Introduction
Telomeres are composed of telomeric DNA and several
binding proteins, which act together as a protective cap on the
ends of chromosomes (1). Healthy
human somatic cells do not express telomerase, and therefore
telomere size decreases with each cell division. Telomere
shortening serves as a checkpoint for the initiation of cell cycle
arrest, which leads to cellular senescence or aging, and apoptosis
or cell death (2). Normal cells
have a finite capacity for replication; however, telomerase
activity is observed in more than 90% of samples from a wide range
of different types of cancer.
Telomerase is a ribonucleoprotein complex whose main
function is to add six nucleotide repeats onto the ends of
chromosomes, in a mechanism that is dependent on its reverse
transcriptase activity (TERT) and intrinsic RNA template (TERC), as
well as the associated proteins, dyskerin, NOP10, NHP2 and GAR1.
TERT is the major catalytic component of telomerase (3), and has been extensively studied, with
several of its functional domains being mapped already. The ectopic
expression of TERT is sufficient to restore telomerase activity in
telomerase-negative cells and increase cell division in a number of
cell types (4). Downregulation of
TERT in telomerase-positive cancer cells results in growth arrest.
These findings demonstrate that TERT or telomerase activity is
required for cancer cell immortalization and proliferation
(5,6).
TERT and telomerase have other biological functions
beyond telomere lengthening, including the protection of
mitochondrial function under oxidative stress conditions, promoting
stem cell proliferation and enhancing DNA repair (7). Recently, several additional activities
for TERT have been reported, which indicates that TERT is able to
exert telomere-independent biological functions, including
promoting cell proliferation (5,8),
extending cell life (6,9), delaying cell aging (10,11)
and modulating cell differentiation (12). Some of these new functions do not
depend on the reverse transcriptase activity of TERT (7,13).
Activating protein 1 (AP-1) is a dimeric
transcription factor composed of proteins from several families
containing a basic leucine zipper (bZIP) domain, which is essential
for dimerization and DNA binding. The Jun (c-Jun, JunB and JunD)
and Fos (c-Fos, FosB, Fra1 and Fra2) subfamilies are the major AP-1
proteins. AP-1 regulates a number of cell processes, including
proliferation, inflammation, differentiation and apoptosis by
contributing to both basal- and stimulus-activated gene expression.
External stimuli, such as growth factors, neurotransmitters,
polypeptide hormones, bacterial and viral infections, as well as a
variety of physical and chemical stresses, activate AP-1 via the
mitogen-activated protein kinase cascades to induce both short- and
long-term gene expression changes (14,15).
TERT and telomerase are overexpressed in 85–90% of
human cancers, and are closely correlated with the development of
laryngeal carcinoma and the proliferation of laryngeal carcinoma
cells. Using siRNA targeting, TERT is capable of inhibiting
laryngeal carcinoma cell proliferation, but the mechanisms are not
well understood (16). Certain
investigators have reported that TERT may induce the expression of
growth-related proteins, such as epidermal growth factor receptor
(EGFR) in human glioma cancer cells (17), and can also interfere with the TGF-β
growth factor network (18). In
this study, we investigated the correlation between TERT and the
major AP-1 proteins (c-Jun and c-Fos) during TERT-promoted
laryngeal carcinoma cell proliferation.
Materials and methods
Cell lines and reagents
The human laryngeal carcinoma cell line, HEp-2, was
constructed in our laboratory and stored in liquid nitrogen. Fetal
bovine serum (FBS) was obtained from HyClone (Logan, UT, USA).
RPMI-1640 media and 0.25% trypsin solution were purchased from
Invitrogen (Carlsbad, CA, USA). The TERT, c-Fos, c-Jun and GAPDH
PCR primers were synthesized by Invitrogen. The TERT antibody was
purchased from Abcam (Cambridge, UK), the AP-1 antibody was
obtained from Sigma-Aldrich (St. Louis, MO, USA), and the c-Fos,
p-c-Fos, c-Jun, p-c-Jun, p-p38, p38, ERK, p-ERK and GAPDH
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA). The cell counting kit-8 was obtained from Dongji
(Kumamaoto, Japan), the quantum dots immunofluorescence detection
kit was purchased from Jiayuan Quantum Dots (Wuhan, China), and the
adenovirus packaging system, the negative control adenovirus Ad-HK
and TERT sh-RNA cDNA were obtained from Genesil (Wuhan, China). The
p38 MAPK inhibitor, SB202190, and the MEK1 and MEK2 inhibitor,
U0126, were purchased from Sigma-Aldrich.
Cell culture
The HEp-2 cell line was cultured in RMPI-1640
supplemented with 10% (FBS), 20 μg/ml ampicillin and 20
μg/ml kanamycin, and maintained in an incubator with 5%
CO2 at 37°C.
Human laryngeal carcinoma tissue
samples
The human laryngeal carcinoma tissue samples were
obtained from 24 laryngeal carcinoma cancer patients undergoing
total laryngectomy or partial laryngectomy, and the diagnosis of
laryngeal carcinoma was confirmed by pathological examination. The
specimens were transfected to liquid nitrogen within 15 min of
excision, and were stored at −80°C. Paraffin blocks created from
these patients were used for the tissue microarray construction.
This study was approved by the ethics review committee of Renmin
Hospital of Wuhan University, China. Informed consent was obtained
from all patients.
Construction of TERT shRNA and
overexpressing adenovirus vectors
Based on the design principles for shRNA
construction, we selected RNAi target sites within the open reading
frames of human TERT. A combination of computer algorithms
and experimental validation were employed to determine the optimal
siRNA sequences complementary to the target mRNA while inducing
minimal immune responses. The specific base sequence of the target
site of TERT was 5′-GTTCCTGCACTGGCTGATG-3′. The full length
human TERT sequence was obtained from The National Center
for Biotechnology Information (GenBank ID: 7015) and synthesized by
Genechem (Shanghai, China).
We constructed Ad-sh-TERT, a recombinant adenovirus
expressing the human TERT shRNA under the control of the immediate
early cytomegalovirus promoter, and Ad-TERT, a recombinant
adenovirus expressing the full length human TERT mRNA under
the immediate early cytomegalovirus promoter. The TERT shRNA
and the TERT full-length cDNA were subcloned into the
HindIII and BamHI restriction sites of the shuttle
vector pGenesil-1 (Genesil) using an adenoviral vector system. The
pGenesil-1 vector was homologously recombined with the pAd/PL-DEST
vector in electro-competent DH5a bacteria and selected on LB plates
containing ampicillin and chloramphenicol. The complete Ad-sh-TERT
and Ad-TERT viruses were recovered by transfection of 10 mg
PacI digested DNA into human embryonic kidney (HEK 293)
cells using Lipofectamine (Invitrogen) (19).
Tissue microarray construction
Tissue microarrays (TMAs) were constructed by
standard procedures using a tissue microarrayer (Beecher
Instruments, Silver Spring, MD, USA) in collaboration with Guilin
Fanpu Biotech Co. Ltd. (Guilin, China). Briefly, all specimens were
reviewed by hematoxylin and eosin (H&E) staining and
representative areas were marked in the formalin-fixed,
paraffin-embedded blocks. Two cores from different invasive areas
were removed from each specimen using 2-mm punch cores along the
greatest dimension of each block. The cores were deposited into the
recipient paraffin blocks in one of 70 cylinders. Two duplicates of
each 2-mm diameter cylinder were included for each case to ensure
reproducibility and homogeneous staining of the slides. Consecutive
sections (4 μm) of the resulting TMA paraffin blocks were
sectioned to create the TMA slides.
Cell proliferation
HEp-2 cells were seeded at 1×103/well in
96-well plates in RPMI-1640 medium supplemented with 10% FBS.
Twenty-four hours later, the cells were transfected with
Ad-sh-TERT, Ad-TERT or Ad-HK, and proliferation was determined at
0, 24, 48 and 72 h post-transfection using the Cell Counting Kit-8,
according to the manufacturer’s instructions.
RNA extraction and RT-PCR
Total RNA was extracted using the TRIzol total RNA
extraction kit (Invitrogen) according to the manufacturer’s
instructions, and cDNA was prepared from 1 μg total RNA
using Taq DNA polymerase (Thermo-Fisher) and oligo (dT)
primers (Thermo-Fisher). The primer sets used were TERT
(forward: 5′-GGAGCAAGTTGCAAAGCA TTG-3′; reverse:
5′-TCCCACGACGTAGTCCATGTT-3′) to amplify a 182-bp product,
c-Fos (forward: 5′-TGCCTCTCC TCAATGACCCTGA-3′; reverse:
5′-ATAGGTCCATGTCTG GCACGGA-3′) to amplify a 162-bp product,
c-Jun (forward: 5′-CTCCAAGTGCCGAAAAAGGAAG-3′; reverse:
5′-CAC CTGTTCCCTGAGCATGTTG-3′) to amplify a 118-bp product and
GAPDH (forward: 5′-CCTGTTCGACAGTCA GCCG-3′; reverse:
5′-CGACCAAATCCGTTGACTCC-3′) to amplify a 101-bp product.
The PCR conditions consisted of an initial
denaturation at 95°C for 5 min; 30 cycles of 94°C for 30 sec, 60°C
for 30 sec and 72°C for 1 min, followed by a final extension at
72°C for 10 min. The PCR products were separated on a 1.5% agarose
gel, and visualized and photographed using a gel documentation
system.
Western blotting
HEp-2 cells were collected and lysed in buffer
containing 1% Nonidet-P40 supplemented with complete protease
inhibitor cocktail (Roche, Basel, Switzerland) and 2 mM
dithiothreitol. The lysates were resolved using 12% SDS-PAGE,
transferred to nitrocellulose membranes and immunoblotted with
primary antibodies against hTERT, c-Jun, c-Fos, p-c-Jun, p-c-Fos,
p-ERK, ERK, p-p38, p38 and GAPDH. Following incubation with
secondary antibodies, the protein bands were detected using
enhanced chemiluminescence (ECL) reagent (Thermo-Fisher). The ERK
and p38 inhibitors were used at a concentration of 20 and 2
μM, respectively, and cells were treated with the inhibitors
24 h prior to transfection with the Ad-TERT or AD-sh-TERT.
Quantum-dots-based
immunofluorescence
TERT and AP-1 double immunofluorescence staining
using 605-QD-SA and 545-QD-SA probes was performed on the head and
neck cancer tissue microarray. The TMA was deparaffinized, antigen
retrieval was performed, blocked with 3% BSA and incubated with
primary mouse anti-human TERT monoclonal antibody. Samples were
then incubated with rabbit anti-human AP-1 monoclonal antibody,
washed and incubated with biotinylated goat anti-rabbit IgG. The
slides were washed and blocked, incubated with 605-QD-SA or
545-QDSA, washed and blocked, then incubated with biotinylated goat
anti-mouse IgG, washed, blocked and incubated with 545-QD-SA or
605-QD-SA, and mounted and observed by fluorescence microscopy
(20). The results were captured
and analyzed using Nuance 2.10 (CRi, Woburn, MA, USA).
Statistical analysis
Values were shown as the means ± SD. One-way ANOVA
and Pearson’s correlation analysis were performed using SPSS (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TERT, c-Fos and c-Jun are overexpressed
in laryngeal carcinoma cells and tissue samples
To test the hypothesis that TERT and AP-1 are
overexpressed in laryngeal carcinoma cells and therefore could
contribute to laryngeal carcinoma cell proliferation, we analyzed
the mRNA and protein expression levels of TERT, c-Fos and c-Jun in
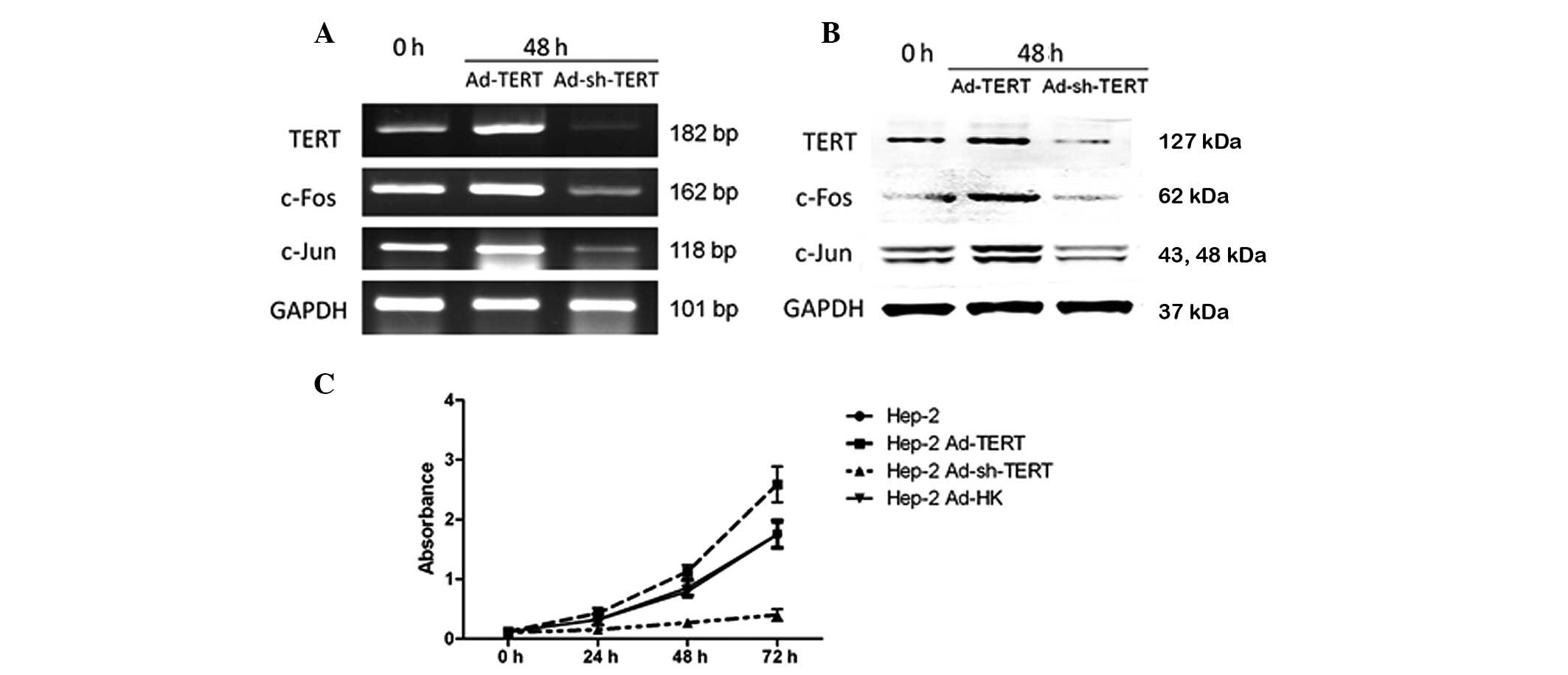
HEp-2 cells and human laryngeal carcinoma tissue samples. TERT,
c-Fos and c-Jun mRNA and protein were all observed to be expressed
at high levels in HEp-2 cells (Fig. 1A
and B) and laryngeal carcinoma tissue samples (Figs. 2A, 3A
and B).
TERT modulates the proliferation of HEp-2
cells
When TERT expression was suppressed by the
transfection of Ad-sh-TERT, HEp-2 cell proliferation was inhibited
in a time-dependent manner (P<0.01). However, when TERT was
overexpressed by the transfection of Ad-TERT, the proliferation of
HEp-2 cells increased at 72 h compared with the negative control
Ad-HK transfected cells (Fig. 1C,
P<0.05).
TERT modulates the expression of c-Fos
and c-Jun
Following treatment with Ad-TERT and Ad-sh-TERT for
48 h, the mRNA and protein levels of TERT, c-Fos and c-Jun changed
significantly in HEp-2 cells (Fig. 1A
and B). Transfection of Ad-TERT led to an increase in TERT,
c-Fos and c-Jun mRNA and protein expression. The transfection of
Ad-sh-TERT led to reduced TERT, c-Fos and c-Jun mRNA and protein
expression.
TERT is co-expressed with AP-1
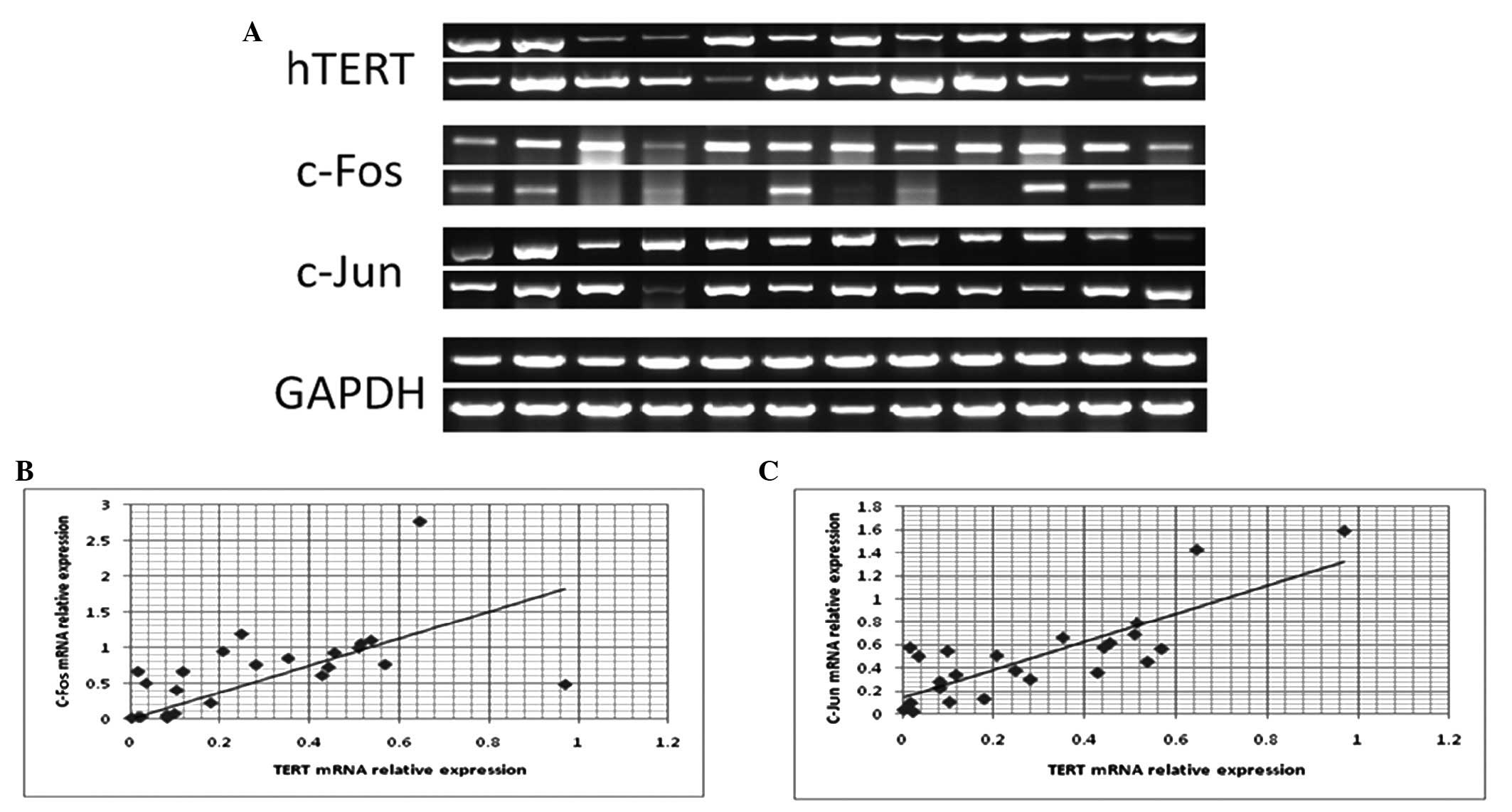
To examine the correlation between TERT and
the AP-1 subunits c-Fos and c-Jun, we analyzed the
correlation between TERT, c-Fos and c-Jun mRNA
expression in 24 laryngeal carcinoma tissue samples using RT-PCR
(Fig. 2A). The correlation
coefficient between TERT and c-Fos mRNA expression in
laryngeal carcinoma tissue samples was 0.574 (Fig. 2B; P<0.01), and the correlation
coefficient between TERT and c-Jun mRNA expression
was 0.809 (Fig. 2C; P<0.01).
These data indicate that TERT expression is significantly
and positively correlated with both c-Fos and c-Jun
expression in laryngeal carcinoma.
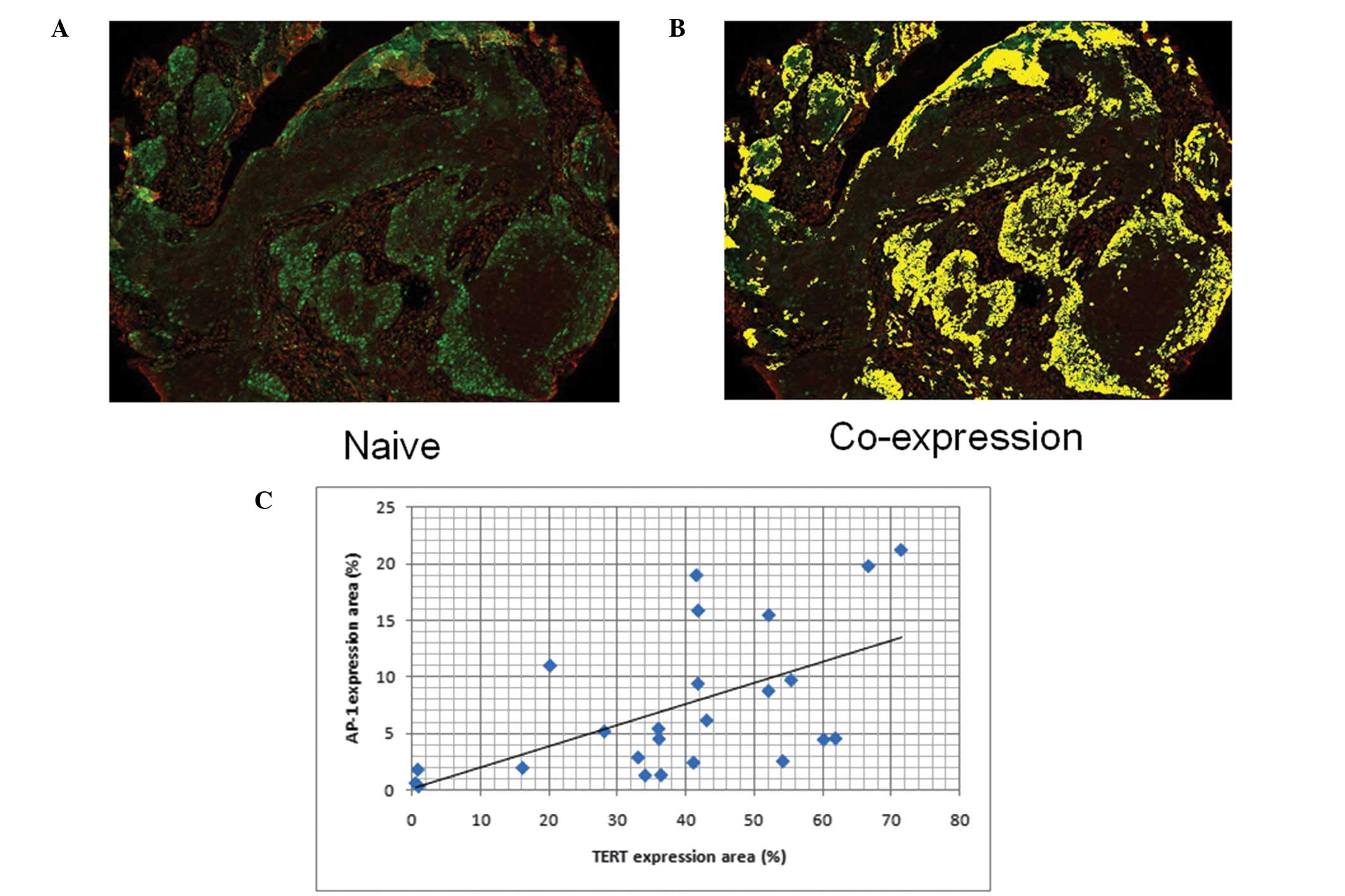
We also determined the correlation between the
protein expression levels of TERT and AP-1 in a laryngeal carcinoma
tissue microarray using quantum-dot based immunofluorescence
(Fig. 3A and B). A significant
positive correlation was observed between TERT and AP-1 expression
in laryngeal carcinoma cells (Fig.
3C, R2= 0.606; P<0.01).
TERT modulates the p38/ERK signaling
pathway
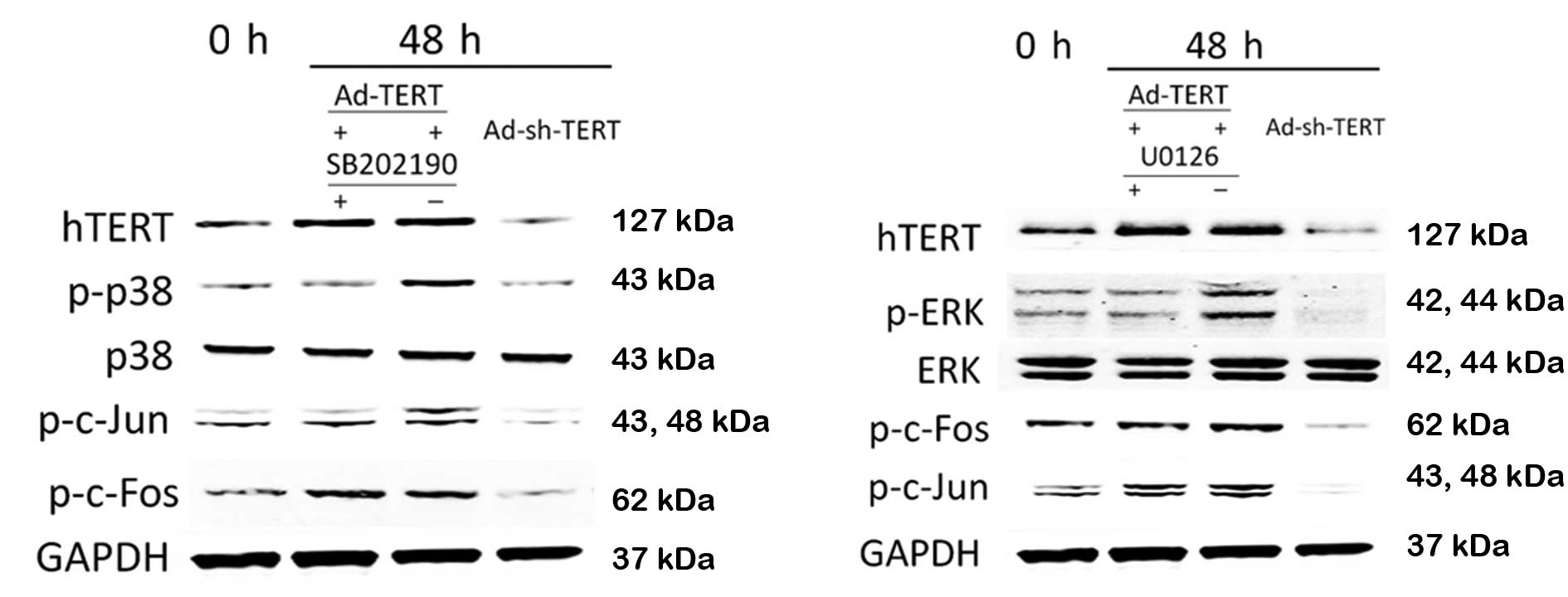
It has been reported that p38/ERK activation induces
AP-1 expression; therefore, we examined the relationship between
TERT, p38, ERK, JNK and AP-1 in HEp-2 laryngeal carcinoma cells.
Compared to the control HEp-2 cells, the levels of phosphorylated
p38 (p-p38), phosphorylated ERK (p-ERK), phosphorylated c-Jun
(p-c-Jun) and phosphorylated c-Fos (p-c-Fos) increased following
transfection with Ad-TERT (Fig. 4).
Conversely, the levels of p-p38, p-ERK, p-c-Jun and p-c-Fos were
reduced following transfection with Ad-sh-TERT. In the presence of
SB202190, a specific inhibitor of p38, overexpression of TERT did
not lead to increased p38 or c-Jun phosphorylation. In the presence
of U1026, a specific inhibitor of ERK, transfection with Ad-TERT
did not lead to increased levels of p-ERK; however, ERK inhibition
did not prevent TERT-induced c-Jun and c-Fos phosphorylation
(Fig. 4).
Discussion
In this study, we overexpressed and silenced the
expression of TERT in the human laryngeal carcinoma cell line,
HEp-2, using adenovirus-based vectors. Overexpression of TERT
markedly accelerated HEp-2 cell proliferation, while the silencing
of TERT significantly decreased the rate of HEp-2 proliferation to
approximately 30% of the levels observed in the control cells.
These results indicate that TERT gene expression is
important in cell proliferation in the HEp-2 human laryngeal
carcinoma cell line. Although the ability of TERT to promote
proliferation has already been proven in various cells, including
fibroblasts, epithelial cells, bone marrow mesenchymal stem cells,
cancer stem cells and tumor cells (4,5,8), the
molecular mechanism remains unclear. The whole genome analysis by
Takano et al (21) indicated
that the expression of genes in the PI3K, Akt and Caspase pathways,
which are associated with cell proliferation and apoptosis, altered
significantly in cells that overexpressed TERT. Yang et al
(4) reported that TERT is capable
of promoting the proliferation of human embryonic stem cells in a
mechanism that is associated with the expression of cyclin D1. This
mechanism may vary between normal and tumor cells, and even between
different tumor cell types.
AP-1 is important in eukaryotic cell proliferation,
cell cycle regulation and resistance to apoptosis. The biological
function of AP-1 is closely related to the major subunits, the
transcription factors c-Jun and c-Fos, which form homologous or
heterogeneous dimers (22). AP-1
regulates a number of cell processes, including proliferation,
inflammation, differentiation and apoptosis (23,24).
In this study, c-Fos and c-Jun expression were significantly
increased in TERT-overexpressing HEp-2 cells, and decreased in
TERT-silenced HEp-2 cells. Additionally, the expression of
c-Fos and c-Jun mRNA were both positively correlated
with TERT expression in human laryngeal carcinoma tissues.
Park et al (25) reported
that TERT interacts with BRG1 to activate transcription of the
Wnt/β-catenin signaling pathway and the downstream target genes.
This indicates that TERT may play a role in this mechanism that is
similar to a transcription factor. Therefore, we may deduce that
TERT acts as a transcription factor to modulate the expression of
c-Fos and c-Jun, as the expression of TERT is closely
correlated with c-Fos and c-Jun mRNA and protein expression in
HEp-2 laryngeal carcinoma cells, and is positively correlated with
c-Fos and c-Jun mRNA expression in human laryngeal
carcinoma tissues.
AP-1 activation occurs at the transcriptional and
post-translational levels. The predominant AP-1 activation signals
are mediated via the mitogen-activated protein kinase (MAPK)
cascade (26). The MAPK pathway
converges on three MAP kinases, extracellular regulated kinase
(ERK), Jun N-terminal kinase (JNK) and p38. AP-1 may be activated
by all of the MAPK pathways (27).
We demonstrated that TERT affects the expression of c-Fos and c-Jun
at both the transcriptional and translational level, and c-Fos and
c-Jun phosphorylation was significantly altered in
TERT-overexpressing and TERT-silenced HEp-2 cells. Therefore, to
identify whether TERT is capable of modulating c-Fos and c-Jun
activation at a post-translational level, we investigated p38, JNK
(data not shown) and ERK expression and phosphorylation. TERT
overexpression promoted p38 phosphorylation and c-Jun
phosphorylation. Phosphorylation of c-Jun in response to TERT
overexpression was inhibited in the presence of a specific p38
inhibitor, indicating a correlation between the phosphorylation of
c-Jun and p38. However, a specific ERK inhibitor did not prevent
phosphorylation of c-Fos or c-Jun in TERT-overexpressing cells. It
is known that p38 phosphorylation specifically leads to c-Jun
phosphorylation, and that ERK phosphorylation is required for c-Fos
phosphorylation (28).
In conclusion, this study indicates that TERT is
important in cell proliferation in laryngeal carcinoma. TERT
induces altered expression and activation of the AP-1 subunits
c-Fos and c-Jun, via the MAPK pathway, which may explain the
increased proliferation observed in cells that overexpress
TERT.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (Nos.
30901662 and 30872851), the Science and Technology Program of Hubei
Province of China (No. 2007AA302B08), the Science and Technology
Program of Wuhan City (Nos. 200951199455 and 200950431168) and the
Self-research Program for Doctoral Candidates (including Mphil-PhD)
of Wuhan University in 2008.
References
|
1.
|
Harley CB: Telomerase and cancer
therapeutics. Nat Rev Cancer. 8:167–179. 2008. View Article : Google Scholar
|
|
2.
|
Liu JP, Chen SM, Cong YS, et al:
Regulation of telomerase activity by apparently opposing elements.
Ageing Res Rev. 9:245–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Montanaro L, Brigotti M, Clohessy J, et
al: Dyskerin expression influences the level of ribosomal RNA
pseudo-uridylation and telomerase RNA component in human breast
cancer. J Pathol. 210:10–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yang C, Przyborski S, Cooke MJ, et al: A
key role for telomerase reverse transcriptase unit in modulating
human embryonic stem cell proliferation, cell cycle dynamics, and
in vitro differentiation. Stem Cells. 26:850–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chang B, Myatt L and Cui XL: Loss of
proliferative capacity in a retroviral immortalized human uterine
smooth muscle cell line derived from leiomyoma is restored by hTERT
overexpression. Reprod Sci. 16:1062–1071. 2009. View Article : Google Scholar
|
|
6.
|
Blagoev KB: Cell proliferation in the
presence of telomerase. PLoS One. 4:e46222009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bollmann FM: The many faces of telomerase:
emerging extratelomeric effects. Bioessays. 30:728–732. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Techangamsuwan S, Haas L, Rohn K,
Baumgartner W and Wewetzer K: Distinct cell tropism of canine
distemper virus strains to adult olfactory ensheathing cells and
Schwann cells in vitro. Virus Res. 144:195–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li N, Yang R, Zhang W, Dorfman H, Rao P
and Gorlick R: Genetically transforming human mesenchymal stem
cells to sarcomas: changes in cellular phenotype and multilineage
differentiation potential. Cancer. 115:4795–4806. 2009. View Article : Google Scholar
|
|
10.
|
Wu YH, Cheng ML, Ho HY, Chiu DT and Wang
TC: Telomerase prevents accelerated senescence in
glucose-6-phosphate dehydrogenase (G6PD)-deficient human
fibroblasts. J Biomed Sci. 16:182009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tomas-Loba A, Flores I, Fernandez-Marcos
PJ, et al: Telomerase reverse transcriptase delays aging in
cancer-resistant mice. Cell. 135:609–622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Li NF, Kocher HM, Salako MA, Obermueller
E, Sandle J and Balkwill F: A novel function of colony-stimulating
factor 1 receptor in hTERT immortalization of human epithelial
cells. Oncogene. 28:773–780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cong Y and Shay JW: Actions of human
telomerase beyond telomeres. Cell Res. 18:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vesely PW, Staber PB, Hoefler G and Kenner
L: Translational regulation mechanisms of AP-1 proteins. Mutat Res.
682:7–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Malnou CE, Brockly F, Favard C,
Moquet-Torcy G, Piechaczyk M and Jariel-Encontre I:
Heterodimerization with different Jun proteins controls c-Fos
intranuclear dynamics and distribution. J Biol Chem. 285:6552–6562.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wang Y, Tao ZZ, Chen SM, Xiao BK, Zhou XH
and Liu JP: Application of combination of short hairpin RNA
segments for silencing VEGF, TERT and Bcl-xl expression in
laryngeal squamous carcinoma. Cancer Biol Ther. 7:896–901. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Beck S, Jin X, Sohn YW, et al: Telomerase
activity-independent function of TERT allows glioma cells to attain
cancer stem cell characteristics by inducing EGFR expression. Mol
Cells. 31:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cassar L, Li H, Jiang FX and Liu JP:
TGF-beta induces telomerase-dependent pancreatic tumor cell cycle
arrest. Mol Cell Endocrinol. 320:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Han JB, Tao ZZ, Chen SM, Kong YG and Xiao
BK: Adenovirus-mediated transfer of tris-shRNAs induced apoptosis
of nasopharyngeal carcinoma cell in vitro and in vivo. Cancer Lett.
309:162–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chen C, Xia HS, Gong YP, et al: The
quantitative detection of total HER2 load by quantum dots and the
identification of a new subtype of breast cancer with different
5-year prognosis. Biomaterials. 31:8818–8825. 2010.PubMed/NCBI
|
|
21.
|
Takano H, Murasawa S and Asahara T:
Functional and gene expression analysis of hTERT overexpressed
endothelial cells. Biologics. 2:547–554. 2008.PubMed/NCBI
|
|
22.
|
Shaulian E: AP-1 - The Jun proteins:
Oncogenes or tumor suppressors in disguise? Cell Signal.
22:894–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zenz R, Eferl R, Scheinecker C, et al:
Activator protein 1 (Fos/Jun) functions in inflammatory bone and
skin disease. Arthritis Res Ther. 10:2012008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Soriano FX, Baxter P, Murray LM, Sporn MB,
Gillingwater TH and Hardingham GE: Transcriptional regulation of
the AP-1 and Nrf2 target gene sulfiredoxin. Mol Cells. 27:279–282.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Park JI, Venteicher AS, Hong JY, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Singh R, Cadeddu RP, Frobel J, et al: The
non-steroidal anti-inflammatory drugs Sulindac sulfide and
Diclofenac induce apoptosis and differentiation in human acute
myeloid leukemia cells through an AP-1 dependent pathway.
Apoptosis. 16:889–901. 2011. View Article : Google Scholar
|
|
27.
|
Liu WH and Chang LS: Piceatannol induces
Fas and FasL up-regulation in human leukemia U937 cells via
Ca2+/p38alpha MAPK-mediated activation of c-Jun and
ATF-2 pathways. Int J Biochem Cell Biol. 42:1498–1506. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Yan H, Zhu Y, Liu B, et al:
Mitogen-activated protein kinase mediates the apoptosis of highly
metastatic human non-small cell lung cancer cells induced by
isothiocyanates. Br J Nutr. 106:1779–1791. 2011. View Article : Google Scholar : PubMed/NCBI
|