Introduction
Although the majority of gene expression regulation
occurs at the time of transcription, translational control of
specific mRNAs through the regulation of mRNA stability,
localization and translation ability determines the spatial and
temporal expression in many cell types (1,2).
The hu antigen R (HuR) is a large, highly conserved
RNA-binding protein that is involved in the shuttling of
transcripts from the nucleus into the cytoplasm (3), as well as the regulation of mRNA
stability and translation (4,5). HuR
binds specifically to translational control elements in the target
mRNA 3′ untranslated regions (UTRs) known as Nanos response
elements (NREs) (4,6). HuR has been implicated in cell growth
and differentiation via the regulation of mRNA expression in the
cytoplasm (7). In human colorectal
carcinoma cells, UV irradiation elevates the rate of p21 mRNA
translation in a HuR-dependent manner (8). In the cytoplasm, HuR-containing mRNA
complexes cofractionate with polysomes (9). Additionally, the binding of p53 mRNA
to polysomes and its increased translation is HuR-mediated
(9). Moreover, high cytoplasmic
levels of HuR have been associated with a higher tumor grade,
increased cyclooxygenase-2 expression and poor survival rates in
breast carcinoma (10), suggesting
a role for HuR in cancer pathogenesis.
The Forkhead box O (FoxO) transcription factor FOXO1
is emerging as an important tumor suppressor that modulates the
expression of genes involved in apoptosis, the cell cycle, DNA
damage repair and oxidative stress (11–13).
FOXO1 can be regulated by a number of mechanisms. It has been
widely accepted that phosphorylation of the three PKB/Akt consensus
sites in FOXO1 following incubation with insulin or other serum
components, results in a rapid export of FOXO proteins from the
nucleus to the cytoplasm (12,14,15),
which inhibits the FOXO-stimulated transcription of target
genes.
In the current study, we demonstrate that HuR
positively regulates FOXO1 expression via the 3′ UTR upon
5-fluorouracil (5-FU) stimulation, which results in enhanced mRNA
stability. Our study suggests that in addition to
post-translational modification, post-transcriptional mechanisms,
including mRNA stability and translation, are critical in the
control of FOXO1 expression.
Materials and methods
Cell culture
The MDA-MB-231 human breast cancer cell line was
grown in Dulbecco’s modified Eagle’s medium supplemented with 10%
inactivated fetal bovine serum, 2 mM L-glutamine, 50 U/ml
penicillin and 50 μg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2.
Plasmids and Stealth siRNAs™
HuR and FOXO1 overexpression vectors (pcDNA-flag-HuR
and pcDNA-flag-FOXO1, respectively) were generated by cloning
PCR-amplified sequences into pcDNA3.0-flag vectors with
EcoRI and BamHI restriction enzymes. The FOXO1 3′ UTR
reporter plasmid (designated as WT) was constructed by cloning
PCR-amplified sequences from the 3′ UTR of FOXO1 cDNA into the
XbaI site of a pGL3 luciferase reporter vector (Promega,
Madison, WI, USA). Two sites of the pGL3-FOXO1-3′ UTR seed sequence
were deleted (designated as Mutant). The siRNA duplex targeting
human HuR is 5′-AAGCCUGUUCAGCAGCAUUGG-3′ (Dharmacon, Inc.,
Lafayette, CO, USA).
Luciferase assays
MDA-MB-231 cells were seeded into 24-well plates and
transiently transfected with 400 ng of FOXO1 3′ UTR reporter
plasmid (WT or Mutant) in combination with increased doses of
pcDNA-flag-HuR. To normalize the transfection efficiency, cells
were cotransfected with 50 ng of pBind containing renilla
luciferase. After 24 h, cells were washed with PBS and lysed using
passive lysis buffer. Luciferase activity was measured using the
Dual-Luciferase Reporter Assay kit (Promega GmbH) and a Wallac
Victor 1420 Multilabel Counter (PerkinElmer, Waltham, MA, USA),
according to the manufacturer’s instructions.
Quantitative reverse transcription
(qRT)-PCR
RNA was isolated using TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. First-strand cDNA synthesis was
conducted using the iScript RT kit (Bio-Rad Laboratories, Hercules,
CA, USA) in 20 μl reaction solutions. Real-time PCR was
conducted with the iQ™ SYBR® Green Supermix (Bio-Rad
Laboratories) in 20 μl reaction solutions using the iCycler
thermal cycler (Bio-Rad Laboratories). The relative RNA amount was
calculated using the ΔΔCt method and normalized using
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal
control.
Actinomycin D (ActD) experiments
MDA-MB-231 cells were seeded into 6-well plates and
transfected with the HuR overexpression vector (pcDNA-flag-HuR)
using Lipofectamine™ 2000 (Invitrogen Life Technologies) according
to the manufacturer’s instructions. After 48 h, MDA-MB-231 cells
were treated with 5 μg/ml transcription inhibitor ActD
(Sigma, St. Louis, MO, USA). Total RNA was isolated at time
intervals of 0, 2, 4 and 6 h following ActD addition. FOXO1 mRNA
was determined using qRT-PCR, and the relative amount of FOXO1 mRNA
without Act D treatment was set to 100%.
Western blot analysis
MDA-MB-231 cells with indicated treatment were
harvested and lysed in ice-cold radioimmunoprecipitation assay
(RIPA) buffer consisting of 1% nonidet P-40, 0.1% SDS, 0.5%
deoxycholate, 150 mM NaCl, 50 mM NaF, 1 mM DTT, 50 mM Tris-HCl, (pH
8.0) and a freshly prepared protease inhibitor mixture (Complete,
Mini; Roche Applied Science, Burgess Hill, UK). Total protein
concentration of lysates was determined using the Bio-Rad protein
assay. A total of 40 μg of protein lysates were separated
using 12% SDS-PAGE and transferred onto nitrocellulose membranes.
For detection of HuR and FOXO1, a rabbit polyclonal anti-HuR
antibody (1:2000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) or a rabbit monoclonal anti-FOXO1 antibody (1:3000; Abcam,
Cambridge, UK) were used. The immuno-reactive proteins on the
western blots were visualized using the enhanced chemiluminescence
(ECL) detection system (Amersham Pharmacia Biotech, Amersham,
UK).
Immunoprecipitation qRT-PCR assay
MDA-MB-231 cells were seeded in 100 mm dishes, and
after 24 h, 1% formaldehyde was added to the medium to crosslink
protein-RNA. Cells were lysed in a buffer containing 10 mM HEPES
(pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.1%
NP-40, 50 mM NaF, 10 mM Na3VO4, 10 mM sodium
pyrophosphate, 50 mM disodium glycerol phosphate, 10 nM okadaic
acid, 0.2% VRC, 100 U/ml RNasin and 1/25 v/v complete EDTA-free
protease inhibitor cocktail. The lysed cells were centrifuged at
12,000 × g for 10 min at 4°C and the supernatants were incubated
with 30 μg of unrelated antibody (IgG; Sigma) or anti-HuR at
4°C for 60 min. Once incubation was complete, agarose beads and 50
μl of protein A/G were added and cells were incubated for a
further 60 min at 4°C. Subsequently, the precipitated beads were
washed with lysis buffer three times. The RNA in the
immunoprecipitated complex and the RNA in the previously saved
input fraction were released by incubating cells at 65°C for 2 h
with 200 mM NaCl and 20 μg proteinase K, which reversed the
cross-linking. The RNAs were extracted as previously described. The
amount of FOXO1 mRNA bound by HuR was determined by RT-PCR using
the following primers: sense, 5′-TTGTTACATAGTCAGCTTG-3′; and
antisense, 5′-TCACTTTCCTGCCCAACCAG-3′. PCR conditions were as
follows: 95°C for 5 min, followed by 25 cycles of 95°C for 15 sec,
55°C for 20 sec and 72°C for 1 min.
Caspase activity assay
MDA-MB-231 cells were seeded into 96-well plates at
a density of 5×103 cells/well for 24 h. Cells were
transfected with control siRNA (siRNA-Con), HuR siRNA (siRNA-HuR)
and a combination of siRNA-HuR and pcDNA-flag-FOXO1 respectively
for 24 h, and treated with 5 μg/ml 5-FU. After 24 h, 50
μl of Caspase-Glo® 3/7 Reagent (Promega GmbH) was
added into each well and incubated for 1 h. The luminescence of
each well was measured using the GENios Pro Multifunctional Reader
(Tecan, Mannedorf, Switzerland).
Annexin V staining
MDA-MB-231 cells were treated as previously
described. After 24 h of 5 μg/ml 5-FU treatment, MDA-MB-231
cells were washed three times with cold PBS. Cells were then
incubated with 100 μl binding buffer containing 2
μg/ml Annexin V-fluorescein isothiocyanate (FITC; Roche
Applied Science) and 10 μg/ml of the vital dye propidium
iodide for 10 min in the dark. Following further washes in PBS, the
cells were analyzed using flow cytometry (BD Pharmingen, San Diego,
CA, USA).
Results
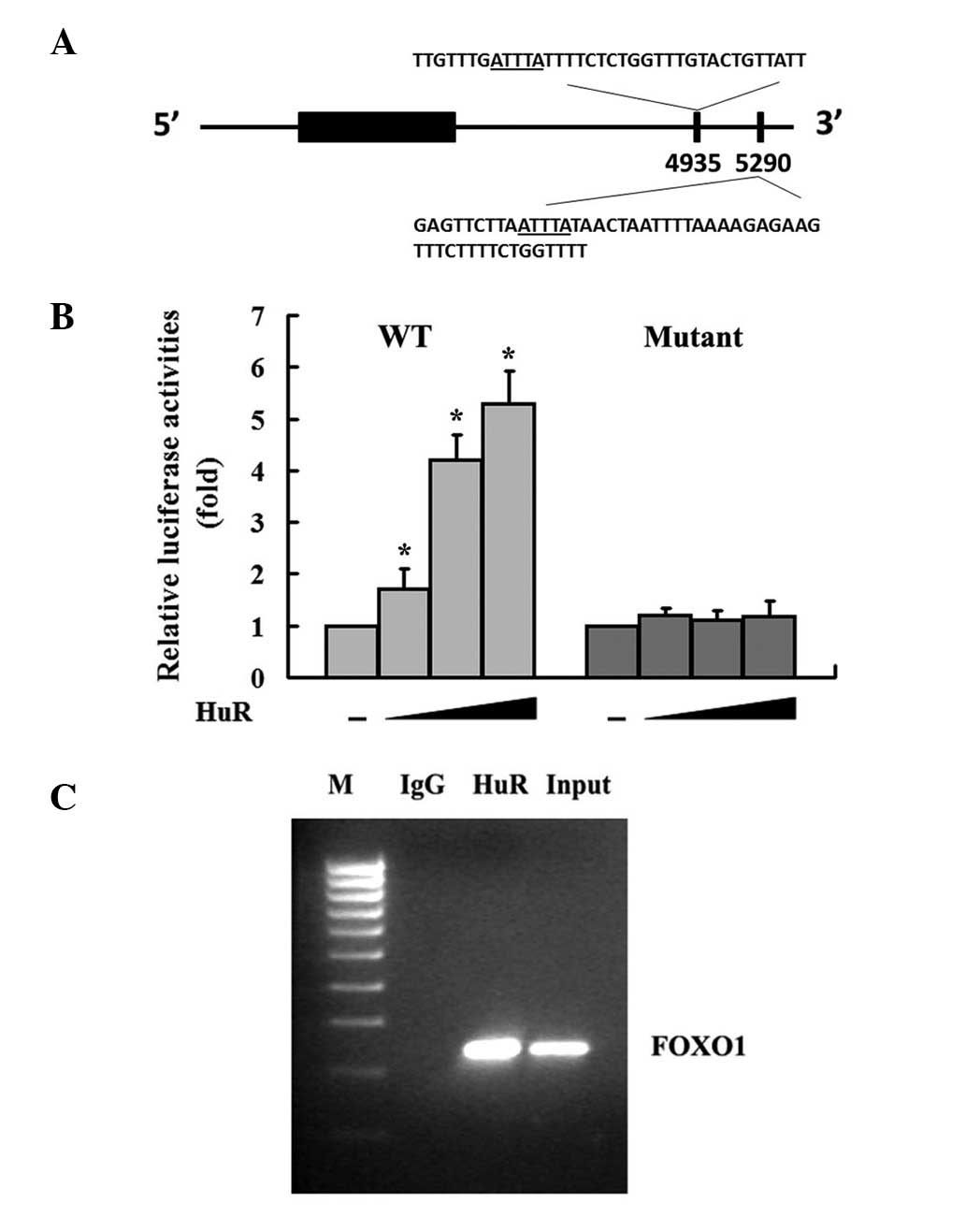
HuR interacts with FOXO1 3′ UTR
Bioinformatics analysis of the human FOXO1 mRNA
revealed that there are two potential AU-rich elements (AREs)
within the 3′ UTR (Fig. 1A);
however, its role in the regulation of FOXO1 gene expression has
not been elucidated. To address this, luciferase report constructs
containing full length FOXO1 3′ UTRs (WT) or deletions of the
AU-rich regions (Mutant) were utilized. Overexpression of HuR in
MDA-MB-231 breast cancer cell lines caused a dose-dependent
increase of luciferase activities (Fig.
1B). However, interference of HuR-FOXO1 interactions by
overexpressing mutant FOXO1 3′ UTR abrogated the effect of HuR on
FOXO1 3′ UTR (Fig. 1B). Taken
together, these results suggest that the AREs within the 3′ UTR of
FOXO1 are responsible for HuR-mediated upregulation of FOXO1. To
further determine if HuR was directly associated with FOXO1 3′ UTR,
we conducted an immunoprecipitation RT-PCR assay with primers that
target the FOXO1 coding region. Using HuR antibodies, we were able
to coimmunoprecipitate FOXO1 mRNA from MDA-MB-231 cells (Fig. 1C). No FOXO1-specific PCR product was
identified when cell lysates were precipitated with IgG antibodies
(Fig. 1C). These findings provide
strong evidence that HuR binds specifically to FOXO1 mRNA in
MDA-MB-231 cells in vivo.
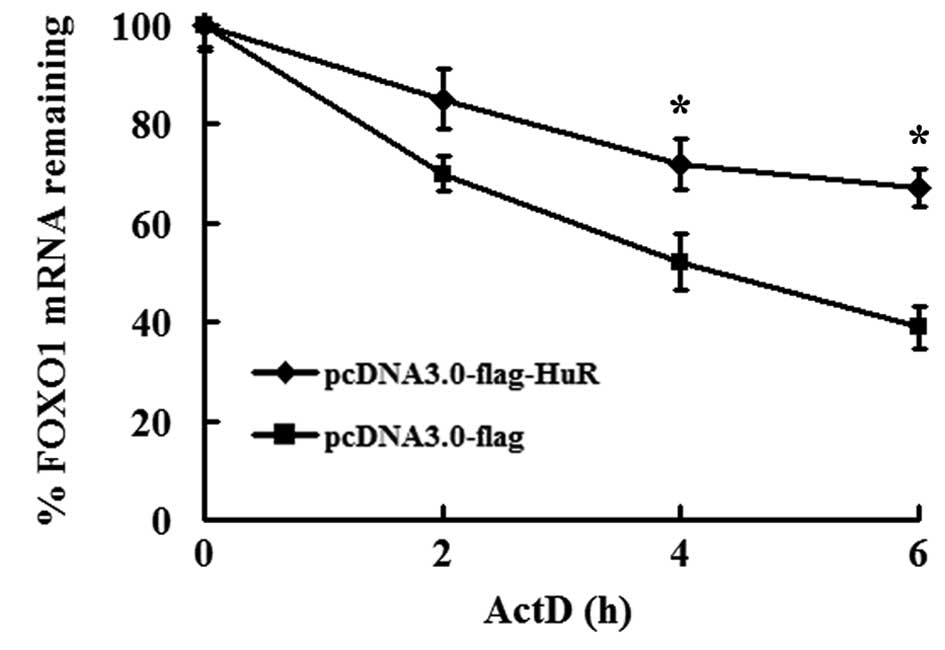
HuR overexpression stabilizes FOXO1
mRNA
Accumulating evidence indicates that HuR controls
mRNA activity by regulating mRNA stability and/or translation
(4,5). We revealed that HuR is involved in
FOXO1 mRNA turnover. To exclude the influence of transcription, the
transcription inhibitor ActD was used. As shown in Fig. 2, the half-life of FOXO1 mRNA in
pcDNA3.0-flag-transfected cells was 4.7±0.3 h, while the half-life
of FOXO1 mRNA in pcDNA3-HuR-flag-transfected cells was much longer
(10.8±0.4 h) compared with the control vector (Fig. 2). These results clearly demonstrate
that HuR stabilizes FOXO1 mRNA, which plays an important role in
the regulation of FOXO1 gene expression.
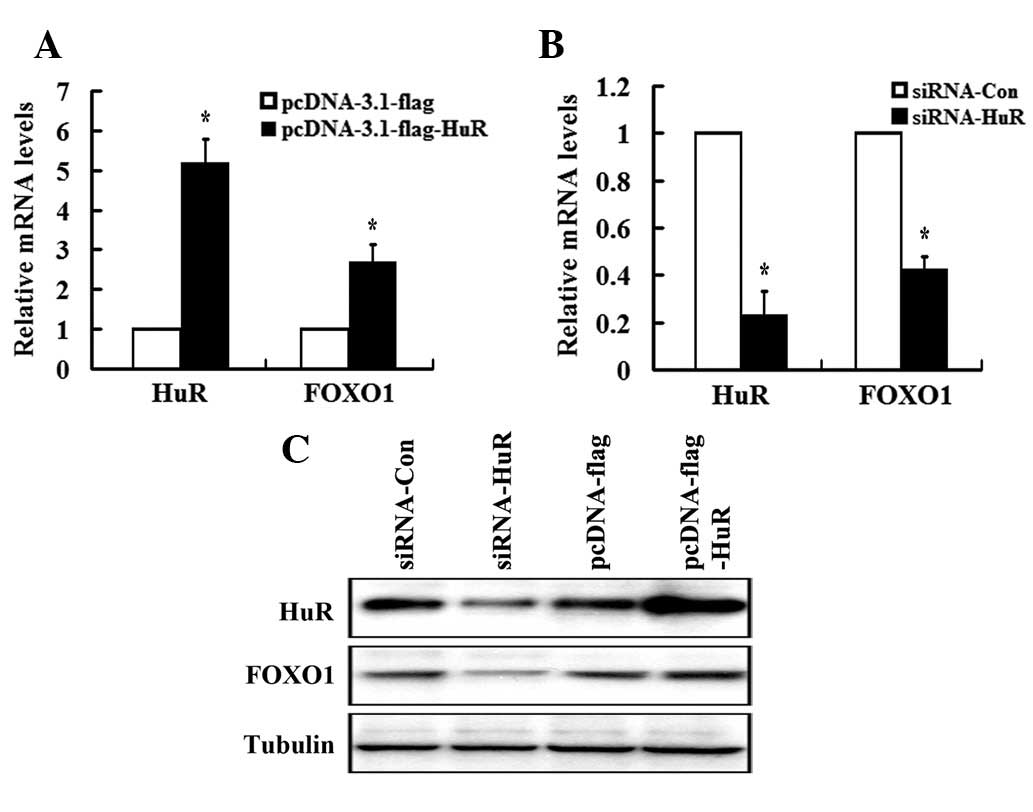
HuR positively regulates FOXO1
expression
Considering the direct association of HuR and FOXO1
3′ UTR, combined with the increased stability of FOXO1 mRNA, we
revealed that HuR positively regulates FOXO1 expression. To study
this, the loss- and gain-of-function of HuR approach was utilized.
HuR expression was confirmed upon transient transfection with
pcDNA3.0-flag or pcDNA3-HuR-flag plasmids (Fig. 3A) and a scrambled siRNA or a
specific siRNA plasmid for HuR (Fig. 3B
and C) by qRT-PCR and western blot analysis. Fig. 3B shows that FOXO1 mRNA levels were
reduced by approximately 60% in siRNA-HuR transfectants relative to
the levels of FOXO1 mRNA in cells transfected with a scrambled
siRNA. In accordance with this, overexpression of HuR resulted in a
2.8- and 2.1-fold increase of FOXO1 expression at mRNA and protein
levels, respectively (Fig. 3A and
C).
5-FU induces FOXO1 expression in a
HuR-dependent manner
Previous studies demonstrate that HuR increases mRNA
stability of target genes upon stimuli. We demonstrated that HuR
regulated FOXO1 expression upon 5-FU treatment in MDA-MB-231 cells.
Notably, 5-FU-induced accumulation of HuR occurred in correlation
with an increase in the FOXO1 mRNA level, which exhibited a dose-
and time-dependent manner (Fig. 4A and
B). To investigate the functional role of HuR in the regulation
of FOXO1 expression and control of apoptosis in MDA-MB-231 cells
upon 5-FU treatment, Annexin V assays were conducted and caspase-3
activity was determined. qRT-PCR analysis revealed that HuR mRNA
was reduced in HuR siRNA-transfected cells to ∼30% of the level
found in cells transfected with a control siRNA (data not shown).
5-FU increased the level of Annexin V positive cells by 3.8-fold
compared with the vehicle control (Fig.
4C); however, 5-FU-induced apoptosis was significantly
inhibited by HuR knockdown (Fig.
4C). Consistently, 5-FU induced a 10.3-fold increase of
caspase-3 activity compared with vehicle treatment (Fig. 4D). Conversely, 5-FU treatment
resulted in a reduced increase of caspase-3 activity upon silencing
HuR (Fig. 4D), which indicates that
HuR knockdown remarkably abrogates 5-FU-induced apoptosis. To
determine whether FOXO1 is involved in HuR-mediated apoptosis upon
5-FU treatment, FOXO1 overexpression lentivirus was utilized. FOXO1
mRNA and protein levels were significantly enhanced upon
transduction of FOXO1 overexpression lentivirus (data not shown).
As expected, overexpression of FOXO1 restores 5-FU-induced
apoptosis upon HuR knockdown determined by Annexin V and caspase-3
activity assays (Fig. 4C and D).
Taken together, our data suggest that HuR-mediated regulation of
FOXO1 plays a critical role in 5-FU-induced apoptosis.
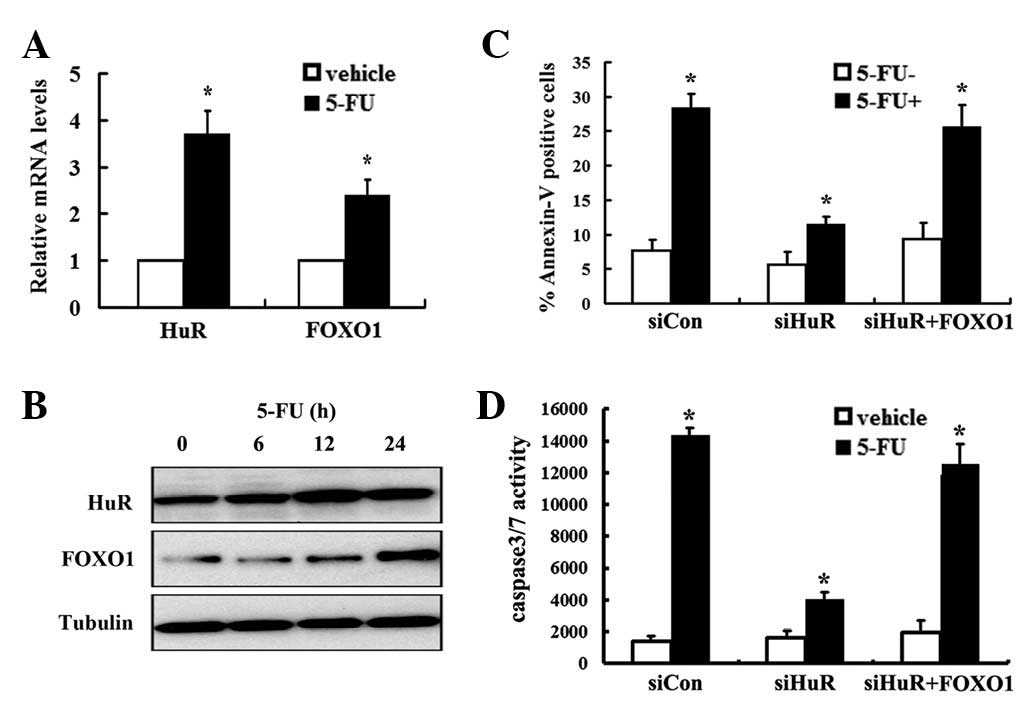 | Figure 4.5-FU induces FOXO1 expression in a
HuR-dependent manner. MDA-MB-231 cells were seeded into 6-well
plates and treated with 5 μg/ml 5-FU. At the indicated time,
cells were collected and (A) RT-PCR and (B) western blot analysis
were conducted to detect HuR and FOXO1 expression. (C) MDA-MB-231
cells were seeded into 6-well plates and transfected with
siRNA-Con, siRNA-HuR and FOXO1 overexpression and control
lentivirus, respectively. After 24 h, cells were treated with 5-FU.
Following 24 h of incubation, the apoptotic cells were measured by
PI and Annexin V-FITC staining and analyzed by flow cytometry.
Values are expressed as mean ± SEM of at least three independent
experiments. *P<0.05. (D) Cells were treated as
described above. Caspase-3 activities were measured using a
Caspase-Glo 3/7 assay kit. Values are expressed as mean ± SEM of at
least three independent experiments. *Indicates
P<0.05. 5-FU, 5-fluorouracil; HuR, human ELAV/Hu protein; FOXO1,
Forkhead box protein O 1; Con, control. RT-PCR, reverse
transcription-PCR; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
Discussion
As a critical transcription factor, FOXO1
orchestratedly regulated genes involved in cell cycle inhibition
(e.g., p27), apoptosis (e.g. Bim, FASL and TATRAIL) and DNA repair
(e.g., GADD45a) under stress or differentiation conditions
(16–20). The majority of previous studies with
regard to FOXO1 function were closely associated with its
phosphorylation and acetylation modification (21,22). A
number of studies conclusively demonstrate that the regulation of
FOXO1 expression by growth factors and other stimuli occurs
predominantly at the post-translational level. However, there is
little understanding of the specific RNA-protein interactions
involved in the regulation of FOXO1 expression and function. We
postulate that post-transcriptional regulation, which controls the
mRNA level of FOXO1, is also critical for its function. A new
finding recently disclosed in endometrial cancer cell lines
suggested that the lack of FOXO1 expression was associated with an
increased mRNA turnover with an unknown mechanism (23). Our study revealed that HuR enhanced
the expression of FOXO1 at the post-transcriptional level by
increasing FOXO1 mRNA stability (Fig.
2), adding a novel molecular mechanism of how FOXO1 is
regulated in addition to translational regulation.
FOXO1 is the most abundant FOXO isoform in
insulin-responsive tissues including hepatic, adipose and
pancreatic cells (21,22). However, as a critical tumor
suppressor, FOXO1 expression has been observed to be undetectable
or extremely low in certain tissues, including prostate, breast and
colon cancer cells (24–26), which suggests that low levels of
FOXO1 may be one of the factors contributing to the oncogenesis and
progression of breast carcinoma. Our study provides the first
evidence that 5-FU treatment enhances FOXO1 expression by
stabilizing its mRNA level via HuR. This suggests that modulating
FOXO1 expression may serve as a novel strategy to sensitize breast
cancers to chemotherapy and/or radiotherapy.
References
|
1.
|
Moore MJ: From birth to death: the complex
lives of eukaryotic mRNAs. Science. 309:1514–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Keene JD: RNA regulons: coordination of
post-transcriptional events. Nat Rev Genet. 8:533–543. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Brennan CM and Steitz JA: HuR and mRNA
stability. Cell Mol Life Sci. 58:266–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gorospe M: HuR in the mammalian genotoxic
response: post-transcriptional multitasking. Cell Cycle. 2:412–414.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Fan XC and Steitz JA: Overexpression of
HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo
stability of ARE-containing mRNAs. EMBO J. 17:3448–3460. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Blackshear PJ: Tristetraprolin and other
CCCH tandem zinc-finger proteins in the regulation of mRNA
turnover. Biochem Soc Trans. 30:945–952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Heinonen M, Bono P, Narko K, et al:
Cytoplasmic HuR expression is a prognostic factor in invasive
ductal breast carcinoma. Cancer Res. 65:2157–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang W, Furneaux H, Cheng H, et al: HuR
regulates p21 mRNA stabilization by UV light. Mol Cell Biol.
20:760–769. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zou T, Mazan-Mamczarz K, Rao JN, et al:
Polyamine depletion increases cytoplasmic levels of RNA-binding
protein HuR leading to stabilization of nucleophosmin and p53
mRNAs. J Biol Chem. 281:19387–19394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Erkinheimo TL, Lassus H, Sivula A, et al:
Cytoplasmic HuR expression correlates with poor outcome and with
cyclooxygenase 2 expression in serous ovarian carcinoma. Cancer
Res. 63:7591–7594. 2003.PubMed/NCBI
|
|
11.
|
Arden KC: Multiple roles of FOXO
transcription factors in mammalian cells point to multiple roles in
cancer. Exp Gerontol. 41:709–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Birkenkamp KU and Coffer PJ: Regulation of
cell survival and proliferation by the FOXO (Forkhead box, class O)
subfamily of Forkhead transcription factors. Biochem Soc Trans.
31:292–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Paik JH, Kollipara R, Chu G, et al: FoxOs
are lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Adachi M, Osawa Y, Uchinami H, Kitamura T,
Accili D and Brenner DA: The forkhead transcription factor FoxO1
regulates proliferation and transdifferentiation of hepatic
stellate cells. Gastroenterology. 132:1434–1446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Finlay D, Patel S, Dickson LM, et al:
Glycogen synthase kinase-3 regulates IGFBP-1 gene transcription
through the thymine-rich insulin response element. BMC Mol Biol.
5:152004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Potente M, Fisslthaler B, Busse R and
Fleming I: 11,12-Epoxyeicosatrienoic acid-induced inhibition of
FOXO factors promotes endothelial proliferation by down-regulating
p27Kip1. J Biol Chem. 278:29619–29625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sengupta A, Molkentin JD, Paik JH, DePinho
RA and Yutzey KE: FoxO transcription factors promote cardiomyocyte
survival upon induction of oxidative stress. J Biol Chem.
286:7468–7478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tang H, Lee M, Budak MT, et al: Intrinsic
apoptosis in mechanically ventilated human diaphragm: linkage to a
novel Fos/FoxO1/Stat3-Bim axis. FASEB J. 25:2921–2936. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Raghavendra PB, Pathak N and Manna SK:
Novel role of thiadiazolidine derivatives in inducing cell death
through Myc-Max, Akt, FKHR, and FasL pathway. Biochem Pharmacol.
78:495–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tran H, Brunet A, Griffith EC and
Greenberg ME: The many forks in FOXO’s road. Sci STKE: RE5.
2003.
|
|
22.
|
Nakae J, Biggs WH 3rd, Kitamura T, et al:
Regulation of insulin action and pancreatic beta-cell function by
mutated alleles of the gene encoding forkhead transcription factor
Foxo1. Nat Genet. 32:245–253. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu P, Kao TP and Huang H: CDK1 promotes
cell proliferation and survival via phosphorylation and inhibition
of FOXO1 transcription factor. Oncogene. 27:4733–4744. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wu Y, Shang X, Sarkissyan M, Slamon D and
Vadgama JV: FOXO1A is a target for HER2-overexpressing breast
tumors. Cancer Res. 70:5475–5485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Abdelnour-Berchtold E, Cerantola Y, Roulin
D, Dormond-Meuwly A, Demartines N and Dormond O: Rapamycin-mediated
FOXO1 inactivation reduces the anticancer efficacy of rapamycin.
Anticancer Res. 30:799–804. 2010.PubMed/NCBI
|
|
26.
|
Zhang H, Pan Y, Zheng L, et al: FOXO1
inhibits Runx2 transcriptional activity and prostate cancer cell
migration and invasion. Cancer Res. 71:3257–3267. 2011. View Article : Google Scholar : PubMed/NCBI
|