with tumor metastasis and is useful as
an informative prognostic indicator for several neoplastic diseases
(15–17, 29).
In the present study, it was revealed that the patients who were
positive for the expression of RECK had less extranodal
lymphomatous involvement and a longer overall survival; these
results were consistent with those of previous reports. The 3-year
overall survival rate of this group of patients was 46% and the
median survival was 18 months. The 5-year survival rate of patients
with PTCLs is reported as ∼30% in the literature (9,10,23–27),
which is consistent with the present study.
Certain limitations have been noted in the design of
the present study. The study was an initial retrospective analysis
of patients with a relatively small sample size, which meant that
the study had limited statistical power and ultimately caused the
data to generate a certain amount of deviation.
In conclusion, a significant correlation between
RECK expression in PTCL and extranodal lymphomatous
involvement of the patients was identified. A positive correlation
between RECK expression and the survival rates of the
patients was also observed. These data are consistent with the
RECK model playing an active role in suppressing the
malignant phenotypes of PTCL cells. Practically, RECK
expression may be a good prognostic indicator in PTCL patients, and
therapeutic strategies based on RECK or its mechanism of
action may be of value in the treatment of this disease. Moreover,
the clinical use of RECK as a prognostic indicator requires
further evaluation.
Acknowledgements
The authors would like to thank Dr Jie
Liu and Jing Xiong, Department of Pathology, Tongji Hospital, for
the preparation of the histological sections and their excellent
technical assistance.
References
|
1
|
Lennert K and Feller AC: The diagnosis of
lymphoma. Histopathology of Non-Hodgkin’s Lymphomas (Based on the
Updated Kiel Classification). 2nd edition. Berlin: Springer-Verlag;
pp. 1–6. 1992
|
|
2
|
Harris NL, Jaffe ES, Stein H, Banks PM,
Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter
KC, et al: A revised European-American classification of lymphoid
neoplasms: a proposal from the International Lymphoma Study Group.
Blood. 84:1361–1392. 1994.PubMed/NCBI
|
|
3
|
Jaffe ES, Harris NL, Stein H and Vardiman
JW: Pathology and genetics of tumours of haematopoetic and
lymphphoid tissues. WHO Classification of Tumours. Kleihues P and
Sobin LH: 3. 1st edition. Lyon, France: IARC Press; pp. 1–351.
2001
|
|
4
|
Pileri S, Ralfkiaer E, Weisenburger D, et
al: Peripheral T-cell lymphoma, not otherwise specified. WHO
Classification of Tumors of Hematopoietic and Lymphoid Tissues.
Swerdlow S, Campo E, Harris NL, et al: 4th edition. Lyon: IARC; pp.
4292008
|
|
5
|
Vose J, Armitage J and Weisenburger D;
International T-Cell Lymphoma Project: International peripheral
T-cell and natural killer/T-cell lymphoma study: pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su IJ, Wang CH, Cheng AL, Chen YC, Hsieh
HC, Chen CJ, Tien HF, Woei-Tsay, Huang SS, Hu CY, et al:
Characterization of the spectrum of postthymic T-cell malignancies
in Taiwan. A clinicopathologic study of HTLV-l-positive and
HTLV-l-negative cases. Cancer. 61:2060–2070. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takagi N, Nakamura S, Ueda R, Osada H,
Obata Y, Kitoh K, Suchi T and Takahashi T: A phenotypic and
genotypic study of three node-based, low-grade peripheral T-cell
lymphomas: angioimmunoblastic lymphoma, T-zone lymphoma, and
lymphoepithelioid lymphoma. Cancer. 69:2571–2582. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura S, Suchi T, Koshikaua T, Suzuki
H, Oyama A, Kojima M, Motoori T, Ueda R and Takahashi T:
Clinicopathologic study of 212 cases of peripheral T-cell lymphoma
among the Japanese. Cancer. 72:1762–1772. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
López-Guillermo A, Cid J, Salar A, López
A, Montalbán C, Castrillo JM, González M, Ribera JM, Brunet S,
García-Conde J, Fernández de Sevilla A, Bosch F and Montserrat E:
Peripheral T-cell lymphomas: initial features, natural history, and
prognostic factors in a series of 174 patients diagnosed according
to the R.E.A.L. Classification. Ann Oncol. 9:849–855.
1998.PubMed/NCBI
|
|
10
|
Gisselbrecht C, Gaulard P, Lepage E,
Coiffier B, Brière J, Haioun C, Cazals-Hatem D, Bosly A, Xerri L,
Tilly H, Berger F, Bouhabdallah R and Diebold J: Prognostic
significance of T-cell phenotype in aggressive non-Hodgkin’s
lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood.
92:76–82. 1998.
|
|
11
|
No authors listed:. Effect of age on the
characteristics and clinical behavior of non-Hodgkin’s lymphoma
patients. The Non-Hodgkin’s Lymphoma Classification Project. Ann
Oncol. 8:973–978. 1997.
|
|
12
|
Takahashi C, Sheng Z, Horan TP, Kitayama
H, Maki M, Hitomi K, Kitaura Y, Takai S, Sasahara RM, Horimoto A,
Ikawa Y, Ratzkin BJ, Arakawa T and Noda M: Regulation of matrix
metalloproteinase-9 and inhibition of tumor invasion by the
membrane anchored glycoprotein RECK. Proc Natl Acad Sci USA.
95:13221–13226. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh J, Takahashi R, Kondo S, Mizoguchi A,
Adachi E, Sasahara RM, Imamura Y, Kitayama H, Alexander DB, Ide C,
Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M,
Itohara S, Takahashi C and Noda M: The membrane-anchored MMP
inhibitor RECK is a key regulator of extracellular matrix integrity
and angiogenesis. Cell. 107:789–800. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masui T, Doi R, Koshiba T, Fujimoto K,
Tsuji S, Nakajima S, Koizumi M, Toyoda E, Tulachan S, Ito D, Kami
K, Mori T, Wada M, Noda M and Imamura M: RECK expression in
pancreatic cancer: its correlation with lower invasiveness and
better prognosis. Clin Cancer Res. 9:1779–1784. 2003.PubMed/NCBI
|
|
15
|
Furumoto K, Arii S, Mori A, Furuyama H,
Gorrin Rivas MJ, Nakao T, Isobe N, Murata T, Takahashi C, Noda M
and Imamura M: RECK gene expression in hepatocellular carcinoma:
correlation with invasion-related clinicopathological factors and
its clinical significance. Reverse inducing - cysteine rich protein
with Kazal motifs. Hepatology. 33:189–195. 2001. View Article : Google Scholar
|
|
16
|
Span PN, Sweep CG, Manders P, Beex LV,
Leppert D and Lindberg RL: Matrix metalloproteinase inhibitor
reversion-inducing cysteine-rich protein with Kazal motifs: a
prognostic marker for good clinical outcome in human breast
carcinoma. Cancer. 97:2710–2715. 2003. View Article : Google Scholar
|
|
17
|
Takenaka K, Ishikawa S, Kawano Y,
Yanagihara K, Miyahara R, Otake Y, Morioka Y, Takahashi C, Noda M,
Wada H and Tanaka F: Expression of a novel matrix metalloproteinase
regulator, RECK, and its clinical significance in resected
non-small cell lung cancer. Eur J Cancer. 40:1617–1623. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeuchi T, Hisanaga M, Nagao M, Ikeda N,
Fujii H, Koyama F, Mukogawa T, Matsumoto H, Kondo S, Takahashi C,
Noda M and Nakajima Y: The membrane-anchored matrix
metalloproteinase (MMP) regulator RECK in combination with MMP-9
serves as an informative prognostic indicator for colorectal
cancer. Clin Cancer Res. 10:5572–5579. 2004. View Article : Google Scholar
|
|
19
|
Li Y, Zhang Y and Zheng Q: Expression of
RECK gene and MMP-9 in hilar cholangiocarcinoma and its clinical
significance. J Huazhong Univ Sci Technolog Med Sci. 25:552–554.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK, Vardiman J, Lister TA and Bloomfield CD:
The World Health Organization classification of neoplastic diseaes
of the hematopietic and lymphoid tissues. Report of the Clinical
Advisor Committee meeting, Airlie House, Virginia, November 1997.
Ann Oncol. 10:1419–1432. 1999. View Article : Google Scholar
|
|
21
|
No author listed:. A predictive model for
aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s
Lymphoma Prognostic Factors Project. N Engl J Med. 329:987–994.
1993.
|
|
22
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melnyk A, Rodriguez A, Pugh WC and
Cabannillas F: Evaluation of the Revised European-American Lymphoma
classification confirms the clinical relevance of immunophenotype
in 560 cases of aggressive non-Hodgkin’s lymphoma. Blood.
89:4514–4520. 1997.PubMed/NCBI
|
|
24
|
Savage KJ, Chhanabhai M, Gascoyne RD and
Connors JM: Characterization of peripheral T-cell lymphomas in a
single North American institution by the WHO classification. Ann
Oncol. 15:1467–1475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gallamini A, Stelitano C, Calvi R, Bellei
M, Mattei D, Vitolo U, Morabito F, Martelli M, Brusamolino E,
Iannitto E, Zaja F, Cortelazzo S, Rigacci L, Devizzi L, Todeschini
G, Santini G, Brugiatelli M and Federico M; Intergruppo Italiano
Linfomi: Peripheral T-cell lymphoma unspecified (PTCL-U): a new
prognostic model from a retrospective multicentric clinical study.
Blood. 103:2474–2479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheung MM, Chan JK, Lau WH, Foo W, Chan
PT, Ng CS and Ngan RK: Primary non-Hodgkin’s lymphoma of the nose
and nasopharynx: clinical features, tumor immunophenotype, and
treatment outcome in 113 patients. J Clin Oncol. 16:70–77.
1998.
|
|
27
|
Chim CS, Ma SY, Au WY, Choy C, Lie AK,
Liang R, Yau CC and Kwong YL: Primary nasal natural killer cell
lymphoma: long-term treatment outcome and relationship with the
International Prognostic Index. Blood. 103:216–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piccaluga PP, Agostinelli C, Gazzola A,
Mannu C, Bacci F, Sabattini E and Pileri SA: Prognostic markers in
peripheral T-cell lymphoma. Curr Hematol Malig Rep. 5:222–228.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasahara RM, Takahashi C and Noda M:
Involvement of the Sp1 site in ras-mediated downregulation of the
RECK metastasis suppressor gene. Biochem Biophys Res Commun.
264:668–675. 1999. View Article : Google Scholar : PubMed/NCBI
|