Introduction
Human nasopharyngeal carcinoma (NPC) is one of the
most common types of cancer in Southern China (1). NPC is a relatively radiosensitive
disease, although the majority of NPC patients suffer from
recurrence and metastasis within 1.5 years of treatment (2). Chemotherapy is a necessary treatment
for NPC patients (3) and the
potential complications and side-effects (e.g., neutropenia and
immunosuppression) of these drugs limit the application of
chemotherapy in NPC. Thus the identification of novel
anticarcinogenic agents with high efficacy, low toxicity and known
mechanisms of action is crucial. A number of studies have focused
on extracting active ingredients from natural plants to prevent and
treat cancer and investigating their anticancer mechanisms
(4).
Curcumin, a phenolic compound extracted from the
plant Curcuma longa, exhibits a wide range of
pharmacological effects, including anti-inflammatory,
anticarcinogenic, hypocholesterolemic and anti-infection
activities. Due to its regulation of multiple cellular pathways,
studies have focused on its clinical importance for the treatment
of different types of cancer (5).
More significantly, curcumin has no or low cytotoxicity in normal
cells in vitro (6) and
inhibited carcinogenesis of various types of cancer without notable
treatment-related toxicity in a phase I study (7). Curcumin is safe in humans; a dose of
10 g/day has been shown not to produce treatment-related toxicity
(8).
Curcumin exhibits photobiological and
photosensitizing activity (9). It
has been reported that curcumin combined with light irradiation
exhibits more marked anticancer effects than curcumin without
irradiation (10). Certain studies
have used curcumin as a photosensitizer in photodynamic therapy to
treat cancer (11,12). Curcumin is sensitive to ultraviolet
and visible light (13). The
greatest absorption peak of curcumin is at 408 nm (14), so in the present study a purple LED
light (405 nm) was used to excite curcumin. Thus far, the direct
cytotoxic effect of curcumin on NPC cells following purple-light
(PL) irradiation has not been reported and this was the main
purpose of the present study.
Materials and methods
Chemicals and reagents
Curcumin,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) and propidium iodide (PI) were obtained from Sigma-Aldrich
Chemical Co. (St. Louis, MO, USA). 2,7-Dichlorodihydrofluorescein
diacetate (DCFH-DA) and Hoechast 33342 were purchased from
Molecular Probes (Invitrogen, Eugene, OR, USA). The culture medium
RPMI-1640, fetal bovine serum (FBS), penicillin-streptomycin and
L-glutamine were purchased from GIBCO BRL (Invitrogen, Grand
Island, NY, USA).
Cell culture
The human NPC cell lines, CNE1 and CNE2, were
obtained from the Cancer Center of Sun Yat-Sen University
(Guangzhou, China) and cultured in RPMI-1640 medium containing 10%
FBS and penicillin-streptomycin sulfate. All cell lines were
incubated at 37°C in an atmosphere of 5% CO2.
Cell viability assays
The MTT assay was used to evaluate the anticancer
effect on cell viability. For the curcumin group, the cells were
seeded at a density of 1×104/well into 96-well plates
for 24 h and incubated with curcumin for 2 h. Fresh medium was then
added into each well. The curcumin followed by PL irradiation
groups were then exposed to PL irradiation at various energy
densities and fresh medium was added. After incubation for 24 h,
MTT reagent was added and the cells were incubated for 4 h, lysed
with DMSO and quantitated using a plate reader.
Morphological changes
The cells were plated on to 6-well plates at a
density of 2×105 cells/well overnight and then divided
into three groups (control, curcumin and curcumin + PL groups).
After 24 h, the cells were fixed with methanol and then stained
with Hoechst 33342 (10 μg/ml for 15 min) and washed with
PBS. A fluorescence microscope was used to observe the apoptotic
morphological changes.
Cell cycle and apoptosis
determination
CNE1 and CNE2 cells (∼3×105 cells/well)
in 6-well plates were incubated with 40 μM curcumin for 2 h
and then irradiated with PL at 0.2 J/cm2. The cells were
harvested by centrifugation and fixed in cold 70% ethanol at 4°C
overnight (≥12 h). The fixed cells were washed with PBS and stained
with PI containing RNase A at 10 μg/ml. The cells were
separated by flow cytometry (FACScalibur, Becton Dickinson, San
Jose, CA, USA) and the results were analyzed using ModFit Software.
The sub-G1 groups (apoptosis) were calculated and
analyzed using CellQuest (Becton-Dickinson) and ModFit Software
(Verity Software House Inc., Topsham, ME, USA).
Detection of reactive oxygen species
(ROS)
CNE1 and CNE2 cells (∼3×105 cells/well)
were seeded into 6-well plates overnight and treated using various
methods. The cells were harvested and washed twice, re-suspended in
500 μl of DCFH-DA (10 μM) and the levels of ROS were
analyzed by flow cytometry.
Statistical analysis
At least three independent experiments were
performed for the statistical evaluation. Data are presented as the
mean ± SEM. The statistical analysis of the results was performed
using the Student’s t-test (two-tailed, unpaired) if two groups
were compared or one-way analysis of variance if there were more
than two groups. P<0.05 was considered to indicate statistically
significant differences.
Results
Enhanced cytotoxicity of curcumin in NPC
cells following PL irradiation
As shown in Fig. 1,
the percentage of viable cells in the curcumin groups decreased
with the IC50 at 157.5 μM in the CNE1 cells and
225.2 μM in the CNE2 cells. Curcumin treatment followed by
PL irradiation enhanced the effect in an energy density-dependent
manner (Fig. 1D). The
IC50 values of the CNE1 and CNE2 cells treated with
curcumin and PL irradiation at 0.2 J/cm2 decreased to
22.52 and 68.2 μM, respectively. Treatment with 40 μM
curcumin and 0.2 J/cm2 energy density was used in the
subsequent experiments.
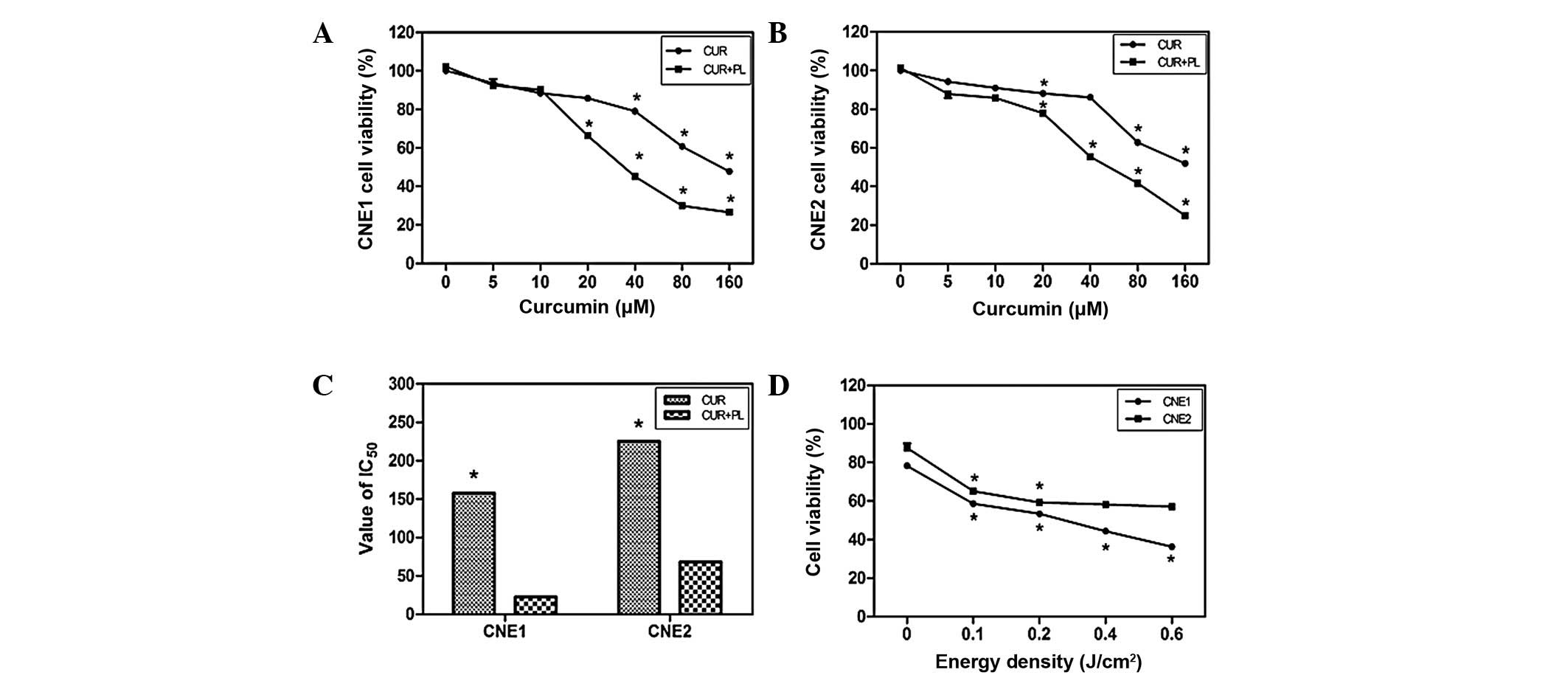 | Figure 1.Viability of CNE1 and CNE2 cells
following various treatments. (A and B) In the curcumin group, the
CNE1 and CNE2 cells were treated with curcumin (5, 10, 20, 40, 80
and 160 μM) for 2 h, then washed with fresh medium and after
24 h the percentage of living cells was determined. In the curcumin
+ PL group, the cells were incubated with curcumin for 2 h, and
washed, followed by PL irradiation at 0.2 J/cm2. The
percentage of living cells in the treated groups vs. the untreated
controls was then measured. (C) IC50 of NPC cells in the
curcumin and curcumin + PL groups. (D) NPC cells were incubated
with curcumin (40 μM) for 2 h, washed, then irradiated with
PL at various energy densities (0.1, 0.2, 0.4 and 0.6
J/cm2) and subsequently the cell viability was
calculated. Data are the mean ± SE; *P<0.01 vs.
control. PL, purple light; NPC, nasopharyngeal carcinaoma; CUR,
curcumin. |
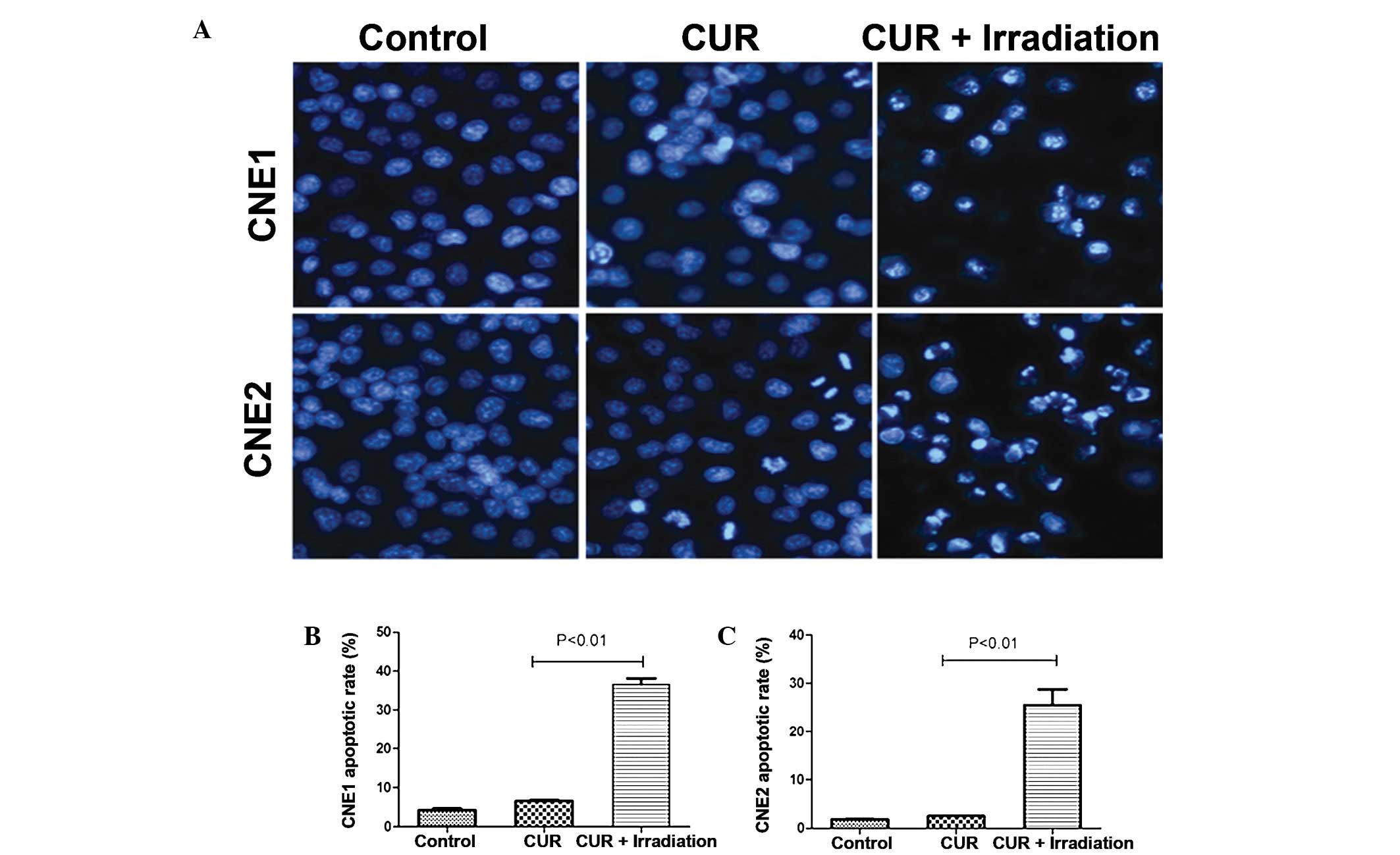
Effect of curcumin on NPC cell morphology
following PL irradiation
Alterations to the cells’ nuclear morphology were
studied using Hoechst 33342 staining to assess whether curcumin
followed by PL irradiation induced NPC cell death by apoptosis. As
shown in Fig. 2A, the typical
morphological features of apoptosis were observed, as characterized
by marked chromatin condensation and nuclear fragmentation. The
number of cells exhibiting nuclei with condensed chromatin
increased significantly after treatment with curcumin followed by
PL irradiation.
Cell cycle arrest and apoptosis of NPC
cells after treatment with curcumin followed by PL irradiation
The sub-G1 peaks indicating the
proportion of apoptotic cells increased to 36.6% in the CNE1 cells
and 25.5% in the CNE2 cells when curcumin (40 μM) was
exposed to 0.2 J/cm2 PL irradiation compared with the
curcumin treatment group (Fig. 2B and
C).
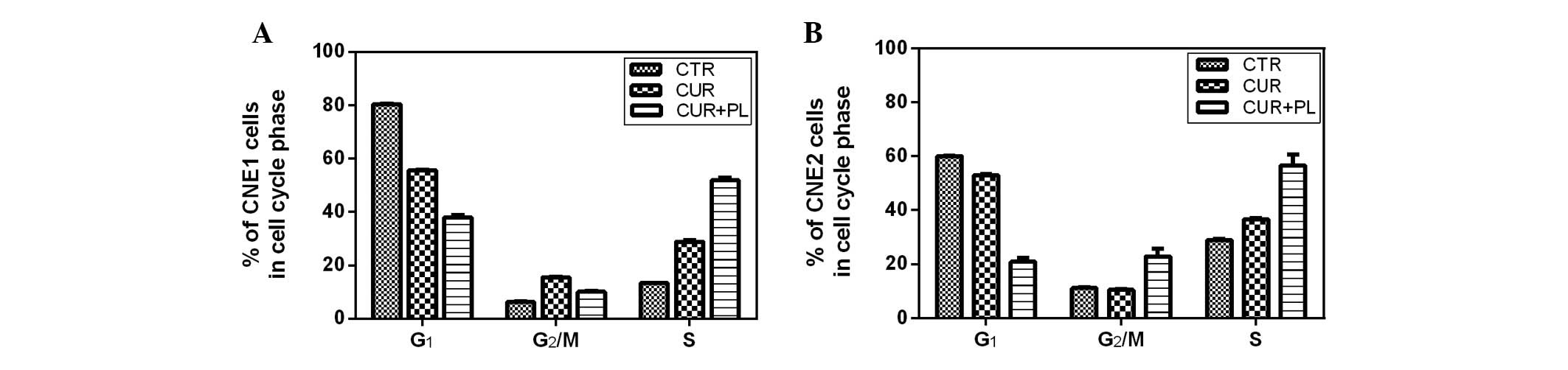
The cell cycle distribution of the CNE1 and CNE2
cells after treatment with curcumin and curcumin followed by PL
irradiation for 24 h is shown in Fig.
3. The majority of CNE1 cells treated with curcumin (40
μM) followed by PL irradiation at 0.2 J/cm2 were
arrested at the S phase and the proportion of S phase cells
increased to 51.9%. The proportion of G2/M phase cells
among the CNE2 cells was double that of the control group treated
with curcumin and the proportion of S phase cells was 56.6%.
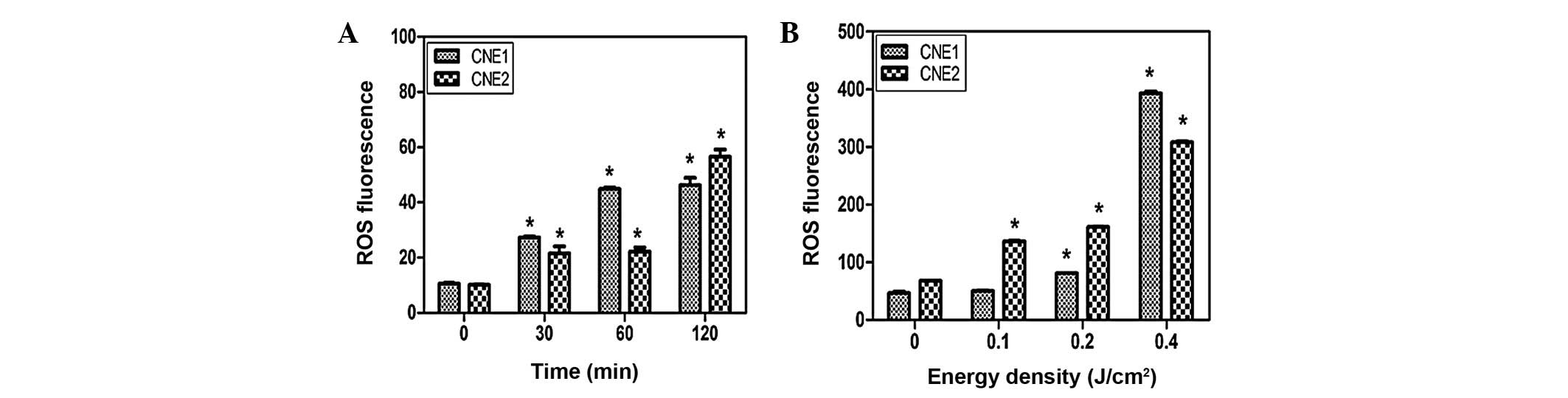
Effect of curcumin on ROS production in
NPC cells following PL irradiation
CNE1 and CNE2 cells were incubated with curcumin (40
μM) for 30, 60 and 120 min. The relative level of ROS
increased from 10.5 to 46.2 in CNE1 cells and 10.1 to 56.5 in CNE2
cells (Fig. 4A) between 0 and 120
min. Furthermore, the ROS fluorescence value of the CNE1 cells
increased from 9.6 to 392.8 between 0 and 0.4 J/cm2,
while in CNE2 cells it increased to 308.1. Compared with the
curcumin group, ROS generation was greatly increased when the cells
were incubated with curcumin for 2 h followed by PL irradiation at
an energy density of 0.2 J/cm2.
Discussion
Curcumin, which possesses anticancer activity, is
widely used as a chemopreventive agent in numerous types of cancer,
including breast, lung, colon, prostate, stomach, kidney, ovary,
brain and blood cancer (15). Few
studies have focused on NPC (16,17).
In China, >95% of NPCs are nonkeratinizing carcinoma while
<5% are keratinizing carcinoma, and thus CNE1 (keratinizing
carcinoma) and CNE2 (nonkeratinizing carcinoma) cells were used to
represent the two main histological types in the present study
(18). As reported previously,
curcumin is sensitive to sun- or UV light (19). When curcumin was combined with
exposure to visible (20) or
blue-filtered light (11), it
exhibited more marked anticancer effects than by itself. In
addition, it was previously reported that the photobiological
activity of curcumin was due to its excited state rather than the
products of the photodegradation of curcumin, such as ferulic acid
and vanillin (21). Koon et
al also clarified that curcumin was rapidly absorbed in the
first 1 h. Due to this, the NPC cells were incubated with curcumin
for 2 h, washed with fresh medium and finally exposed to PL to
produce the excited state of curcumin (11).
In the present study, it was observed that curcumin
was cytotoxic towards CNE1 and CNE2 cells in a dose-dependent
manner and the cytotoxicity in CNE1 cells was more marked than that
in CNE2 cells. The cytotoxic effect of curcumin following PL
irradiation was greater than that of curcumin alone. Curcumin
treatment followed by PL irradiation enhanced the effect in an
energy density-dependent manner and exhibited increased
cytotoxicity compared with the curcumin group.
The most studied property of photo-actived curcumin
is its pro-apoptotic effect. Park and Lee observed the
photosensitizer effect of curcumin in UVB-irradiated HaCaT cells
via the activation of caspase pathways (12). Dujic et al demonstrated the
effect on apoptosis, showing the enhanced activation of caspase-9
(22). By contrast, Chan and Wu
reported that curcumin inhibited apoptosis in photosensitized A431
cells (23). Thus curcumin had a
two-sided effect which was dependent on the concentration, cell
lines and cellular micro-environment. The present data demonstrated
that curcumin treatment followed by PL irradiation induced
apoptosis in NPC cells and the apoptotic effect was more marked
than that of curcumin alone.
Curcumin passes through the plasma membrane and
induce ROS generation. Intracellular ROS damage mitochondrial and
nuclear DNA and lead to apoptosis (24). The photoexcited state of curcumin is
able to increase the level of singlet-oxygen (14). Atsumi T also demonstrated that
visible light irradiation following curcumin treatment greatly
enhanced the pro-apoptotic effect due to the increase in ROS
levels. The ROS levels were measured to demonstrate the important
role of ROS in curcumin treatment followed by PL
irradiation-induced apoptosis in NPC cells. From the present data,
we suggest that ROS may be more important in photoactivated
curcumin-induced apoptosis compared with curcumin alone.
Besides apoptosis, the dysregulation of the cell
cycle also contributes to tumorigenesis (25). CNE1 and CNE2 cells were arrested at
the S and G2/M phases as reported in breast cancer by
Mehta et al (26).
Furthermore, CNE1 cells treated with curcumin followed by PL
irradiation were mainly arrested at the S phase, while CNE2 cells
were arrested at the G2/M and S phases. Apoptotic
induction and cell cycle arrest contribute to the anticancer effect
of curcumin following PL irradiation.
In summary, curcumin treatment followed by PL
irradiation enhances the cytotoxicity against CNE1 and CNE2 cells
through the potential induction of apoptosis and ROS generation.
The treatment promoted S or G2/M phase arrest in the two
cell lines. Taken together, the data indicate that curcumin PL
exposure may be a potentially effective therapy for NPC.
Acknowledgements
The authors would like to thank
Professor Huiling Yang for critical reading of the manuscript.
References
|
1.
|
Her C: Nasopharyngeal cancer and the
Southeast Asian patient. Am Fam Physician. 63:1776–1782.
2001.PubMed/NCBI
|
|
2.
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270.
1992.PubMed/NCBI
|
|
3.
|
Ahmad A and Stefani S: Distant metastases
of nasopharyngeal carcinoma: a study of 256 male patients. J Surg
Oncol. 33:194–197. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
6.
|
Kunwar A, Barik A, Mishra B, Rathinasamy
K, Pandey R and Priyadarsini KI: Quantitative cellular uptake,
localization and cytotoxicity of curcumin in normal and tumor
cells. Biochim Biophys Acta. 1780:673–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cheng AL, Hsu CH, Lin JK, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
8.
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008.
|
|
9.
|
Dahl TA, Bilski P, Reszka KJ and Chignell
CF: Photocytotoxicity of curcumin. Photochem Photobiol. 59:290–294.
1994. View Article : Google Scholar
|
|
10.
|
López-Jornet P, Camacho-Alonso F and
Gómez-Garcia F: Effect of curcumin and irradiation in PE/CA-PJ15
oral squamous cell carcinoma. Acta Odontol Scand. 69:269–273.
2011.PubMed/NCBI
|
|
11.
|
Koon H, Leung AW, Yue KK and Mak NK:
Photodynamic effect of curcumin on NPC/CNE2 cells. J Environ Pathol
Toxicol Oncol. 25:205–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Park K and Lee JH: Photosensitizer effect
of curcumin on UVB-irradiated HaCaT cells through activation of
caspase pathways. Oncol Rep. 17:537–540. 2007.PubMed/NCBI
|
|
13.
|
Jain V, Prasad V, Pal R and Singh S:
Standardization and stability studies of neuroprotective lipid
soluble fraction obtained from Curcuma longa. J Pharm Biomed
Anal. 44:1079–1086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nardo L, Andreoni A, Bondani M, Másson M
and Hjorth Tønnesen H: Studies on curcumin and curcuminoids. XXXIV.
Photophysical properties of a symmetrical, non-substituted curcumin
analogue. J Photochem Photobiol B. 97:77–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
López-Lázaro M: Anticancer and
carcinogenic properties of curcumin: considerations for its
clinical development as a cancer chemopreventive and
chemotherapeutic agent. Mol Nutr Food Res. 52(Suppl 1): S103–S127.
2008.PubMed/NCBI
|
|
16.
|
Yang FW, Huang JZ, Lin XL, Zhen ZN and
Chen XM: Apoptosis in nasopharyngeal carcinoma cell line NCE
induced by curcumin and its molecular mechanism. Zhonghua Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 41:612–616. 2006.(In Chinese).
|
|
17.
|
Lin YT, Wang LF and Hsu YC: Curcuminoids
suppress the growth of pharynx and nasopharyngeal carcinoma cells
through induced apoptosis. J Agric Food Chem. 57:3765–3770. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zheng XK, Chen LH, Wang WJ, Ye F, Liu JB,
Li QS and Sun HW: Impact of prolonged fraction delivery times
simulating IMRT on cultured nasopharyngeal carcinoma cell killing.
Int J Radiat Oncol Biol Phys. 78:1541–1547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chignell CF, Bilski P, Reszka KJ, Motten
AG, Sik RH and Dahl TA: Spectral and photochemical properties of
curcumin. Photochem Photobiol. 59:295–302. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Atsumi T, Tonosaki K and Fujisawa S:
Comparative cytotoxicity and ROS generation by curcumin and
tetrahydrocurcumin following visible-light irradiation or treatment
with horseradish peroxidase. Anticancer Res. 27:363–371.
2007.PubMed/NCBI
|
|
21.
|
Khurana A and Ho CT: High performance
liquid chromatography analysis of curcuminoids and their
photo-oxidative decomposition compounds in Curcuma longa L.
J Liq Chromatogr. 11:2295–2304. 1988. View Article : Google Scholar
|
|
22.
|
Dujic J, Kippenberger S, Ramirez-Bosca A,
et al: Curcumin in combination with visible light inhibits tumor
growth in a xenograft tumor model. Int J Cancer. 124:1422–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chan WH and Wu HJ: Anti-apoptotic effects
of curcumin on photosensitized human epidermal carcinoma A431
cells. J Cell Biochem. 92:200–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Thayyullathil F, Chathoth S, Hago A, Patel
M and Galadari S: Rapid reactive oxygen species (ROS) generation
induced by curcumin leads to caspase-dependent and -independent
apoptosis in L929 cells. Free Radic Biol Med. 45:1403–1412. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mehta K, Pantazis P, McQueen T and
Aggarwal BB: Antiproliferative effect of curcumin
(diferuloylmethane) against human breast tumor cell lines.
Anticancer Drugs. 8:470–481. 1997. View Article : Google Scholar : PubMed/NCBI
|