Introduction
Radiotherapy is an effective treatment for head and
neck cancer and also forms the first-line treatment for
nasopharyngeal carcinoma (NPC). During NPC radiotherapy, the
thyroid may be partially or fully exposed to the radiation field
due to excessive cervical lymph drainage. Various thyroid
dysfunctions are induced by damage resulting from the relatively
high doses of radiation administered to the thyroid during NPC
radiotherapy, among which hypothyroidism is the most common.
Radiation-induced damage to the pituitary gland may also cause
central hypothyroidism. However, hyperthyroidism induced by NPC
radiotherapy is extremely rare (1–3). The
present study reports a case of Grave’s disease induced by NPC
radiotherapy in a patient admitted to The Second Xiangya Hospital
of Central South University (Hunan, China). This study was approved
by the ethics committee of the SecondXiangya Hospital of Central
South University, Changsha, China. Written informed consent was
obtained from the patient.
Case report
Clinical presentation, diagnosis and
treatment
A 40-year-old male was admitted to The Second
Xiangya Hospital of Central South University with a neck mass that
had been present for 4 months and retractable epistaxis that had
been observed for 2 months. The patient had a history of good
health with no history of thyroid-related disease. Upon physical
examination, several enlarged lymph nodes that were solid and
difficult to move, could be palpated in the upper neck. The largest
lymph node was located in the upper right side of the neck and
measured ∼4.3 cm in size. The largest lymph node in the upper left
side of the neck measured ∼3.2 cm. A CT scan of the nasopharynx
revealed a mass on the right wall and narrowing of the right
parapharyngeal space. An ultrasound of the superficial lymph nodes
demonstrated several enlarged lymph nodes in the neck. An
examination using a nasopharyngeal fiberscope revealed a mass on
the right wall of the nasopharynx and the biopsy results
demonstrated a poorly-differentiated squamous cell carcinoma. ECG,
plain chest film X-rays and ultrasound examinations of the liver,
gall bladder, pancreas, spleen and kidneys all yielded normal
results. According to the observed clinical manifestations, the
patient was diagnosed with NPC (poorly-differentiated squamous cell
carcinoma, T2N2M0, stage III). Concurrent chemoradiation therapy
was administered to the patient. Routine radiotherapy at a dose of
DT 72 Gy/36 F/7.5 W (6 MV X-ray) was administered to the primary
site and a dose of DT 50 Gy/25 F/5 W was administered to the lower
neck tangential radiation field (6 MV X-ray). The radiation dose
administered to the posterior upper neck was 66 Gy/33 F/7 W (36
Gy/18 F X-ray and 30 Gy/15 F β-ray). Following radiation therapy,
residual lymph nodes in the right posterior neck were evident;
therefore, a dose of 10 Gy/5 F/10 MeV β-ray was additionally
administered. During radiotherapy, two cycles of concurrent
chemotherapy, including docetaxel 75 mg/m2 IVGTT on day
1 (d1) every 3 weeks and carboplatin 300 mg/m2 d1 IVGTT
every 3 weeks, were administered. The treatment of the patient was
terminated in June 2010. A physical examination subsequent to the
treatment demonstrated that the enlarged lymph nodes previously
present in the neck were significantly reduced in size. An
examination using a nasopharyngeal fiberscope revealed a smooth
mucous membrane with no masses.
Patient follow-up
The patient was regularly followed up at The Second
Xiangya Hospital of Central South University, and no local relapse
or metastasis was observed. In February 2012, the patient started
to complain of photophobia and swelling of the eyes. The symptoms
became aggravated in May 2012, and several additional symptoms,
including fatigue, insomnia, irritability, palpitations and an
increased appetite were noted. A physical examination revealed
protrusion of the eyes, with a 16-mm exophthalmos of the left eye
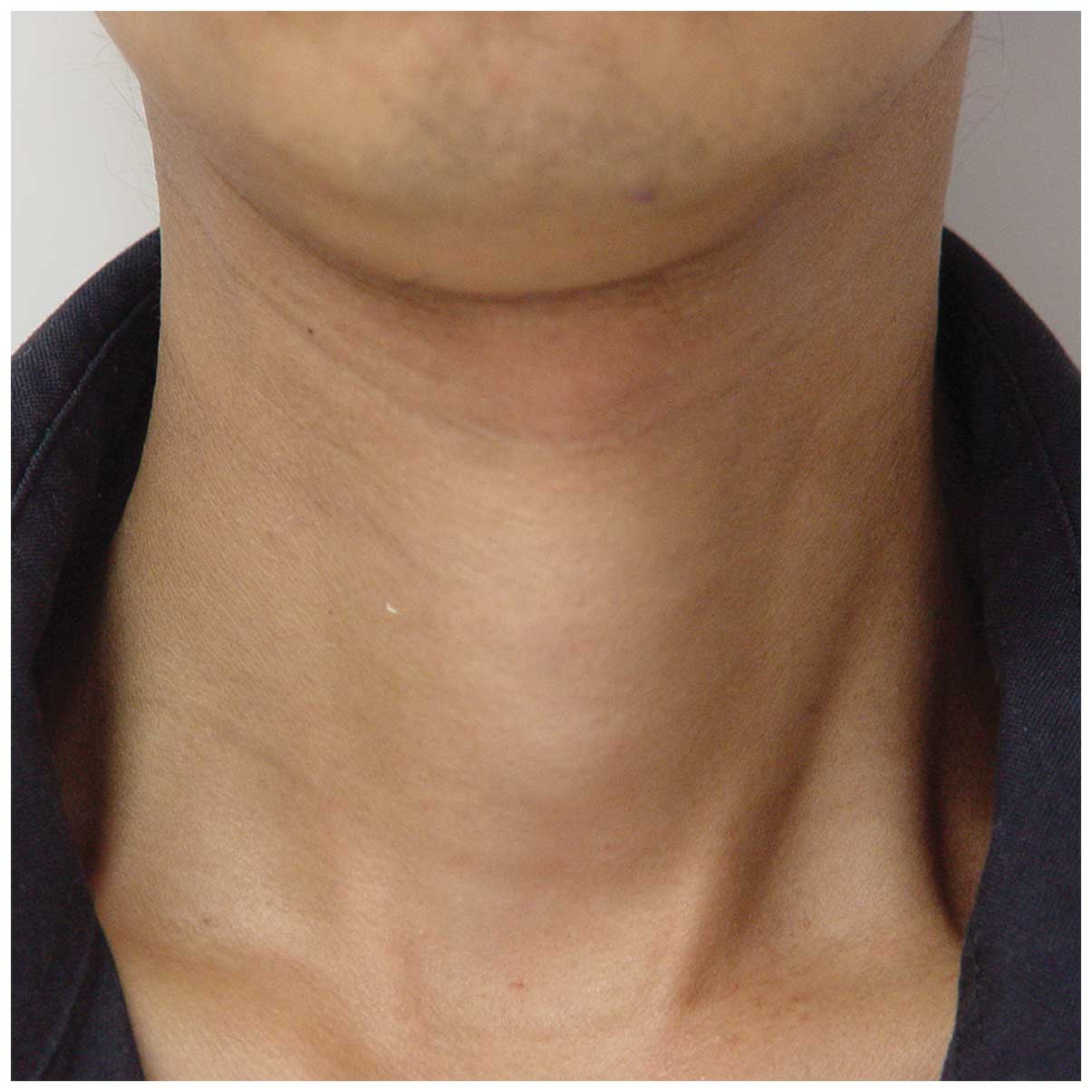
and a 17-mm exophthalmus of the right eye. The thyroid was palpated
Iº enlargement (Fig. 1). The
patient’s heart rate was measured at 102 bpm. The patient felt a
tremor in his hands and tongue. No positive signs of rales,
tenderness or tension in the lung and abdomen were observed upon
examination. An examination of thyroid function revealed the
following: Thyroid-stimulating hormone (TSH) <0.005 mIU/l
(normal, 0.27–4.20 mIU/l); triiodothyronine (T3), 6.47 nmol/l
(normal, 1.30–3.10 nmol/l); thyroxine (T4), 300.5 nmol/l (normal,
66.0–181.0 nmol/l); free (F)T3, 24.90 pmol/l (normal, 3.10–6.80
pmol/l); and FT4, 61.0 pmol/l (normal, 12.0–22.0 pmol/l). An
examination of the thyroid-related antibody levels revealed the
following results: anti-thyroglobulin (TG), 262.500 IU/l (normal,
0.000–115.000 IU/l); anti-thyroid peroxidase (TPO), 246.300 IU/l
(normal, 0.000–34.000 IU/l); anti-thyrotropin receptor (TSHR),
7.930 IU/l (normal, 0.000–1.750 IU/l); and TSH t30<0.011 mIU/l
(normal, 2–10 mIU/l). Ultrasonography demonstrated a mild diffusive
swelling of the thyroid with abundant blood perfusion. A
radionuclide scan of the thyroid showed uniform density with no
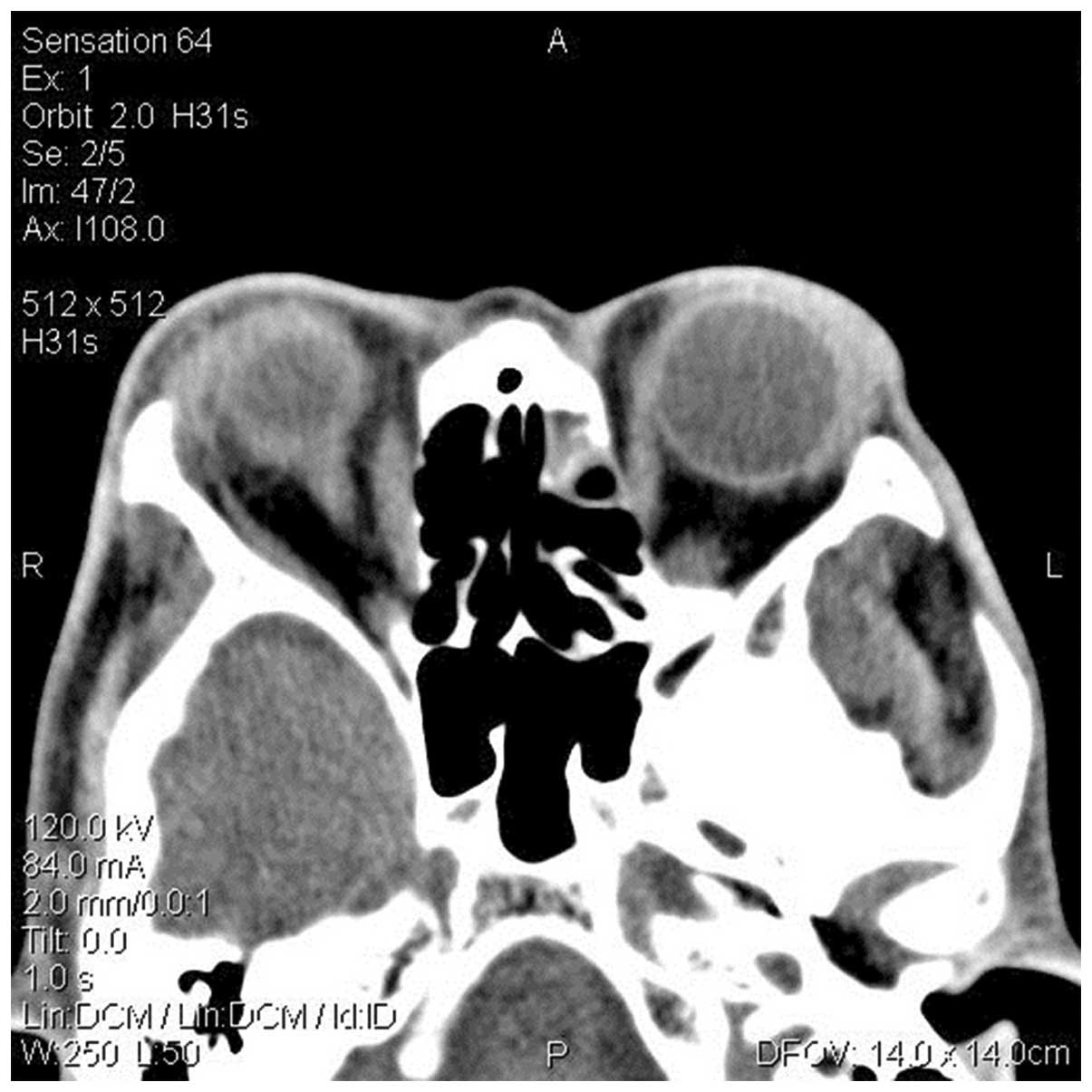
obvious abnormal uptake or sparse defects. A CT scan revealed
rectal thickening (Fig. 2). The
patient was diagnosed with Grave’s disease, and propylthiouracil
(100 mg orally, three times daily) and propranolol (10 mg orally,
twice daily) were consequently administered. One month later, a
thyroid function test showed TSH levels of 0.007 mIU/l (normal,
0.27–4.20 mIU/l) and T3, T4, FT3 and FT4 levels within the normal
ranges. The patient’s symptoms improved, including those of
palpitations, insomnia and fatigue. A physical examination
demonstrated a heart rate of 80 bpm. The swelling of the eyes did
not improve however, therefore, prednisone was administered.
Discussion
The thyroid is the largest endocrine organ in the
human body. Various thyroid dysfunctions, including hypothyroidism,
thyroiditis, benign thyroid tumors and thyroid carcinoma, may be
induced by damage caused by head and neck cancer radiotherapy
(1). Hypothyroidism is the most
commonly observed dysfunction with an incidence of 20–30% (1). However, hyperthyroidism induced by
radiotherapy is extremely rare and the earliest studies reporting
this condition date back to the 1970s (2,3). In
the present study, the patient developed hyperthyroidism and/or
Grave’s disease following external radiation therapy for a
non-thyroid carcinoma (2,3). Grave’s disease is the most common
cause of hyperthyroidism, constituting 80–85% of all cases. The
incidence of radiotherapy-induced Grave’s disease is 0.1–0.5%
(4). It has been reported that the
incidence of Grave’s disease in the population that has received
thyroid radiation is 7–20-times higher than for those who have not
received thyroid radiation (45 neck carcinoma may lead to thyroid
damage. The majority of patients present with clinical or
subclinical hypothyroidism, while a limited number present with
thyrotoxic symptoms, including hyperactivation of the neural,
circulatory and digestive systems and hypermetabolism (1). Thyrotoxicity may be divided into two
subtypes, hyperthyroidism and non-hyperthyroidism, according to the
function of the thyroid. It has been reported that non-hyperthyroid
thyrotoxicosis (transient thyroiditis or unsymptomatic thyroiditis)
may be induced following head and neck radiotherapy for thorax
cancer (6), Hodgkin’s lymphoma
(7–9) and tonsil carcinoma (10). Slacmeulder et al (11) reported the cases of 2 children who
presented with hyperthyroidism following head and neck
radiotherapy; three years after the radiotherapy, one of the
patients presented with medulloblastoma, hyperthyroidism, diffusive
thyroid enlargement and negative activity of the anti-TSH receptor
antibody. Methimazole was effective in treating the symptoms. All
the observed clinical manifestations met with the diagnostic
criteria of Grave’s disease.
To the best of our knowledge, the present study is
the first in China to report that Grave’s disease may be induced by
radiotherapy of the head and neck. In the present patient,
hyperthyroidism, diffusive thyroid enlargement, protrusion of the
eyes and positive anti-thyroid antibody activity, appeared 2 years
after the radiotherapy for NPC. Taken together, these symptoms led
to the diagnosis of Grave’s disease. The pathophysiological
mechanism of radiotherapy-induced hyperthyroidism is unclear. One
potential mechanism is that the release of antigen subsequent to
radial thyroid damage facilitates the production of thyroid-related
antibodies, which leads to chronic autoimmune thyroiditis (12). Another potential mechanism is
considered to be the direct cytotoxic activity of the radiation
(13). The precise mechanism of
radiation-induced hyperthyroidism requires further
investigation.
Hyperthyroidism is a rare complication of head and
neck cancer, which may be neglected as a result of exhibiting
similar clinical manifestations to other complications during and
following the treatment of cancer. In addition, other thyroid
dysfunctions, including hypothyroidism, are commonly observed
following head and neck radiotherapy. It is therefore necessary to
monitor thyroid function following head and neck radiotherapy.
References
|
1.
|
Jereczek-Fossa BA, Alterio D, Jassem J,
Gibelli B, Tradati N and Orecchia R: Radiotherapy-induced thyroid
disorders. Cancer Treat Rev. 30:369–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wasnich RD, Grumet FC, Payne RO and Kriss
JP: Graves’ ophthalmopathy following external neck irradiation for
nonthyroidal neoplastic disease. J Clin Endocrinol Metab.
37:703–713. 1973.
|
|
3.
|
Jackson R, Rosenberg C, Kleinmann R,
Vagenakis AG and Braverman LE: Ophthalmopathy after neck
irradiation therapy for Hodgkin’s disease. Cancer Treat Rep.
63:1393–1395. 1979.
|
|
4.
|
Hancock SL, Cox RS and McDougall IR:
Thyroid diseases after treatment of Hodgkin’s disease. N Engl J
Med. 325:599–605. 1991.
|
|
5.
|
Hancock SL, McDougall IR and Constine LS:
Thyroid abnormalities after therapeutic external radiation. Int J
Radiat Oncol Biol Phys. 31:1165–1170. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fachnie JD and Rao SD: Painless
thyroiditis with hyperthyroidism following external irradiation to
the neck. Henry Ford Hosp Med J. 28:149–151. 1980.PubMed/NCBI
|
|
7.
|
Constine LS, Donaldson SS, McDougall IR,
Cox RS, Link MP and Kaplan HS: Thyroid dysfunction after
radiotherapy in children with Hodgkin’s disease. Cancer.
53:878–883. 1984.
|
|
8.
|
Blitzer JB, Paolozzi FP, Gottlieb AJ,
Zamkoff KW and Chung CT: Thyrotoxic thyroiditis after radiotherapy
for Hodgkin’s disease. Arch Intern Med. 145:1734–1735.
1985.PubMed/NCBI
|
|
9.
|
Petersen M, Keeling CA and McDougall IR:
Hyperthyroidism with low radioiodine uptake after head and neck
irradiation for Hodgkin’s disease. J Nucl Med. 30:255–257.
1989.PubMed/NCBI
|
|
10.
|
Bryer-Ash M, Lodhi W, Robbins K and
Morrison R: Early thyrotoxic thyroiditis after radiotherapy for
tonsillar carcinoma. Arch Otolaryngol Head Neck Surg. 127:209–211.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Slacmeulder M, Brauner R, Guilhaume B,
Habrand JL, Kalifa C and Hartmann O: Post-radiotherapy
hyperthyroidism: a rare complication of cancer treatment in the
child. Arch Pediatr. 10:42–44. 2003.(In French).
|
|
12.
|
Nishiyama K, Tanaka E, Tarui Y, Miyauchi K
and Okagawa K: A prospective analysis of subacute thyroid
dysfunction after neck irradiation. Int J Radiat Oncol Biol Phys.
34:439–444. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hopewell JW: The importance of vascular
damage in the development of late radiation effects in normal
tissues. Radiation Biology in Cancer Research. Meyn RE and Withers
HR: Raven Press; New York: pp. 449–459. 1980
|