Introduction
Lung cancer is one of the most harmful malignant
tumors to human health and life and its incidence is increasing
every year, ranking first in large- and medium-sized cities in the
incidence of malignant tumors (1,2).
Two-thirds of patients are in the advanced stage when diagnosed
with lung cancer and lose the opportunity for surgical treatment.
Non-small cell lung cancer (NSCLC) comprises 80% of all lung
cancers. Chemotherapy is primarily used for the treatment of
advanced lung cancer. Although new anticancer drugs and
chemotherapies have been introduced, the outcomes for certain
patients are not always satisfactory and patients become less able
to tolerate treatment as the chemotherapy extends (3).
Therefore, targeted therapy for lung cancer has
become a research hotspot in recent years. Erlotinib and gefitinib
are widely used in lung cancer therapy, although they are only
effective for specific pathological situations and patients; the
outcomes for the rest of the patients remain unsatisfactory. The
issue of subsequent resistance to chemotherapy and the mechanisms
involved have yet to be elucidated. Novel treatment strategies
targeting this aggressive disease are expected to offer long-term
disease control or possibly even a cure.
Rhein, one of the major bioactive constituents of
the rhizome of rhubarb (4,5), inhibits the proliferation of various
human cancer cells (6–10). Our previous studies showed that
rhein lysinate (RHL), a salt of rhein and lysine that is easily
dissolved in water, exhibits anticancer activity in breast cancer,
ovarian cancer, hepatocellular carcinoma and cervical cancer in
vivo and in vitro (11–14).
We previously showed that RHL was highly active in
targeting the MEK/extracellular signal-regulated kinase (ERK)
signal pathway and induced apoptosis and cell cycle arrest in human
ovarian cancer cells (12). The
present study aimed to determine whether RHL has additive or
synergistic effects with Taxol and to determine the molecular
mechanisms by which RHL enhances Taxol-induced cytotoxicity and
apoptosis.
Materials and methods
Chemicals and reagents
Rhein (purity, 98%) was purchased from Nanjing
Qingze Medicine Ltd., (Nanjing, Jiangsu, China), while lysine was
purchased from Beijing Solarbio Science and Technology Co.
(Beijing, China). RHL was made in our department (patent no.
200810089025.8). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
and dimethyl sulfoxide (DMSO) were obtained from Sigma Aldrich
(Shanghai, China). Antibodies targeting poly(ADP-ribose) polymerase
(PARP), caspase-3, Bcl-2, NF-κB, MEK, p-MEK, ERK, p-ERK and β-actin
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Secondary antibodies against rabbit or mouse IgG were
purchased from Cell Signaling Technology (Danvers, MA, USA). The
prestained protein marker p7708V was purchased from New England
Biolabs Ltd. (Beijing, China). All other chemicals were of standard
analytical grade.
Cell culture
The human lung carcinoma cell lines, H460 and A549,
were obtained from the Cell Center of the Institute of Basic
Medical Sciences, Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China). The cells were cultured in
RPMI 1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis,
MO, USA), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
Cell proliferation assays were performed using the
MTT method, according to the manufacturer’s instructions. The cells
were seeded in 96-well plates (Costar, Cambridge, MA, USA) with
2,500 cells/well. Subsequent to overnight incubation, triplicate
wells were treated with various concentrations of RHL for 48 h.
Next, 20 μl MTT solutions (5 mg/ml in PBS) were added to
each well and incubated for 4 h at 37°C. The MTT formazan was
dissolved in 150 μl DMSO and the absorbance was measured
with a microplate reader (Multiskan MK3; Thermo Labsystem, Waltham,
MA USA) at a wavelength of 570 nm.
FITC-Annexin V/PI apoptosis assay
The cells were collected and resuspended in 200
μl binding buffer, then 10 μl FITC-labeled enhanced
Annexin V (Baosai Biotechnology Ltd., Beijing, China) and 100 ng
propidium iodide (PI) were added. Upon incubation in the dark for
15 min at room temperature or 30 min at 4°C, the samples were
diluted with 300 μl binding buffer. Flow cytometry was
performed on a FACScan instrument (Becton-Dickinson, Franklin
Lakes, NJ, USA) and the data were processed using
WinMDI/PC-software.
Western blot analysis
The cells were harvested and washed with PBS
solution. The whole cellular extracts were prepared by incubating
the cells on ice in lysis buffer containing 50 mM Tris-HCl (pH
7.5), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM dithiothreitol, 1%
Nonidet P-40, 0.1% SDS, protease inhibitors (1 mMPMSF, 5
μg/ml aprotinin, 5 μg/ml leupeptin and 5 μg/ml
pepstatin) and phosphatase inhibitors (20 mM β-glycerophosphate, 50
mM NaF and 1 mM Na3VO4). The supernatant was
collected through centrifugation at 12,000 x g for 12 min. Protein
concentrations were determined with the Bradford protein assay.
Equal amounts of lysate (40 μg) were resolved by SDS-PAGE
and transferred to polyvinylidene difluoride membrane (Millipore
Corp., Bedford, MA, USA). The membranes were blocked in TBST
containing 5% skim med milk at room temperature for 2 h and probed
with primary antibodies overnight at 4°C. The membranes were then
blotted with an appropriate horseradish peroxidase-linked secondary
antibody (Santa Cruz Biotechnology, Inc.). Proteins were visualized
using enhanced chemiluminescence western blotting detection
reagents (Amersham Pharmacia Biotech, Inc., Piscataway, NJ,
USA).
Results
Taxol-induced growth inhibition is
potentiated by RHL in H460 and A549 cells
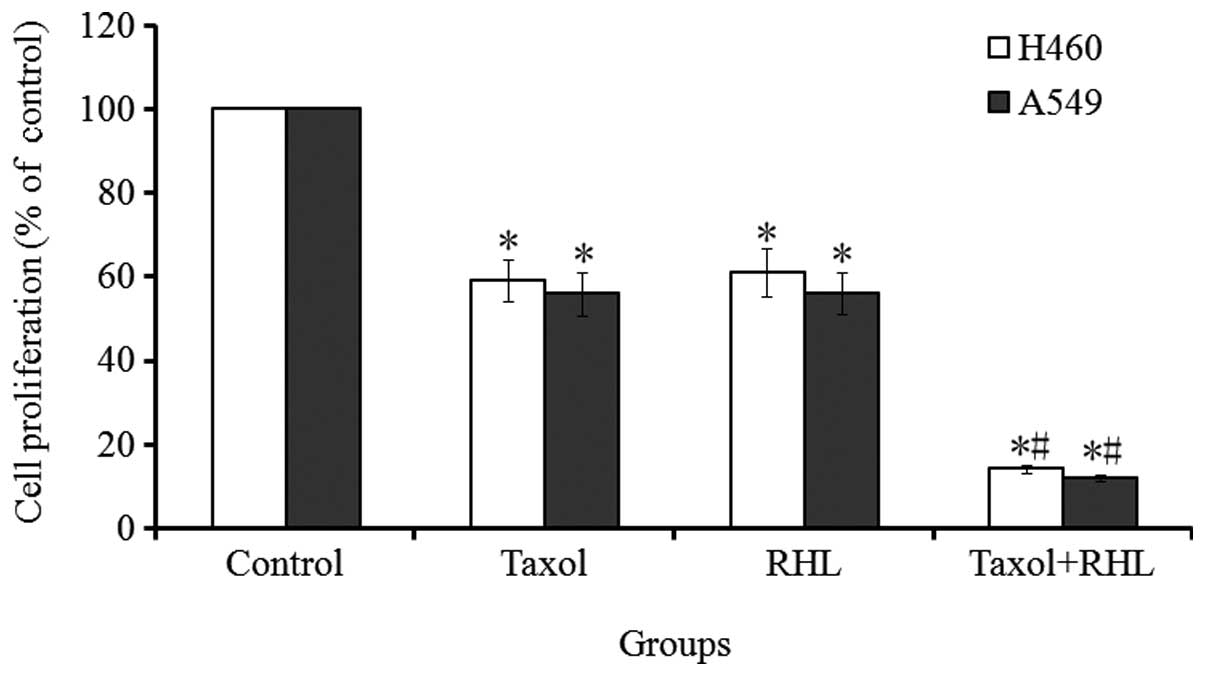
The growth of the H460 and A549 cells treated with
RHL (100 μmol/l), Taxol (1 μmol/l) or a combination
of the two was determined via MTT assays. The dose used in the
present study was selected based upon a preliminary dose escalation
study. A significant reduction in growth was observed in the cells
treated with RHL and Taxol in combination compared with treatment
with RHL or Taxol alone (Fig. 1).
The combined drug intoxication (CDI) value was <0.7, indicating
that the two drugs have a synergistic effect.
Taxol-induced apoptosis is sensitized by
RHL in H460 and A549 cells
The induction of apoptosis was observed in the lung
cancer cells treated with either Taxol, RHL or a combination of the
two. Relative to the single agents, the combined treatment induced
more apoptosis in the two cell lines, as shown by flow cytometry
combined with FITC-Annexin V/PI staining (Fig. 2). The ratios of apoptosis were 52.31
and 52.51% in the combined treatment groups, whereas the ratios of
the Taxol groups were 18.98 and 18.86% in the H460 and A549 cells,
respectively. These results were consistent with the cell growth
inhibition experiments using MTT, suggesting that the loss of
viable cells due to RHL and Taxol treatment is partly due to the
induction of an apoptotic cell death mechanism.
Taxol-induced apoptosis signaling is
augmented by RHL in H460 and A549 cells
In an attempt to investigate the mechanism of the
enhanced apoptotic process induced by the treatment of the cells
with RHL and Taxol, the levels of caspase-3 and PARP were assessed
in the H460 and A549 cells. The results showed that the combined
treatment was able to increase the levels of cleaved PARP and
caspase-3 significantly. The results for the anti-apoptotic Bcl-2
and NF-κB proteins also showed downregulation in the combination
group relative to the single-agent treatments and untreated control
(Fig. 3).
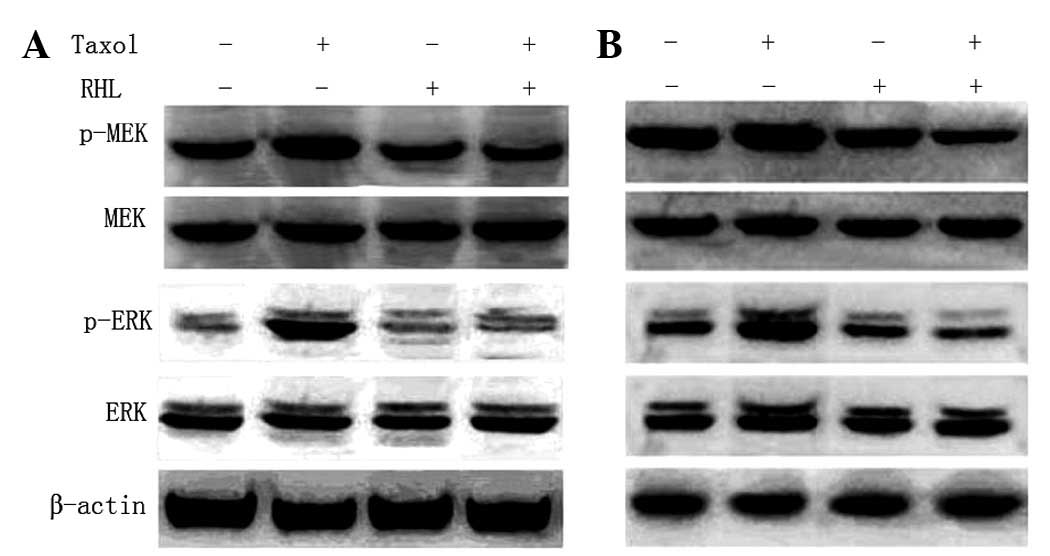
Effects of Taxol, RHL and a combination
of the two on the MEK/ERK signaling pathway
In the present study, it was observed that Taxol
treatment enhanced the activation of ERK in the H460 and A5449
cells. However, Taxol in combination with RHL prevented the
Taxol-induced ERK activation through the inhibition of MEK
phosphorylation (Fig. 4).
Discussion
Lung cancer remains the leading cause of
cancer-related mortality worldwide despite advances in the field of
cancer therapeutics (2).
Traditional treatment with empirically selected cytotoxic
chemotherapeutic agents has provided small, but real survival
benefits (2). Moreover, cancer
recurrence and subsequent resistance to chemotherapy remain
problematic and the mechanisms are not clear. New agents are
required to offer long-term disease control or even possibly a
cure. Advances and insights into the molecular pathogenesis of lung
cancers have provided certain novel molecular targets, offering new
strategies and agents that are tumor specific.
Certain studies have demonstrated that tumor cells
are able to produce resistance to Taxol by activating the MEK/ERK
signal pathway. The activation of ERK was demonstrated to be
important in mediating proliferation in cancer cells (15–18).
Inhibiting MEK/ERK signaling may therefore enhance Taxol-induced
cytotoxicity in lung cancer cells.
Rhein, one of the major bioactive constituents of
the rhizome of rhubarb (4,5), inhibits the proliferation of various
human cancer cells (6–10). We previously demonstrated that RHL,
a salt of rhein and lysine that is easily dissolved in water, has
anticancer activity in breast cancer, ovarian cancer, cervical
cancer and hepatocellular carcinoma in vivo and in
vitro (11–14). We also showed that RHL was highly
active in targeting the MEK/ERK signal pathway and that it induced
apoptosis and cell cycle arrest in human ovarian cancer cells
(12).
In the present study, it was observed that RHL
improved the anti-tumor activity of Taxol in lung cancer.
Mechanically, RHL potentiated Taxol-induced cell killing by
reducing the phosphorylation of ERK and increasing the levels of
cleaved caspase-3 and PARP.
These caspases belong to a family of cysteine
proteases whose activation induces cellular apoptosis.
Specifically, proteolytically cleaved caspase-3 and caspase-7, the
active forms of pro-caspase-3 and pro-caspase-7, are key molecules
for identifying the activation of apoptosis (19–21).
In addition, PARP is one of the main substrates of activated
caspase pathways and a well-established indicator of apoptotic cell
death.
The results for the anti-apoptotic Bcl-2 and NF-κB
proteins also showed downregulation in the combined treatment group
compared with the signal-agent treatment and untreated control
groups.
It is known that members of the Bcl-2 protein family
act as key regulators of cellular apoptosis and are important
determinants of cellular sensitivity or resistance to chemotherapy
drugs (22–24). The overexpression of Bcl-2, an
anti-apoptosis member of this family, is commonly observed in human
lung cancer and Bcl-2 overexpression correlates with
chemoresistance in this disease. In addition, NF-κB has been shown
to inhibit apoptosis in response to chemotherapeutic agents.
Compounds targeting the NF-κB pathway are able to sensitize lung
tumor cells by counteracting resistance mechanisms, and therefore,
deserve further evaluation with regard to chemotherapy and the
possible chemoprevention of lung cancer (25–27).
In conclusion, the present findings showed a
synergistic effect between RHL and Taxol in certain lung cancer
cell lines. This synergy is likely to be associated with the
downregulation of ERK activation. Accordingly, further mechanistic
studies may be useful in the treatment of patients with lung
carcinoma.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (81001439)
and the General Program of Natural Science Foundation of Hebei
Province of China (H2012401030).
References
|
1.
|
Hazra S, Peebles KA, Sharma S, Mao JT and
Dubinett SM: The role of PPARgamma in the cyclooxygenase pathway in
lung cancer. PPAR Res. 2008:7905682008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Vijayalakshmi R and Krishnamurthy A:
Targetable “driver” mutations in non small cell lung cancer. Indian
J Surg Oncol. 2:178–188. 2011.
|
|
3.
|
Yuan SF, Chen WJ, Zhu LJ, Zheng WE, Chen H
and Xiong JP: Effects of monoclonal antibodies against human
stathmin combined with paclitaxel on proliferation of the QG-56
human lung carcinoma cell line. Asian Pac J Cancer Prev.
13:2967–2971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kuo PL, Hsu YL, Ng LT and Lin CC: Rhein
inhibits the growth and induces the apoptosis of Hep G2 cells.
Planta Med. 70:12–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Huang Q, Lu G, Shen HM, Chung MC and Ong
CN: Anti-cancer properties of anthraquinones from rhubarb. Med Res
Rev. 27:609–630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lai WW, Yang JS, Lai KC, et al: Rhein
induced apoptosis through the endoplasmic reticulum stress,
caspase-and mitochondria-dependent pathways in SCC-4 human tongue
squamous cancer cells. In Vivo. 23:309–316. 2009.
|
|
7.
|
Ip SW, Weng YS, Lin SY, et al: The role of
Ca2+ on rhein-induced apoptosis in human cervical cancer
Ca Ski cells. Anticancer Res. 27:379–389. 2007.
|
|
8.
|
Lin ML, Chen SS, Lu YC, et al: Rhein
induces apoptosis through induction of endoplasmic reticulum stress
and Ca2+-dependent mitochondrial death pathway in human
nasopharyngeal carcinoma cells. Anticancer Res. 27:3313–3322.
2007.PubMed/NCBI
|
|
9.
|
Cichewicz RH, Zhang Y, Seeram NP and Nair
MG: Inhibition of human tumor cell proliferation by novel
anthraquinones from daylilies. Life Sci. 74:1791–1799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Floridi A, Gentile PF, Bruno T, et al:
Cytotoxic effect of the association of BCNU with rhein or
lonidamine on a human glioma cell line. Anticancer Res. 11:789–792.
1991.PubMed/NCBI
|
|
11.
|
Lin YJ and Zhen YS: Rhein lysinate
suppresses the growth of breast cancer cells and potentiates the
inhibitory effect of Taxol in athymic mice. Anticancer Drugs.
20:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lin YJ, Zhen YZ, Shang BY and Zhen YS:
Rhein lysinate suppresses the growth of tumor cells and increases
the anti-tumor activity of Taxol in mice. Am J Chin Med.
37:923–931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lin YJ, Huang YH, Zhen YZ, Liu XJ and Zhen
YS: Rhein lysinate induces apoptosis in breast cancer SK-Br-3 cells
by inhibiting HER-2 signal pathway. Yao Xue Xue Bao. 43:1099–1105.
2008.(In Chinese).
|
|
14.
|
Zhen YZ, Lin YJ, Gao JL, Zhao YF and Xu
AJ: Rhein lysinate inhibits cell growth by modulating various
mitogen-activated protein kinases in cervical cancer cells. Oncol
Lett. 2:129–133. 2011.PubMed/NCBI
|
|
15.
|
Brognard J and Dennis PA: Variable
apoptotic response of NSCLC cells to inhibition of the MEK/ERK
pathway by small molecules or dominant negative mutants. Cell Death
Differ. 9:893–904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lee MW, Kim DS, Min NY and Kim HT: Akt1
inhibition by RNA interference sensitizes human non-small cell lung
cancer cells to cisplatin. Int J Cancer. 122:2380–2384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ozaki K, Kosugi M, Baba N, et al: Blockade
of the ERK or PI3K-Akt signaling pathway enhances the cytotoxicity
of histone deacetylase inhibitors in tumor cells resistant to
gefitinib or imatinib. Biochem Biophys Res Commun. 391:1610–1615.
2010. View Article : Google Scholar
|
|
18.
|
Lunghi P, Giuliani N, Mazzera L, et al:
Targeting MEK/MAPK signal transduction module potentiates
ATO-induced apoptosis in multiple myeloma cells through multiple
signaling pathways. Blood. 112:2450–2462. 2008. View Article : Google Scholar
|
|
19.
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wolf BB, Schuler M, Echeverri F and Green
DR: Caspase-3 is the primary activator of apoptotic DNA
fragmentation via DNA fragmentation factor-45/inhibitor of
caspase-activated DNase inactivation. J Biol Chem. 274:30651–30656.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
McDonnell TJ, Beham A, Sarkiss M, Andersen
MM and Lo P: Importance of the Bcl-2 family in cell death
regulation. Experientia. 52:1008–1017. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Reed JC, Miyashita T, Takayama S, et al:
BCL-2 family proteins: regulators of cell death involved in the
pathogenesis of cancer and resistance to therapy. J Cell Biochem.
60:23–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Halasova E, Adamkov M, Matakova T, et al:
Expression of Ki-67, Bcl-2, Survivin and p53 Proteins in Patients
with Pulmonary Carcinoma. Adv Exp Med Biol. 756:15–21. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Arlt A, Gehrz A, Müerköster S, et al: Role
of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma
cell lines against gemcitabine-induced cell death. Oncogene.
22:3243–3251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Linardopoulos S: Aurora-A kinase regulates
NF-kappaB activity: lessons from combination studies. J BUON.
12(Suppl 1): S67–S70. 2007.PubMed/NCBI
|
|
27.
|
Deng LL, Shao YX, Lv HF, Deng HB and Lv
FZ: Over-expressing CYLD augments antitumor activity of TRAIL by
inhibiting the NF-κB survival signaling in lung cancer cells.
Neoplasma. 59:18–29. 2012.PubMed/NCBI
|