Introduction
Human papillomavirus (HPV) infects numerous females
worldwide and is generally transmitted through sexual contact
(1). The majority of HPV-induced
lesions disappear 6–12 months after development, however, a small
number progress to become high-grade squamous intraepithelial
lesions (HSIL) and cervical cancer (2). The interaction of HPV with the host
cells represents a significant cascade of molecular events that
culminate in the natural history of cervical cancer development
(3).
The high-risk HPV types encode two oncoproteins, E6
and E7. The E6 oncoprotein binds to the p53 tumor suppressor,
resulting in its inactivation and the prevention of cellular
apoptosis (4). The E7 oncoprotein
binds to the retinoblastoma protein (pRb) tumor suppressor, leading
to continuous cell cycling without any repair check-points
(5). In an attempt to prevent this
continuous cell cycling, p16, a pRb regulator, is overexpressed and
accumulates inside the cells (6).
p16 is a protein that is expressed in low concentrations in healthy
cells, but is overexpressed in cervical cancer and high-grade
precursor lesions. Consequently, p16 overexpression is a
significant marker of cervical lesions and is considered to be a
useful test that may facilitate an improved diagnosis of severe
cervical lesions (7). The HPV E6
oncoprotein is involved in a complementary pathway that is
associated with cell cycle deregulation, where p53 is abrogated.
The immunohistochemical expression of E6 has been proposed to be
useful for determining a diagnosis and/or prognosis (8). Finally, the hybrid capture 2 (HC2)
test is a well-known molecular test that identifies a pool of
high-risk HPVs. The test is used in combination with a liquid-based
cytology examination to ascertain the HPV status in patients
treated for HPV-induced lesions with undetermined cytology
[atypical squamous cells of undetermined significance (ASC-US),
atypical squamous cells, cannot exclude HSIL (ASC-H) or atypical
glandular cell (AGC)], and in the primary screening of cervical
lesions (9).
The aim of the present study was to characterize p16
expression in patients treated by conization with large loop
excision, and to compare the p16 performance with the E6
immunohistochemical and HC2 test results in combination with the
Pap smear examination.
Patients and methods
Patients
Between March 2006 and May 2009, 114 females were
treated for high-grade cervical intraepithelial neoplasia (CIN 2/3)
by conization with large loop excision of the transformation zone
(LLETZ) at the Department of Gynecology, Faculty of Medicine, São
Paulo University (Cerqueira César, Brazil). Following surgery, the
patients returned within 30–45 days for post-operative evaluation.
A follow-up was conducted every 6 months for 2 years. Each
follow-up appointment comprised a Pap smear, colposcopy and HPV DNA
test.
The procedure was explained to all the patient and
written informed consent was provided. The study was approved by
the ethics committee of the Clinics Hospital, Faculty of Medicine,
São Paulo University. Immunohistochemical examinations, including
E6 and p16 staining, were performed on the surgical specimens.
Hybrid capture assay
The HC2 test was conducted according to the
manufacturer’s instructions (Quiagen, Gaithersburg, MD, USA). Only
high-risk HPVs were examined and the carcinogenic types included
types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68.
Immunohistochemistry
p16 CINTEC test
The immunohistochemical reaction for p16 was
performed with the CINtec® Histology kit according to
the manufacturer’s instructions (Roche MTM Laboratories,
Heidelberg, Germany). Briefly, subsequent to the use of retrieval
solution at 125°C for 3 min and 90°C for 20 min, the slides were
cooled at room temperature and washed in wash buffer (1:10
dilution), for 5 min. Endogenous peroxidase was blocked with
peroxidase-blocking reagent at a volume of 30 ml per slide for 5
min. Primary ready-to-use p16 antibody was added after 30 min at
room temperature. Visualization reagent was utilized for signal
amplification. The revelation with diaminobenzidine (DAB) was
performed with 15 ml 3,3′-diaminobenzidine (DAB) chromogen and
counterstained with hematoxylin.
E6 immunohistochemistry
Antigen retrieval was conducted using a microwave
and a solution of 10 mM citric acid (pH 6.0; Merck KGaA, Darmstadt,
Germany) for three minutes at 125°C. Endogenous peroxidase blocking
was performed with 6% hydrogen peroxide
(H2O2). The primary E6 monoclonal antibody
(C1P5) sc460, from mouse E6 HPV 16 and HPV 18 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used at a 1:200
dilution. The revelation was performed with an ADVANCE™ horseradish
peroxidase (HRP) kit (Dako, Carpinteria, CA, USA).
Evaluation of the p16 and E6
immunostaining
The p16 reaction was evaluated as positive when
nuclear or cytoplasmic immunostaining was clearly demonstrated. The
scoring was conducted as previously demonstrated by Longatto-Filho
et al, with slight modifications (10): Negative (no reaction or ≤1% positive
cells), sporadic (>1% but ≤25% positive cells), moderate
(>25% but ≤50% positive cells) and diffuse (>50% positive
cells).
Dichotomic negative/positive evaluation was adapted
to determine E6 immunoreaction as suggested by Lin et al
(8). Brown nuclear staining was
considered as a positive reaction to E6 HPV 16/18 proteins.
Statistical analysis
The Fisher’s exact test was performed to compare
categorical variables. To calculate the parameters of the hybrid
capture accuracy (sensitivity, specificity, positive predictive
value and negative predictive value), the follow-up Pap smear was
adopted as the gold standard. In all statistical tests, P<0.05
was considered to indicate a statistically significant
difference.
Results
The HC2 HPV DNA test (developed in 1997 by Digene
Corporation, Gaithersburg, MD, USA) was performed in 112 of the
included patients prior to the surgical procedure. A total of 108
patients tested positive for HPV DNA and four tested negative prior
to the procedure. Two cases had no HC2 HPV DNA test performed.
Table I presents a description of
the population involved in the study.
 | Table I.Population description data. |
Table I.
Population description data.
| Characteristic | Value |
|---|
| Age (years) | |
| Range | 20–57 |
| Mean (SD) | 33.89 (8.593) |
| Age at first sexual
intercourse (years) | |
| Range | 9–29 |
| Mean (SD) | 16.5 (2.836) |
| Number of sexual
partners | |
| Range | 1–40 |
| Mean (SD) | 4.07 (5.221) |
| Number of births | |
| Range | 0–7 |
| Mean (SD) | 2.29 (1.538) |
| Smoking status, n
(%)a | |
| Non-smoker | 76 (66.7) |
| Smoker | 37 (32.5) |
| Birth control
methods, n (%)b | |
| None | 32 (28.1) |
| Hormonal | 42 (36.8) |
| Others (IUD, tubal
ligation, condom) | 38 (33.3) |
The cytological results prior to the surgical
procedure were as follows: 71 patients presented with HSIL, 2 with
HSIL and AGC and 14 with low-grade squamous intraepithelial lesions
(LSIL). Another 6 patients exhibited ASC-H, 1 exhibited ASC-H +
AGC, and 6 exhibited ASC-US. Only 1 patient presented with ASC-US +
AGC, 4 presented with AGC and 9 were classified as having normal
cytology. The patients who had normal, ASC-US or LSIL cytology
presented with CIN 2/3 in their biopsy samples. The cytological and
histological findings prior to treatment are listed in Table II.
 | Table II.Cytological and histological findings
prior to surgery. |
Table II.
Cytological and histological findings
prior to surgery.
| Tumor
characteristic | No. of patients
(%) |
|---|
| Cytology | |
| Low-grade | 29 (25.4) |
| High-grade | 85 (74.6) |
| Histology | |
| Low-grade | 15 (13.2) |
| High-grade | 93 (81.6) |
| Non-realized | 6 (5.3) |
The pathological examination of the excised cervical
specimens revealed the following diagnoses: 18 (15.8%) patients
with chronic cervicitis; 11 (9.6%) with CIN 1; 19 (16.7%) with CIN
2; 64 (56.1%) CIN 3; one (0.9%) with CIN 3 and adenocarcinoma in
situ (AIS), and one (0.9%) with micro-invasive carcinoma.
Table III shows the results of the
HC2 HPV DNA tests performed prior to the surgical procedure, and
those of the E6 and p16 immunohistochemical tests on the tissue
samples of the surgical specimens. The correlation between the
expression of the E6 and p16 proteins in the surgical specimen is
shown in Table IV. As predicted,
the negative expression of p16 was significantly correlated with
the negative expression of the E6 oncoprotein. In addition, the
positive expression of p16 was significantly correlated with the
positive expression of the E6 oncoprotein.
 | Table III.HC2 HPV DNA test prior to the surgical
procedure, and the subsequent E6 and p16 immunohistochemical test
data. |
Table III.
HC2 HPV DNA test prior to the surgical
procedure, and the subsequent E6 and p16 immunohistochemical test
data.
| Test | Positive, n (%) | Negative, n (%) | Not performed, n
(%) | Total, n (%) |
|---|
| HC2 | 108 (94.7) | 4 (3.5) | 2 (1.8) | 114 (100.0) |
| E6 | 45 (39.5) | 69 (60.5) | - | 114 (100.0) |
| p16 | 74 (64.9) | 40 (35.1) | - | 114 (100.0) |
 | Table IV.Correlation between p16 and E6 protein
immunohistochemical expression. |
Table IV.
Correlation between p16 and E6 protein
immunohistochemical expression.
| E6, n (%)
| Total, n (%) |
|---|
| Negative | Positive |
|---|
| p16 | | | |
| Negative | 30 (75.0) | 10 (25.0) | 40 (100.0) |
| Positive | 39 (52.7) | 35 (47.3) | 74 (100.0) |
| Total | 69 (60.5) | 45 (39.5) | 114 (100.0) |
The results of the p16 and E6 immunohistochemical
reactions, the HC2 HPV DNA tests prior to the surgical procedure
and the histopathological findings in the surgical specimens are
presented in Table V. The positive
expression of p16 was correlated with lesions of increased severity
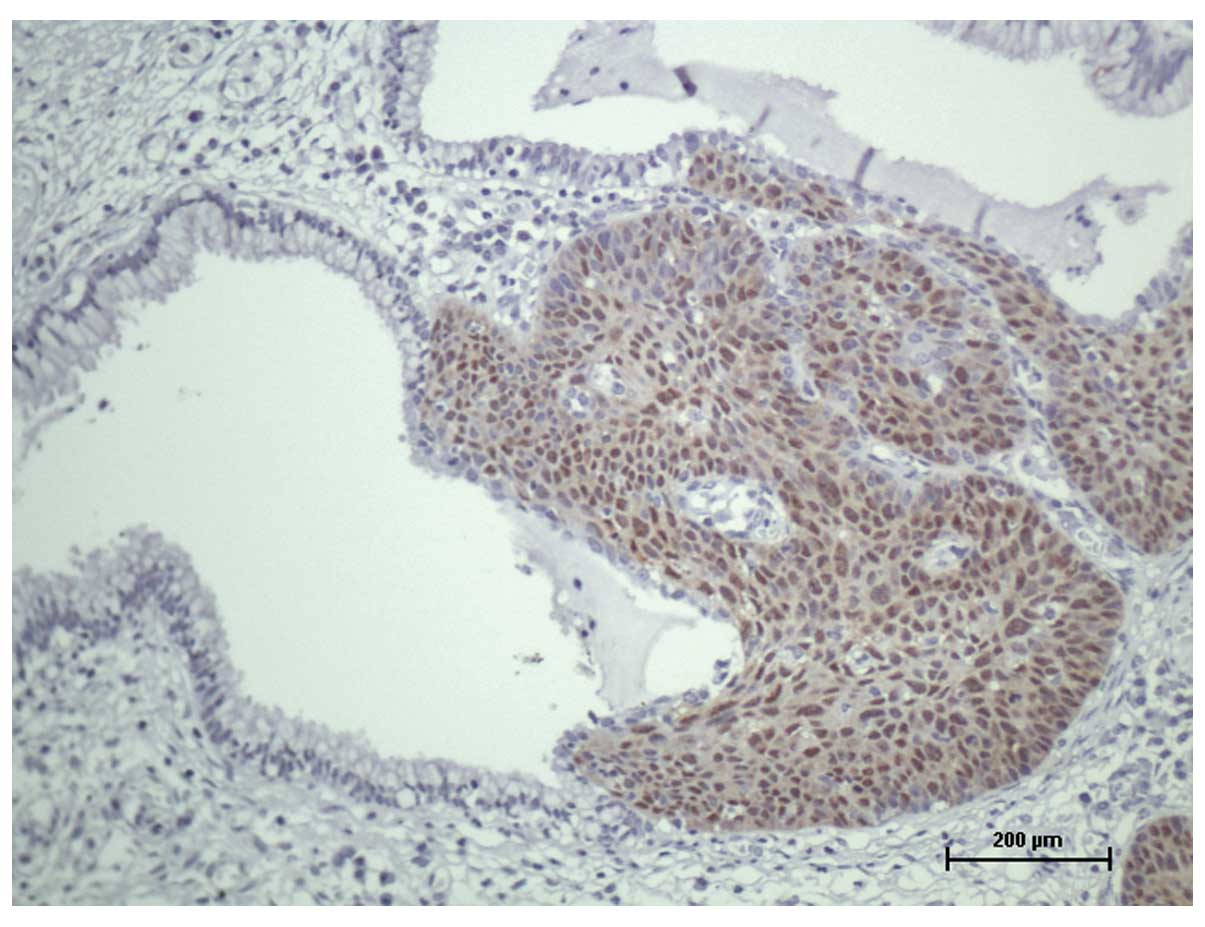
identified in the surgical specimen (P=0.0001; Fig. 1), however, no such correlation was
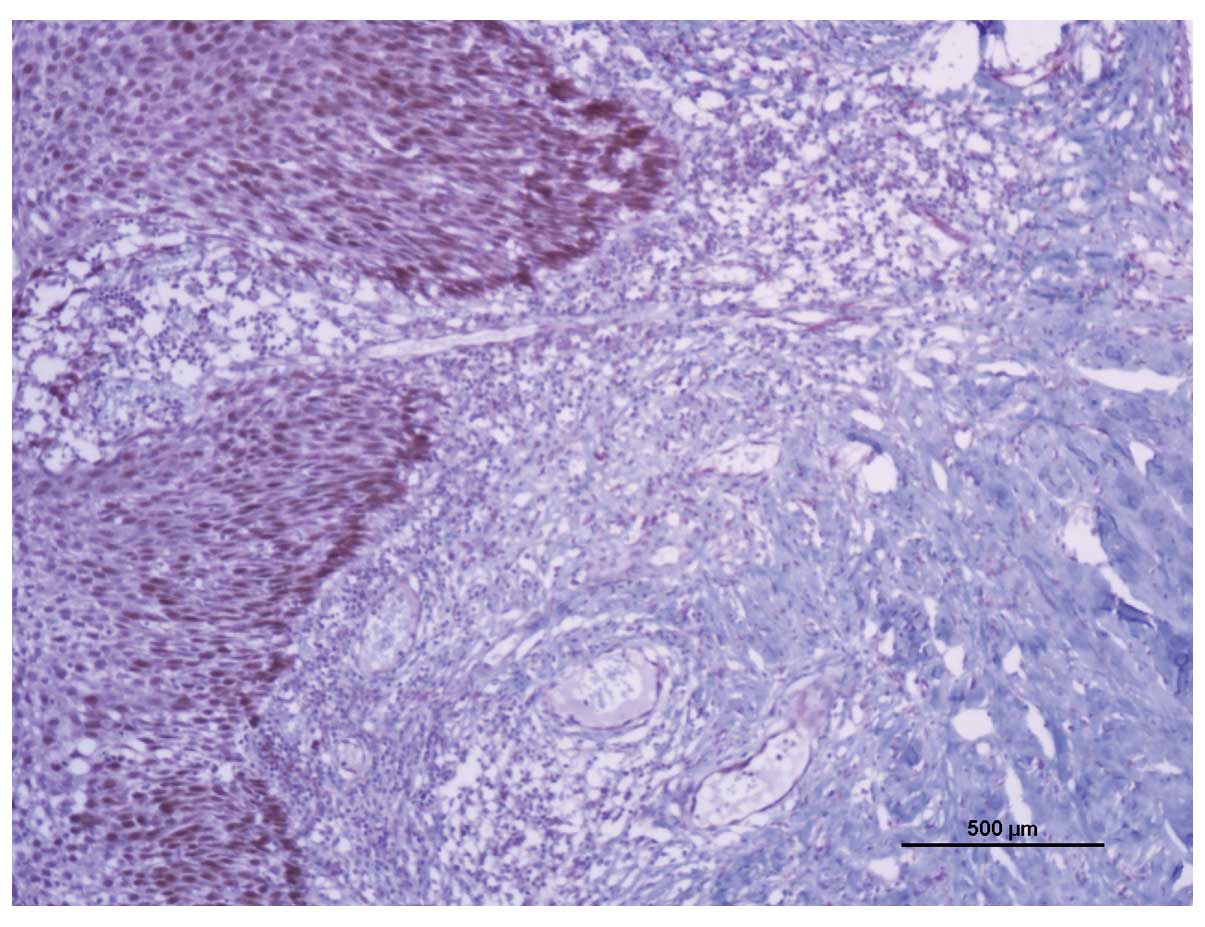
identified with E6 expression (P=0.131; Fig 2).
 | Table V.Correlation between p16 and E6
expression and HC2 status, and the histopathological findings in
the surgical specimen. |
Table V.
Correlation between p16 and E6
expression and HC2 status, and the histopathological findings in
the surgical specimen.
A, p16
|
| Negative, n
(%) | Positive, n
(%) | Total, n (%) | P-value |
|
| Histopathological
diagnosis | | | | |
| Cervicitis/CIN
1 | 23 (79.3) | 6 (20.7) | 29 (100.0) | |
| CIN 2/CIN
3/AIS/Ca microinvasor | 17 (20.0) | 68 (80.0) | 85 (100.0) | |
| Total | 40 (35.1) | 74 (64.9) | 114 (100.0) | 0.0001 |
|
B, E6
|
| Negative, n
(%) | Positive, n
(%) | Total, n (%) | P-value |
| Histopathological
diagnosis | | | | |
| Cervicitis/CIN
1 | 21 (72.4) | 8 (27.6) | 29 (100.0) | |
| CIN 2/CIN
3/AIS/Ca microinvasor | 48 (56.5) | 37 (43.5) | 85 (100.0) | |
| Total | 69 (60.5) | 45 (39.5) | 114 (100.0) | 0.131 |
|
C, DNA HPV test
|
| Negative, n
(%) | Positive, n
(%) | Total, n (%) | P-value |
|
| Histopathological
diagnosis | | | | |
| Cervicitis/CIN
1 | 4 (14.8) | 23 (85.2) | 27 (100.0) | |
| CIN 2/CIN
3/AIS/Ca microinvasor | 0 (0.0) | 85 (100.0) | 85 (100.0) | |
| Total | 4 (3.6) | 108 (96.4) | 112
(100.0)a | 0.0001 |
Table VI presents
the comparison between the cytological diagnoses prior to surgery
and the HPV-related markers; p16, E6 and HC2 status.
 | Table VI.Comparison among the cytological
diagnoses prior to surgery and the HPV related-markers, p16, E6 and
HC2. |
Table VI.
Comparison among the cytological
diagnoses prior to surgery and the HPV related-markers, p16, E6 and
HC2.
| Cytological
diagnosis | HPV-related markers
(n)
|
|---|
p16
| E6
| HC2
| Total |
|---|
| Positive | Negative | Positive | Negative | Positive | Negative |
|---|
| Negative | 5 | 4 | 6 | 3 | 9 | 0 | 9 |
| ASC-US | 3 | 3 | 2 | 4 | 5 | 1 | 6 |
| ASC-US+AGC | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| ASC-H | 4 | 2 | 4 | 2 | 6 | 0 | 6 |
| ASC-H+AGC | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| LSIL | 9 | 5 | 6 | 8 | 14 | 0 | 14 |
| HSIL | 48 | 23 | 24 | 47 | 66 | 3 | 71a |
| HSIL+AGC | 1 | 1 | 0 | 2 | 2 | 0 | 2 |
| AGC | 3 | 1 | 2 | 2 | 4 | 0 | 4 |
| Total | 74 | 40 | 45 | 69 | 108 | 4 | |
| 114 | 114 | 112a | |
The accuracy values of the HC2 test in predicting
cytological abnormalities over the 2-year follow-up are shown in
Table VII. The HC2 test was results
were compared with the cytology. The tests were conducted
simultaneously during the follow-up period.
 | Table VII.The accuracy values of the HC2 test
in predicting cytological abnormalities over a 2-year follow-up
period. |
Table VII.
The accuracy values of the HC2 test
in predicting cytological abnormalities over a 2-year follow-up
period.
| Time (months) | Positive predictive
value (%) | Negative predictive
value (%) | Sensitivity
(%) | Specificity
(%) | Total no. of
patients |
|---|
| 6 | 50.0 | 97.3 | 83.3 | 87.8 | 94.0 |
| 12 | 42.9 | 96.4 | 75.0 | 87.1 | 70.0 |
| 18 | 33.3 | 100.0 | 100.0 | 86.0 | 61.0 |
| 24 | 54.5 | 98.0 | 85.7 | 90.7 | 61.0 |
According to the results of the HPV DNA hybrid,
collected in the first post-operative follow-up as a predictor of
the cytological abnormalities found in the 24-month follow-up
period, a sensitivity of 55.6%, a specificity of 84.8%, a positive
predictive value of 33.3% and a negative predictive value of 93.3%
were recorded. In comparison, Table
VII describes the accuracy of the HC2 test in predicting
cytological abnormalities at each follow-up examination performed
over a 2-year period.
Discussion
In our previous study, containing partial
information from the present study, we identified that patients
with a combination of negative cytology and negative hybrid capture
test results did not exhibit high-grade lesions at the conization
follow-up examination (11). The
results of the present study supported the use of the HC2 HPV DNA
test, collected in the first post-operative assessment, as a marker
of disease recurrence or a disease-free status (11). Additionally, the results
demonstrated that the characterization of p16 in a well-controlled
population that underwent cervix conization due to the HSIL
alteration was concordant with previous results. This indicated
that the p16 marker was strongly expressed in high-grade lesions,
and that it had the potential to identify severe lesions when
associated with a positive hybrid capture test (12). Furthermore, p16 was expressed in 80%
of CIN 2+ biopsy-diagnosed cases, which reinforced its
potential use as an accurate marker of high-grade cervical
lesions.
p16 changes in the methylation profile of cervical
HPV-induced lesions have been implicated in transcription and
replication control, potentially triggering the neoplastic
transformation (13). This is a
noteworthy finding, as different HPV methylomes are linked to the
various stages of squamous intraepithelial lesion differentiation,
including those of a high-grade phenotype. However, the enhanced
expression of the viral E6 oncogene in advanced lesions of
persistent HPV infections was not observed in our specimens as we
had predicted (13). The
immunohistochemical expression of the E6 oncoprotein has been
recorded in different types of tumors, presumably induced by
persistent high-risk HPV infection; however, the frequency of a
positive immunoreaction was low (14–17).
For the E6 immunohistochemical evaluation in cervical HPV-induced
lesions, we did not identify any studies comparable with the
present study; however, the negative p16 and E6 reactions were
observed in combination in 75% of cases, but only 52.7% of positive
reactions were identified in combination. This may be due to a
limitation in sensitivity for the immunohistochemical reaction, as
37 of the CIN 2+ cases (43.5%) were E6-positive. It has
been suggested that differences in E6 variants prevalent in
cervical carcinoma are not correlated with the carcinogenic
potential of the E6 protein. Moreover, E6 variants have revealed
comparable abilities in preventing growth arrest and inhibiting the
induced p53 elevation. Differences were detected in the ability to
deregulate stratification and differentiation, as well as in
modulating apoptosis and hyperactivating the Wnt signaling cascade
(18). The absence of a correlation
between p16 and E6 expression was not predicted, however, the
reason for this discrepancy may be attributed to the low
sensitivity of E6 immunohistochemical expression (15).
The present study demonstrated the predictive
potential of the negative values in the hybrid capture test during
the follow-up of the patients that underwent conization. In
addition, specificity was observed in each clinical visit. Repeated
detection of high-risk HPV was demonstrated to be significantly
more specific, but less sensitive, in identifying females at risk
for CIN 2/3 as compared with a single time-point measurement.
Moreover, sensitivity has been estimated to decrease and
specificity to increase when the testing intervals were increased
from 12 to 24 months (19). The
variations in sensitivity and specificity, observed in the present
study during the 24-month visit following conization, did not
demonstrate such significant disparity.
In conclusion, the current study supported the
critical function of p16INK4A as a highly specific marker of CIN.
However, the immunohistochemical expression of p16 has been
previously demonstrated to have no prognostic value in predicting
the clearance of high-risk HPV following conization (20). Overexpression of p16 in human tumors
as a whole has been demonstrated to be correlated with high-grade
pre-malignant lesions, high-grade tumors and senescence (21).
References
|
1.
|
zur Hausen H: Papillomaviruses causing
cancer: evasion from host-cell control in early events in
carcinogenesis. J Natl Cancer Inst. 92:690–698. 2000.PubMed/NCBI
|
|
2.
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
3.
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 70:890–907. 2007. View Article : Google Scholar
|
|
4.
|
Tungteakkhun SS and Duerksen-Hughes PJ:
Cellular binding partners of the human papillomavirus E6 protein.
Arch Virol. 153:397–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Doorbar J: The papillomavirus life cycle.
J Clin Virol. 32(Suppl 1): S7–S15. 2005. View Article : Google Scholar
|
|
6.
|
Cuschieri K and Wentzensen N: Human
papillomavirus mRNA and p16 detection as biomarkers for the
improved diagnosis of cervical neoplasia. Cancer Epidemiol
Biomarkers Prev. 17:2536–2545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Carozzi F, Confortini M, Dalla Palma P,
Del Mistro A, Gillio-Tos A, De Marco L, et al: Use of p16-INK4A
overexpression to increase the specificity of human papillomavirus
testing: a nested substudy of the NTCC randomised controlled trial.
Lancet Oncol. 9:937–945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lin HP, Wang YP and Chiang CP: Expression
of p53, MDM2, p21, heat shock protein 70, and HPV 16/18 E6 proteins
in oral verrucous carcinoma and oral verrucous hyperplasia. Head
Neck. 33:334–340. 2011.PubMed/NCBI
|
|
9.
|
Schiffman M, Wentzensen N, Wacholder S,
Kinney W, Gage JC and Castle PE: Human papillomavirus testing in
the prevention of cervical cancer. J Natl Cancer Inst. 103:368–383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Longatto-Filho A, Etlinger D, Pereira SM,
Kanamura CT, di Loreto C, Santos Gda C, et al: The association of
p16(INK4A) and fragile histidine triad gene expression and cervical
lesions. J Low Genit Tract Dis. 11:151–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Roncaglia MT, Tacla M, Vieira da Motta E,
Caiaffa H, Ab’Saber A, Alves VA, Longatto Filho A and Baracat EC:
Evaluation of the combination of cytology and hybrid capture to
safely predict the high-grade lesion status of patients treated
with conization with large loop excision of the transformation
zone. Acta Cytol. 55:421–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Reuschenbach M, Clad A, von Knebel
Doeberitz C, Wentzensen N, Rahmsdorf J, Schaffrath F, Griesser H,
Freudenberg N and von Knebel Doeberitz M: Performance of
p16INK4a-cytology, HPV mRNA, and HPV DNA testing to identify high
grade cervical dysplasia in women with abnormal screening results.
Gynecol Oncol. 119:98–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Vinokurova S and von Knebel Doeberitz M:
Differential methylation of the HPV 16 upstream regulatory region
during epithelial differentiation and neoplastic transformation.
PLoS One. 6:e244512011. View Article : Google Scholar
|
|
14.
|
Wu QJ, Guo M, Lu ZM, Li T, Qiao HZ and Ke
Y: Detection of human papillomavirus-16 in ovarian malignancy. Br J
Cancer. 89:672–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Qi ZL, Huo X, Xu XJ, Zhang B, Du MG, Yang
HW, Zheng LK, Li J and Shen ZY: Relationship between HPV16/18 E6
and 53, 21WAF1, MDM2, Ki67 and cyclin D1 expression in esophageal
squamous cell carcinoma: comparative study by using tissue
microarray technology. Exp Oncol. 28:235–240. 2006.PubMed/NCBI
|
|
16.
|
Lawson JS, Glenn WK, Heng B, Ye Y, Tran B,
Lutze-Mann L and Whitaker NJ: Koilocytes indicate a role for human
papilloma virus in breast cancer. Br J Cancer. 101:1351–1356. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Akil N, Yasmeen A, Kassab A, Ghabreau L,
Darnel AD and Al Moustafa AE: High-risk human papillomavirus
infections in breast cancer in Syrian women and their association
with Id-1 expression: a tissue microarray study. Br J Cancer.
99:404–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zehbe I, Lichtig H, Westerback A, Lambert
PF, Tommasino M and Sherman L: Rare human papillomavirus 16 E6
variants reveal significant oncogenic potential. Mol Cancer.
10:772011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Marks M, Castle PE, Schiffman M and
Gravitt PE: Evaluation of any or type-specific persistence of
high-risk human papillomavirus for detecting cervical precancer. J
Clin Microbiol. 50:300–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Branca M, Ciotti M, Santini D, Di Bonito
L, Giorgi C, Benedetto A, Paba P, Favalli C, Costa S, Agarossi A,
Alderisio M and Syrjänen K: p16(INK4A) expression is related to
grade of cin and high-risk human papillomavirus but does not
predict virus clearance after conization or disease outcome. Int J
Gynecol Pathol. 23:354–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Romagosa C, Simonetti S, López-Vicente L,
Mazo A, Lleonart ME, Castellvi J and Ramon y Cajal S: p16(Ink4a)
overexpression in cancer: a tumor suppressor gene associated with
senescence and high-grade tumors. Oncogene. 30:2087–2097. 2011.
View Article : Google Scholar : PubMed/NCBI
|