Introduction
Almost 97% of all reported endometrial cancers are
classed as carcinomas, which encompass a heterogenous group of
tumors that display a range of biological, morphological and
pathological characteristics (1–4).
It is well known that tumor transformation and
progression do not represent singular events, but involve several
steps and complex interactions, including the inflammatory process
and angiogenesis, associated with tumors that are establishing a
growth-favorable local microenvironment. Macrophages, as a major
component of the tumor microenvironment, release growth factors
that affect tumor cells or the endothelium of tumor vessels and
promote the recruitment of secondary inflammatory cells, including
mast cells and neutrophils (5).
In addition, tumor-associated macrophages (TAMs)
appear to play a key role in tumor angiogenesis by modulating tumor
growth and invasion (6–13). However, mast cells are also known to
be able to synthesize and release strong angiogenic cytokines
(14), such as tryptase. Tryptase
has been reported to be released by mast cells in areas of
angiogenesis and also to play a significant role in
neovascularization (15).
The aim of the present study was to assess tumor
angiogenesis quantified by microvessel density (MVD), mast cell
density (McMD) and macrophage density (MphMD), and to assess any
putative correlations these factors may have with the tumor stage
and grade in patients with endometrioid endometrial carcinoma
(EEC).
Material and methods
Patients
The cases of 52 patients who were diagnosed with
endometrial carcinoma between 2003 and 2011 in the Laboratory of
Pathology from the County Emergency Clinical Hospital Craiova
(Craiova, Romania) were analyzed. Furthermore, five samples of
normal endometrium and myometrium were used as controls. The
biological material was obtained from hysterectomy fragments that
had been fixed in 10% buffered neutral formalin and then
classically processed for paraffin embedding and hematoxylin-eosin
staining.
The grade and stage of the neoplasias were used as
the morphoclinical parameters and were classified according to the
guidelines by the World Health Organization (WHO) (16). In Romania at present, there is no
National Register for patients who have been diagnosed with
endometrial adenocarcinoma. Approval for the present study was
obtained from the ethics committee of the University of Medicine
and Pharmacy of Craiova, and written informed consent was obtained
from all patients.
Immunohistochemistry
The panel of antibodies that were used for the
immunohistochemical analysis are presented in Table I. The analysis of the morphology,
topography and density of the elements of interest was based on
sequential double immunohistochemical reactions using the CD105,
mast cell tryptase and CD68 antibodies successively. To detect the
first antibody, an LSAB2-HRP amplification and detection system
(K0675; Dako, Redox Bucharest, Romania) was used with DAB chromogen
development (3467; Dako). Detection of the second antibody was
performed using a species-specific LSAB2-AP System (K0674; Dako)
and chromogen Vulcan Fast Red (FR805S; Biocare Medical, MedicaRom,
Bucharest, Romania). An avidin-biotin blocking step was included
between the two procedures to mask any available biotin that
remained following the first round of the reaction (X0590; Dako).
Positive and negative external controls, which omitted the primary
antibodies, were also included (data not shown).
 | Table I.Panel of antibodies used for the
immunohistochemistry. |
Table I.
Panel of antibodies used for the
immunohistochemistry.
| Antibody | Clone,
manufacturer | Dilution | Antigenic
retrieval | External positive
control |
|---|
| CD105
(endoglin) | Polyclonal, Thermo
Scientific | 1:50 | Citrate, pH 6 | Kidney |
| Mast cell
tryptase | AA1, Dako | 1:100 | Citrate, pH 6 | Skin |
| CD68 | KP1, Dako | 1:50 | Citrate, pH 6 | Skin |
The immunohistochemical analysis allowed the
visualization of the blood vessels (stained with CD105), mast cells
(stained with tryptase) and macrophages (stained with CD68) in all
investigated cases. Thus, the McMD, MphMD and MVD in the tumor were
determined. Microvascularity was defined as a single endothelial
cell or group of endothelial cells that was positive for CD105 and
formed a visible lumen that was clearly separated from the adjacent
microvessels.
Microdensity measurement and image
acquisition
The microdensity measurements were performed
intratumorally and at the advancing edge. The intratumoral area was
defined as stromal tissue that contained two or more neoplastic
islands, and the invasion front/advancing edge was considered to be
a positive structure located on the limit of the tumor-free
tissue.
Image acquisition was performed using a Nikon
Eclipse E600 microscope, equipped with the Lucia 5 image analysis
software (Nikon, Apidrag, Bucharest, Romania).
The microdensities of the elements of interest were
determined using the method described by Weidner et al
(17). The slides were initially
scanned at x100 magnification to identify the areas with the
highest densities. The quantification was reported as the average
value (area or number) for 10 hot-spot fields, with an area of
0.7386 mm2, using a 20X objective lens.
To assess the reproducibility of the method, the
specimens were counted independently by two observers with a
correction factor κ-value of 0.07.
Statistical analysis
For the statistical analysis, a paired Student’s
t-test, one way ANOVA and Pearson’s correlation index were
performed using SPSS 10 software (SPSS, Inc., Chicago, IL, USA).
The values were reported as mean ± standard deviation. The data
averages of the groups were used in order to create data groups and
classes for further tests.
Results
Normal tissue immunoprofile
The CD105+ MVD values in the normal
uterus specimens were higher in the endometrium (7.6±2.1; x200
magnification) compared with the myometrium (4.3±1.3; x200
magnification). The analysis of McMD in the normal uterus specimens
indicated higher average values in the myometrium (9.7±1.7; x200
magnification) compared with the endometrium (3.7±1.3; x200
magnification), while MphMD had higher values in the endometrium
(93±43.7; x200 magnification) compared with the myometrium
(3.2±1.2; x200 magnification).
Clinical data
The average age of the patients with endometrioid
carcinoma was 57.8 years (range, 43–75 years). In terms of the
degree of differentiation, the well- and moderately-differentiated
tumors of stages IA (17 cases) and IB (27 cases), respectively,
were most prevalent, as the majority were diagnosed in the early
stages.
Immunohistochemistry
The analysis of the CD105+ MVD,
Try+ McMD and CD68+ MphMD values revealed no
significant differences, but there were variations in their
distribution when the data were grouped into tumor topography
(intratumoral vs. advancing edge), grade and tumor stage
subcategories.
The morphology and topography of the vessels that
were stained using CD105 indicated the presence of numerous
vascular structures, which were more concentrated at the advancing
edge (19.9±6.3; x200 magnification) compared with the intratumoral
areas (12.8±4.6; x200 magnification). The vessels from the
advancing edge had significantly larger lumens, and were more
branched and dilated compared with the intratumoral vessels, which
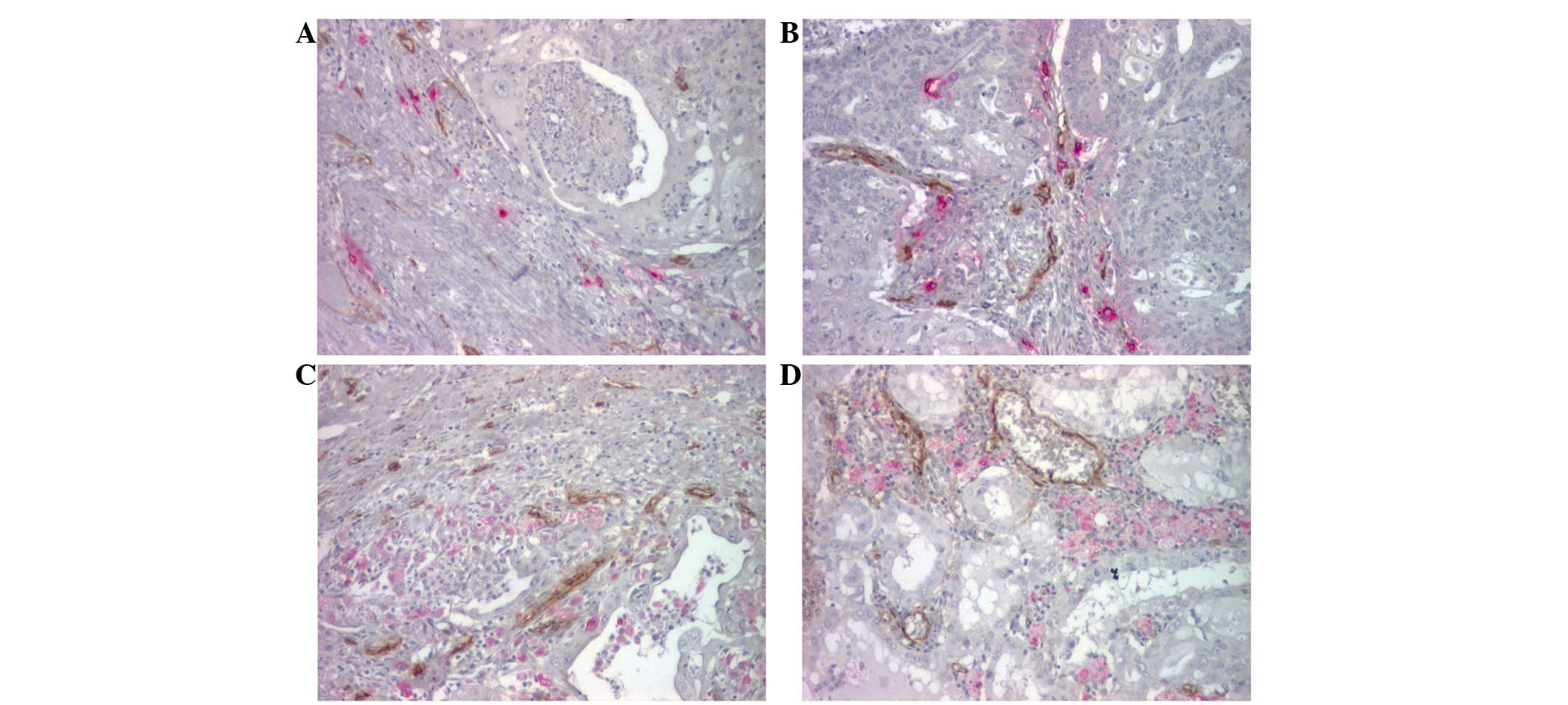
were less branched with a collapsed lumen (Fig. 1).
The analysis of the Try+ mast cells
revealed that they were present in all the studied cases, with
various shapes, sizes, degrees of degranulation and aspects of
mastocytoclasia. Overall, the mast cells were located mainly around
the vessels (Fig. 1A and B),
isolated or associated with the inflammatory site, and were more
numerous at the advancing edge (7.4±4.5; x200 magnification)
compared with the intratumoral location (4.7±3.8; x200
magnification).
The CD68+ macrophages were also present
in all cases studied, with a dendritic morphology, abundant
cytoplasm and round-oval nuclei. Variable densities of the
macrophages were observed, being more numerous in the intratumoral
areas (11.2±9.2; x200 magnification) and predominantly perivascular
(Fig. 1C and D) or adjacent to
areas of necrosis compared with the advancing edge of the tumor
(8.3±10.2; x200 magnification).
The data from the immunohistochemical analysis for
the mean microdensities of the morphological elements of interest,
depending on the grade and tumor stage, are presented in Table II.
 | Table II.Correlations of MVD, McMD and MphMD
in EEC with clinico-morphological investigated parameters. |
Table II.
Correlations of MVD, McMD and MphMD
in EEC with clinico-morphological investigated parameters.
| Parameter | Tumoral degree | Tumoral stage |
|---|
|
|
|---|
| WD | MD | PD | IA | IB | II | III |
|---|
| No. | 30 | 13 | 9 | 17 | 27 | 5 | 3 |
| MVD | | | | | | | |
| IT | 11.8±4.2 | 12.8±3.2 | 16.2±6.4 | 13.9±5.6 | 12.2±4.5 | 14.0±2.0 | 10.3±0.6 |
| AE | 20.1±6.7 | 17.5±5.0 | 22.8±6.1 | 19.0±7.2 | 20.1±6.6 | 21.2±4.2 | 21.7±1.5 |
| P-value |
1.938×10−7 | 0.004 | 0.019 | 0.014 |
1.875×10−6 | 0.004 | 0.000 |
| McMD | | | | | | | |
| IT | 3.7±3.3 | 6.8±3.7 | 5.3±4.7 | 4.4±3.1 | 5.2±4.2 | 5.0±5.5 | 2.3±0.6 |
| AE | 7.5±5.2 | 5.8±2.8 | 9.7±3.8 | 9.0±5.6 | 6.8±4.4 | 5.6±1.1 | 8.0±1.0 |
| P-value | 0.000 | 0.221 | 0.020 | 0.002 | 0.088 | 0.408 | 0.000 |
| MphMD | | | | | | | |
| IT | 13.7±8.9 | 10.2±10.9 | 4.6±2.0 | 11.0±8.2 | 11.3±7.9 | 17.4±17.7 | 2.3±0.6 |
| AE | 9.6±12.6 | 6.8±6.6 | 6.5±3.9 | 3.2±3.6 | 12.3±12.5 | 7.2±5.2 | 4.0±5.2 |
| P-value | 0.075 | 0.169 | 0.108 | 0.000 | 0.358 | 0.125 | 0.305 |
Clinico-morphological correlations
According to the histological grade of the analyzed
endometrioid carcinomas, the Student’s t-test indicated that
CD105+ MVD for all degrees of differentiation and
Try+ McMD for well- and poorly-differentiated tumors had
significantly lower intratumoral values compared with the advancing
edge (Fig. 2A).
The analysis of CD105+ MVD,
Try+ McMD and CD68+ MphMD, depending on the
tumor stage using a Student’s t-test, revealed that the values of
CD105+ MVD for all stages and of Try+ McMD
for stages IA and III, were lower intratumorally compared with the
advancing edge (Fig. 2B). In
contrast, the CD68+ MphMD values in stage IA tumors
showed significant differences and were higher intratumorally
compared with the advancing edge.
Overall, using Pearson’s test, CD105+ MVD
indicated a weak correlation with Try+ McMD
[r(50)=0.287, P=0.039] at the advancing edge of the tumor.
When considering the data grouped into tumor stages,
Pearson’s test indicated that CD105+ MVD exhibited an
intratumoral, indirect correlation with Try+ McMD stage
IA [r(15)=−0.538, P=0.026] and II [r(3)=−0.951, P=0.013] tumors.
CD105+ MVD also presented an indirect correlation with
CD68+ MphMD at the advancing edge for
well-differentiated forms [r(28)=−0.403, P=0.027] as well with
lesions from stage IB tumors [r(25)=−0.458, P=0016].
Pearson’s test showed that CD68+ MphMD
varied in direct correlation with Try+ McMD in
well-differentiated [r(28)=0.502, P=0.005] and stage II
[r(3)=0.952, P=0.012] tumors. In contrast, at the advancing edge of
the stage II tumors, this correlation was indirect [r(3)=−0.959,
P=0.010].
Discussion
Angiogenesis and inflammation have numerous common
pathways, as they are biological processes that are closely
associated with cancer. Angiogenesis is critical for the continuous
growth of tumors and the development of metastases (18). The initiation of angiogenesis is
primarily regulated by the balance between pro- and anti-angiogenic
factors, and represents an early and essential event in tumor
development and progression (19,20). A
significant role in this process appears to be played by the
interaction between tumor cells and the tumor microenvironment,
which indirectly contributes to the induction of angiogenesis.
Macrophages and mast cells are pivotal inflammatory
cells in the tumor stroma, and are present in the majority of
malignant neoplasms. In the second half of the 1990s, a correlation
was identified between mast cells and angiogenesis in various
malignancies (21). Mast cell
tryptase was shown to be a potent angiogenic factor (15). Furthermore, several studies have
demonstrated the association between the intensity of tumor
vascularization and macrophage infiltration in numerous cancers,
including endometrial cancer (22),
suggesting that TAMs increase the angiogenic potential of tumors by
producing pro-angiogenic factors, including vascular-endothelial
growth factor (VEGF) (23).
In the present study, certain differences were
identified between the distribution of CD105+ MVD and
Try+ McMD in relation to the tumor grade and stage.
CD105+ MVD for tumors of all degrees of differentiation,
and Try+ McMD in well- and poorly-differentiated tumors,
had significantly lower intratumoral values compared with the
advancing edge. Depending on the tumor stage, CD105+ MVD
for all stages and Try+ McMD for stages IA and II were
lower intratumorally compared with the advancing edge. Overall, the
Pearson correlation test indicated the existence of a weak
correlation between CD105+ MVD and Try+ McMD
levels in the advancing edge. In contrast, the analysis of the
correlation with regard to the tumor stage between
CD105+ MVD and Try+ McMD indicated an
intratumoral, indirect correlation for stage IA and II tumors.
Although angiogenesis, quantified by CD105+ MVD and
Try+ McMD, was more active at the advancing edge, only a
weak statistical correlation was observed between the two
parameters. This would argue in favor of the pro-angiogenic effect
of mast cells in the advancing edge of endometrial carcinomas.
Certain studies have indicated the existence of a
correlation between MVD and McMD and the degree of differentiation
of endometrial carcinomas (24),
while others have recorded its absence (25–27).
Ribatti et al (24) report that endometrial carcinoma
angiogenesis, measured by the number of CD31+
microvessels, is strongly correlated with the number of
Try+ mast cells, with poorly-differentiated tumors
containing a higher number of vessels compared with
well-differentiated tumors. In contrast, no correlation was
observed in other studies between the density of
C-kit+(26) or
try+(27) mast cells or
the number of CD31+ microvessels and the tumor grade.
Gosku et al (25) reported a
more intense vascularity in the tumor stroma and myometrium of
high-grade endometrial cancers compared with low-grade tumors, and
also that try+ mast cells do not increase in parallel
with the histological grade of tumors. The lack of correlation
between mast cell density, angiogenesis and the histological tumor
grade seem to suggest that mast cells are not a significant
prognostic factor in tumor progression (25).
Data obtained from the literature on the correlation
between mast cells density and tumor stage are also contradictory.
Ribatti et al (24) reported
that angiogenesis and try+ mast cell number increases
with tumor progression. Similarly, Cinel et al (26) indicated a statistically significant
correlation between a high density of C-kit+ mast cells
and the presence of myometrial invasion, with high levels in 54% of
the analyzed cases, 94% of which were also associated with
myometrial invasion. In contrast, other studies have reported no
correlation between Try+ McMD and myometrial invasion
(25,27).
In the present study, similar to the Try+
McMD analysis, the test for the CD68+ MphMD-grouped data
showed certain differences with regard to the tumor stage. The
levels of CD68+ MphMD were higher intratumorally than in
the advancing edge in stage IA tumors. With regard to the
association between CD105+ MVD and CD68+
MphMD, the existence of certain indirect correlations at the
advancing edge for well-differentiated forms, as well as for stage
IB tumors, were identified. Overall, the lack of correlation
between CD105+ MVD and CD68+ MphMD values
would indicate a minor role of these cells in the angiogenesis
process.
In the case of macrophages, data from various
studies with regard to the correlation between the tumor grade and
stage are also controversial. Espinosa et al (28) reported high CD168+
macrophage infiltration in high-grade endometrioid carcinomas and
the presence of a greater number of CD31+ vessels
compared with low-grade endometrioid tumors. Hashimoto et al
(22) reported that TAMs from tumor
nests and stroma were significantly increased in high-grade tumors.
A univariate analysis by Soeda et al (29) observed that a statistically
significant correlation existed between the number of intratumoral
TAMs and the tumor grades defined by the International Federation
of Gynecology and Obstetrics (FIGO), and also between the number of
intratumoral TAMs and MVD. Another study has shown that the
moderate or strong expression of VEGF is significantly associated
with a high MVD and an increased number of CD68+
macrophages for aggressive tumor subgroups (30). The literature reports a correlation
between macrophages and myometrial invasion, but not as a
prediction factor for prognosis (22,28,29).
Certain studies have provided statistically
significant results for macrophage infiltration and myoinvasive vs.
non-myoinvasive tumors (28) and
the depth of myometrial invasion (22,29),
suggesting that tumor angiogenesis is triggered and enhanced by
stromal macrophages, which regulate the progression of endometrial
carcinoma (28). Ohno et al
(31) reported that only the
CD68+ macrophages from within the areas of necrosis were
associated with the clinical stage and level of myometrial
invasion, while Soeda et al (29) identified this correlation for the
CD68+ macrophages in the intratumoral and advancing edge
areas.
In the present study, Try+ McMD and
CD68+ MphMD at the intratumoral level were correlated in
well-differentiated and stage II forms. At the advancing edge,
there was an indirect correlation in stage II tumors. Although
there were no significant correlations between McMD and MphMD, an
indirect correlation was observed between the two parameters in the
stage II tumors at the advancing edge, and a direct correlation
intratumorally. This would emphasize the dual agonist-antagonist
roles of the two cell populations in the processes of angiogenesis
and tumor progression in endometrial carcinoma.
The existence of conflicting results in the
literature is most likely to be caused by the large variation in
tumor types and stages, the location of the inflammatory cells and
the methods used to label the inflammatory cells and blood vessels,
in addition to the lack of a standardized assessment of
angiogenesis or the inflammatory cells (25,32). A
significant limitation is the variation in the methods for
reporting the topography of the mast cells and macrophages
(31). In addition, labeling the
vessels for CD31 (24,25,27,28) or
CD105 (33,34), the mast cells for tryptase (24,25,35),
toluidine blue (27) or C-kit
(26) and the macrophages for CD68
(29–31) or CD163 (28) may cause discrepancies.
In conclusion, angiogenesis was observed to be more
intense in the advancing edge, where it showed a weak correlation
with Try+ McMD. The correlation between CD68+
MphMD and angiogenesis was indirect and only identified at the
intratumoral level in stage IA and II tumors. A direct correlation
existed between Try+ McMD and CD68+ MphMD at
the intratumoral level in well-differentiated and stage II tumors,
while in the advancing edge, this correlation was indirect and only
identified in stage II tumors. The present data require a
quantification of angiogenesis and McMD and MphMD in endometrial
carcinomas, in order to determine the prognosis and future
development of alternative therapies that may target these
denominators, with potential benefits for patients.
References
|
1.
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sherman ME: Theories of endometrial
carcinogenesis: a multi-disciplinary approach. Mod Pathol.
13:295–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lax SF: Molecular genetic pathways in
various types of endometrial carcinoma: from a phenotypical to a
molecular-based classification. Virchows Arch. 444:213–223. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers and the implications for
pathogenesis, classification, and targeted therapies. Cancer
Control. 16:8–13. 2009.PubMed/NCBI
|
|
5.
|
Theoharides TC and Conti P: Mast cells:
the Jekyll and Hyde of tumor growth. Trends Immunol. 25:235–241.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Crowther M, Brown NJ, Bishop ET and Lewis
CE: Microenvironmental influence on macrophage regulation of
angiogenesis in wounds and malignant tumors. J Leukoc Biol.
70:478–490. 2001.PubMed/NCBI
|
|
7.
|
Kataki A, Scheid P, Piet M, Marie B,
Martinet N, Martinet Y and Vignaud JM: Tumor infiltrating
lymphocytes and macrophages have a potential dual role in lung
cancer by supporting both host-defense and tumor progression. J Lab
Clin Med. 140:320–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Murdoch C, Giannoudis A and Lewis CE:
Mechanisms regulating the recruitment of macrophages into hypoxic
areas of tumors and other ischemic tissues. Blood. 104:2224–2234.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Murdoch C, Muthana M and Lewis CE: Hypoxia
regulates macrophage functions in inflammation. J Immunol.
175:6257–6263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Condeelis J and Pollard JW: Macrophages:
obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dirkx AE, Oude Egbrink MG, Wagstaff J and
Griffioen AW: Monocyte/macrophage infiltration in tumors:
modulators of angiogenesis. J Leukoc Biol. 80:1183–1196. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev. 4:71–78. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ribatti D and Crivellato E: The
controversial role of mast cells in tumor growth. Int Rev Cell Mol
Biol. 275:89–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Blair RJ, Meng H, Marchese MJ, Ren S,
Schwartz LB, Tonnesen MG and Gruber BL: Human mast cells stimulate
vascular tube formation. Tryptase is a novel, potent angiogenic
factor. J Clin Invest. 99:2691–2700. 1997. View Article : Google Scholar
|
|
16.
|
Silverberg SG, Kurman RJ, Nogales F,
Mutter GL, Kubik-Huch RA and Tavassoli FA: Tumors of the uterine
corpus. Epithelial tumors and related lesions. WHO Classification
of Tumors: Pathology and Genetics of Tumors of the Breast and
Female Genital Organs. Tavassoli FA and Devilee P: IARC Press;
Lyon, France: pp. 221–232. 2003
|
|
17.
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
18.
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
21.
|
Ribatti D, Vacca A, Marzullo A, Nico B,
Ria R, Roncali L and Dammacco F: Angiogenesis and mast cell density
with tryptase activity increase simultaneously with pathological
progression in B-cell non-Hodgkin’s lymphomas. Int J Cancer.
85:171–175. 2000.PubMed/NCBI
|
|
22.
|
Hashimoto I, Kodama J, Seki N, Hongo A,
Miyagi Y, Yoshinouchi M and Kudo T: Macrophage infiltration and
angiogenesis in endometrial cancer. Anticancer Res. 20:4853–4856.
2000.PubMed/NCBI
|
|
23.
|
Gargett CE, Lederman F, Heryanto B,
Gambino LS and Rogers PA: Focal vascular endothelial growth factor
correlates with angiogenesis in human endometrium. Role of
intravascular neutrophils. Hum Reprod. 16:1065–1075. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ribatti D, Finato N, Crivellato E,
Marzullo A, Mangieri D, Nico B, Vacca A and Beltrami CA:
Neovascularization and mast cells with tryptase activity increase
simultaneously with pathologic progression in human endometrial
cancer. Am J Obstet Gynecol. 193:1961–1965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Goksu Erol AY, Tokyol C, Ozdemir O,
Yilmazer M, Arioz TD and Aktepe F: The role of mast cells and
angiogenesis in benign and malignant neoplasms of the uterus.
Pathol Res Pract. 207:618–622. 2011.PubMed/NCBI
|
|
26.
|
Cinel L, Aban M, Basturk M, Ertunc D,
Arpaci R, Dilek S and Camdeviren H: The association of mast cell
density with myometrial invasion in endometrial carcinoma: a
preliminary report. Pathol Res Pract. 205:255–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pansrikaew P, Cheewakriangkrai C,
Taweevisit M, Khunamornpong S and Siriaunkgul S: Correlation of
mast cell density, tumor angiogenesis, and clinical outcomes in
patients with endometrioid endometrial cancer. Asian Pac J Cancer
Prev. 11:623–626. 2010.
|
|
28.
|
Espinosa I, José Carnicer M, Catasus L,
Canet B, D’angelo E, Zannoni GF and Prat J: Myometrial invasion and
lymph node metastasis in endometrioid carcinomas: tumor-associated
macrophages, microvessel density, and HIF1A have a crucial role. Am
J Surg Pathol. 34:1708–1714. 2010.
|
|
29.
|
Soeda S, Nakamura N, Ozeki T, Nishiyama H,
Hojo H, Yamada H, Abe M and Sato A: Tumor-associated macrophages
correlate with vascular space invasion and myometrial invasion in
endometrial carcinoma. Gynecol Oncol. 109:122–128. 2008. View Article : Google Scholar
|
|
30.
|
Salvesen HB and Akslen LA: Significance of
tumour-associated macrophages, vascular endothelial growth factor
and thrombospondin-1 expression for tumour angiogenesis and
prognosis in endometrial carcinomas. Int J Cancer. 84:538–543.
1999. View Article : Google Scholar
|
|
31.
|
Ohno S, Ohno Y, Suzuki N, Kamei T, Koike
K, Inagawa H, Kohchi C, Soma G and Inoue M: Correlation of
histological localization of tumor-associated macrophages with
clinico-pathological features in endometrial cancer. Anticancer
Res. 24:3335–3342. 2004.PubMed/NCBI
|
|
32.
|
Erol AY and Ozdemir O: Do mast cell
phenotypes play a role in concomitantly increased microvessel
density and progression of non-small cell lung cancer? Hum Pathol.
42:1056–1057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Saad RS, Jasnosz KM, Tung MY and Silverman
JF: Endoglin (CD105) expression in endometrial carcinoma. Int J
Gynecol Pathol. 22:248–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Salvesen HB, Gulluoglu MG, Stefansson I
and Akslen LA: Significance of CD 105 expression for tumour
angiogenesis and prognosis in endometrial carcinomas. APMIS.
111:1011–1018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Ribatti D, Nico B, Finato N and Crivellato
E: Tryptase-positive mast cells and CD8-positive T cells in human
endometrial cancer. Pathol Int. 61:442–444. 2011. View Article : Google Scholar : PubMed/NCBI
|