Introduction
Deleted in liver cancer-1 (DLC-1) is a
potential tumor suppressor gene, which has been isolated from human
hepatocellular carcinoma and identified by representational
difference analysis. DLC-1 is localized on human chromo-some
8p21.3-22. The full-length cDNA for DLC-1 contains 3,800 bp and
encodes a 1,091-amino acid protein that has 86% homology with the
rat pl22RhoGAP gene (1).
DLC-1 (also known as ARHGAP7 and STARD12) contains
three functional domains: The RhoGTPase-activating protein (RhoGAP)
domain, the steroidogenic acute regulatory-related lipid transfer
(START) domain and the sterile α-motif (SAM) domain (2,3).
Studies have demonstrated that the RhoGAP domain is necessary for
inhibiting tumor cell growth, as well as for actin fiber and focal
adhesion formation (4–6). RhoGAPs negatively regulate the Rho
family of small GTPases, enhancing the hydrolysis of bound GTP to
convert Rho proteins to their inactive GDP-bound state (7,8).
DLC-1 mRNA is expressed in the majority of normal
human tissues and is downregulated or absent in a number of common
types of human cancer, including brain, lung, breast, liver,
stomach, colon and prostate cancers. The aberrant expression of
DLC-1 is associated with either genomic deletion or promoter
hypermethylation (9–14). Increasing evidence has shown that
DLC-1 negatively regulates tumor cell growth and in vivo
tumorigenicity (15–17).
However, DLC-1 has been less intensively examined in
pancreatic cancer. To obtain further evidence that DLC-1 functions
as a tumor suppressor gene, in the present study, a recombinant
plasmid (pcDNA3.1/DLC-1) was constructed and transduced into PANC-1
cells, in order to observe the effect of the DLC-1 gene on cell
growth and tumorigenicity.
Materials and methods
Cell line and culture
The human pancreatic carcinoma cell line, PANC-1,
was obtained from the Shanghai Institute of Biochemistry and Cell
Biology (Chinese Academy of Sciences, Shanghai, China). The cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and antibiotics at
37°C, in a humidified incubator with 5% CO2.
Plasmid construction
A 3.4-kb fragment of the full-length coding sequence
of the DLC-1 gene was amplified by PCR from human liver PCR-Ready
cDNA (Invitrogen, Carlsbad, CA, USA). The primers included
NheI and KpnI linkers. Subsequent to purification and
restriction digestion, the PCR product was ligated to the
pcDNA3.1(+) vector (Invitrogen). The sequence and orientation of
the DLC-1 recombinant were confirmed by DNA sequencing and
restriction enzyme digestion.
Cell transfection
The cells (105) were seeded into 24-well
plates one day prior to transfection. The cells were transfected
with 1 μg plasmid DNA in Lipofectamine 2000 (Invitrogen),
according to the manufacturer’s instructions.
RNA extraction and RT-PCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen). The total RNA (2 μg) was used as a
template in the first strand cDNA synthesis using a First-Strand
cDNA Synthesis kit (Shinegene, Shanghai, China) according to the
manufacturer’s instructions. Total RNA (2 μg) was combined
with 0.1 μg oligo(dT)18 primer and
diethylpyrocarbonate (DEPC) H2O and preheated at 65°C
for 5 min. The mixture was then placed at 20°C for 10 min, then 10
μl 2X First-Strand Buffer and 1 μl RT mix was added
for a total volume of 20 μl. The mixture was incubated at
42°C for 50 min, then the reaction was stopped by heating at 90°C
for 5 min. The cDNA stock was stored at −20°C. A pair of primers
(forward, GGAATAACGGCTCTGTGAA and reverse, TCTCCGACCACTGATTGAC) was
used to amplify the 400-bp fragment of DLC-1. As a control, a pair
of primers (forward, GTGGACATCCGCAAAGAC and reverse, AAA
GGGTGTAACGCAACTAA) was used to amplify the 200-bp fragment of
β-actin. PCR was performed using a PTC-200 PCR machine (MJ Research
Inc, Waltham, MA, USA). The reaction conditions were as follows:
94°C for 3 min, then 35 cycles of 94°C for 1 min, 55°C for 30 sec
and 72°C for 1 min, followed by a final extension step for 10 min
at 72°C.
Western blot analysis
The cells were harvested and solubilized in cold
RIPA buffer. Proteins were resolved by SDS-PAGE and transferred to
polyvinylidene fluoride (PVDF) membranes. DLC-1 was detected by
western blotting using mouse anti-human DLC-1 antibody (BD
Biosciences, Franklin Lakes, NJ, USA). β-actin staining served as
the internal standard for all membranes.
MTT assays
The cells were plated in 96-well microtiter plates
at a density of 104 cells/well, then cultured for 48 h
and incubated with 20 μl MTT solution (5 mg/ml) for 4 h. The
cells were lysed in 150 μl DMSO, and the absorbance at 490
nm was determined with an ELISA plate reader. The absorbance values
for the cell lines transfected with pcDNA3.1(+) alone,
pcDNA3.1(+)/DLC-1 and the untransfected cells were compared. The
entire experiment was performed three times independently.
Preparation of liposome:plasmid
complexes
Plasmids were purified using alkaline lysis.
Liposomes were composed of 1,2-dioleoyl-3-trimethylammonium-propane
and cholesterol in a 1:1 molar ratio, and the dried lipid film was
resuspended with 5% dextrose in water. Following the hydration of
the lipids, using a bath sonicator, the liposomes were sonicated
until clear. The liposomes were then extruded through
poly-carbonate membranes and stored at 4°C until use. Prior to
injection, the mixture containing liposomes in a 3:1 mass ratio
with the plasmid DNA was incubated at room temperature for 20
min.
Tumorigenicity assay
The PANC-1 cells (107) were inoculated
subcutaneously into the right oxter of four-week-old female Balb/c
athymic nude mice. Eight days subsequent to the injection of cells,
the mice were randomly divided into three groups: i) The
liposome:pcDNA3.1(+)/DLC-1 group; ii) the liposome:pcDNA3.1(+)
group; and iii) the isosmotic saline treatment group. Each group
contained 10 mice and each mouse received seven intravenous
injections via the tail vein, five days apart. Each injection (200
μl) consisted of liposomes (150 μg) complexed to 50
μg of a plasmid encoding DLC-1 or a control plasmid. Tumor
size was measured in two dimensions prior to each injection and
five days subsequent to the last injection, using a vernier
caliper. This study was approved by the ethics committee of West
China Hospital, Sichuan University, China.
Statistical analysis
Data are expressed as the mean ± SD. All statistical
analyses were performed with standard statistical programs (SPSS
for Windows, version 17.0; SPSS, Inc., Chicago, IL, USA). A one-way
ANOVA was used for the statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
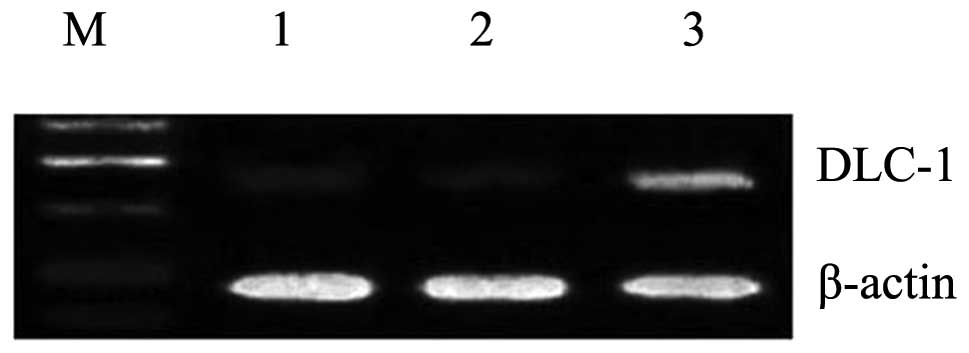
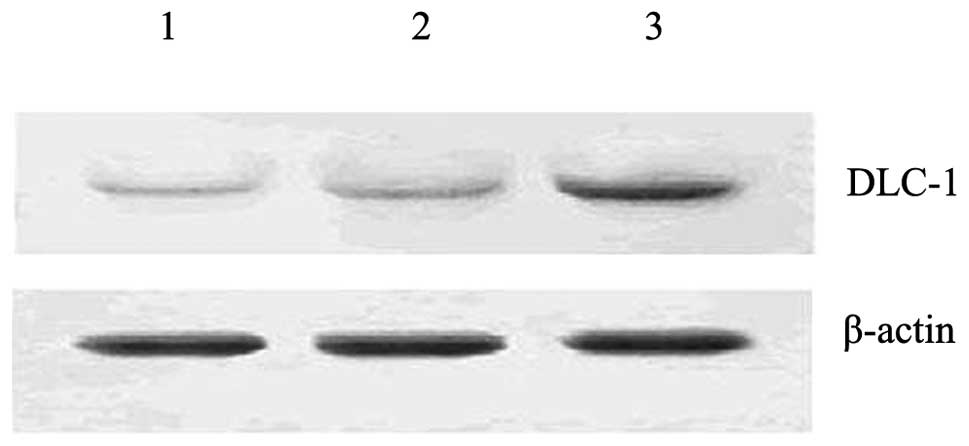
Overexpression of the DLC-1 gene by
liposome-mediated transfection
Subsequent to 48 h of transfection, the transfection
of the DLC-1 gene into the PANC-1 cells was detected by
semi-quantitative RT-PCR and western blotting, respectively. As
shown in Figs. 1 and 2, a successful transfer of DLC-1 by the
liposome complex was demonstrated. In the
pcDNA3.1(+)/DLC-1-transfected cells, the mRNA and protein
expression levels of DLC-1 were upregulated. By contrast, only weak
bands were observed in the empty vector-transfected and
untransfected cells.
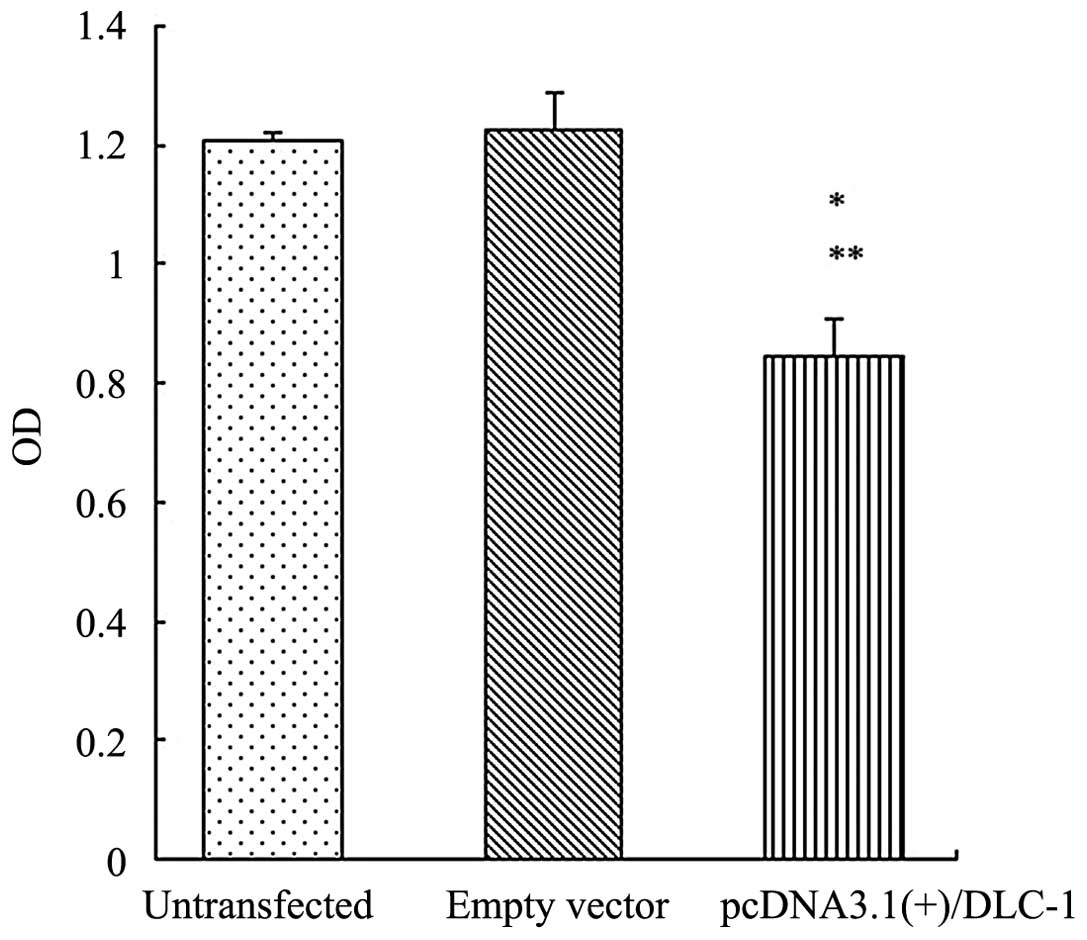
Overexpression of the DLC-1 gene inhibits
cell proliferation
To investigate whether DLC-1 was involved in the
cell proliferation of PANC-1 cells, an MTT assay was performed
subsequent to 48 h of transfection. As shown in Fig. 3, the OD value was lower in the cells
transfected with pcDNA3.1(+)/DLC-1 compared with the cells
transfected with the empty vector and the untransfected cells
(P<0.05). The results demonstrated that DLC-1 had an effect on
cell proliferation.
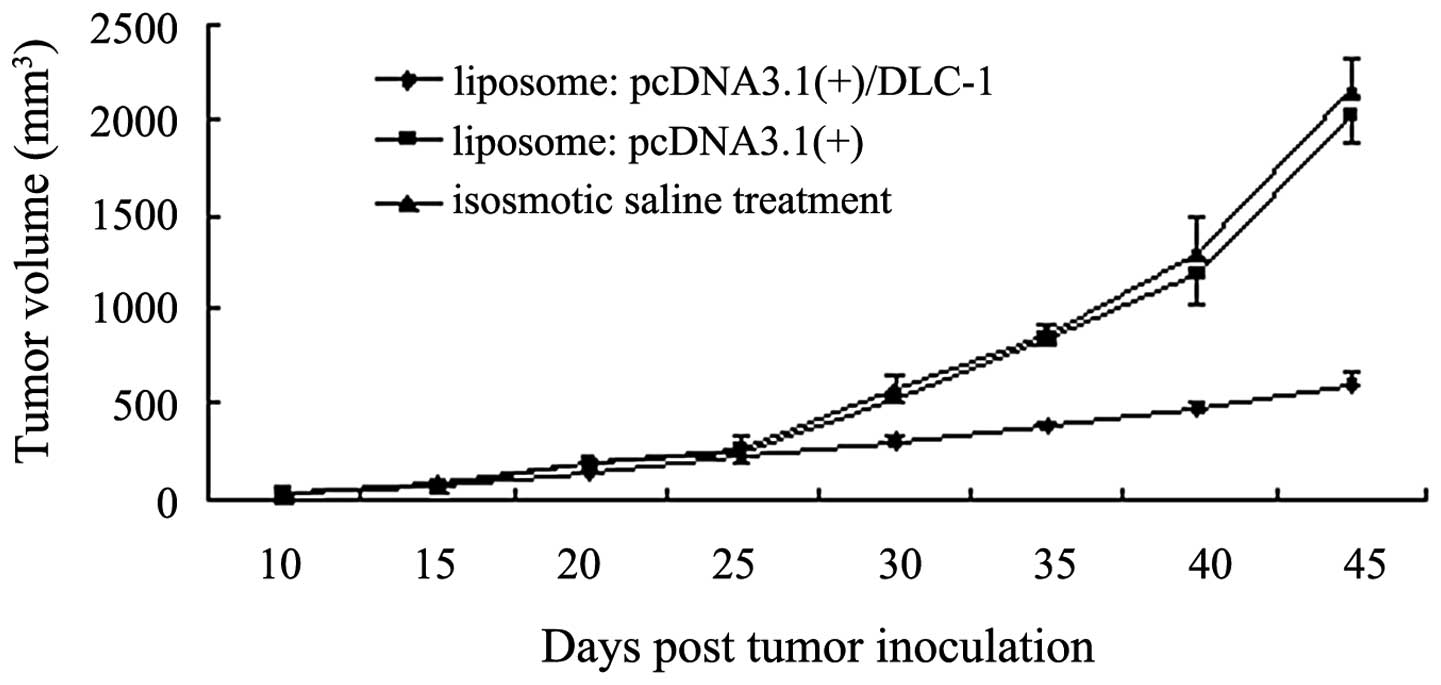
Inhibition of in vivo tumorigenicity by
the DLC-1 gene
To investigate the effect of DLC-1 on tumor growth,
the liposome:DNA complex was injected into athymic nude mice via
the tail vein using pre-established human PANC-1 pancreatic
carcinoma cells. As shown in Fig.
4, the tumors from the liposome:pcDNA3.1(+)/DLC-1 group were
smaller than those from the liposome:pcDNA3.1(+) and isosmotic
saline treatment groups after the fifth injection (P<0.05). This
result indicates that DLC-1 gene therapy using liposomes as a
carrier effectively inhibits tumor growth in vivo.
Discussion
In the United States, 42,470 patients were diagnosed
with and 35,240 patients succumbed to pancreatic cancer in 2009
(18). Pancreatic cancer is the
fourth most common cause of cancer-related mortality in the United
States. Due to the lack of effective screening modalities, the
majority of patients are diagnosed with pancreatic cancer at a
regional or distant stage of the disease. The overall five-year
relative survival rate for patients with pancreatic cancer is 5%
(18). At present, surgery is the
only curative therapeutic approach. However, only 5 to 25% of
patients with pancreatic cancer are suitable for resection at the
time of diagnosis (19).
With recent developments in molecular biology
techniques and following the mapping of the entire human genome,
gene therapy for pancreatic cancer is becoming available. It has
been reported that DLC-1 is expressed in a number of normal human
tissues and is downregulated or absent in various types of human
cancer (9–11). Reduced mRNA levels have also been
observed in certain tumor cell lines (15,20,21).
These results suggest that DLC-1 may function as a tumor
suppressor.
In the present study, the overexpression of the
DLC-1 gene in the PANC-1 cell line resulted in the inhibition of
cell growth in vitro. This result is consistent with other
studies. Wong et al showed that the overexpression of DLC-1
in SMMC-7721 human HCC cells that lack endogenous DLC-1 expression
was able to inhibit cell proliferation and invasiveness (5). In addition, Healy et al
observed that the restoration of DLC-1 expression in non-small cell
lung cancer cell lines impaired anchorage-dependent and
-independent growth, as well as invasion in vitro (22). It was demonstrated that the
suppressive function may be attributable to the biological
functions of the DLC-1 gene, which include the organization of the
cytoskeleton, the formation of focal adhesions and the induction of
apoptosis (4,17,23).
Liposome-mediated intravenous gene delivery in
animals usually results in expression in the major organs,
including the lung, kidney, spleen and liver, and is not associated
with autoimmunity and toxicity (24). Transgenes have continued to be
expressed in large numbers of cells in multiple tissues for at
least nine weeks without any apparent treatment-related toxicity
following a single intravenous injection of liposome:plasmid
complexes (25). Chen et al
noted that i.v. injections of liposomes complexed to PCI-endostatin
inhibited the growth of MDA-MB-435 tumors implanted in the mammary
fat pads of nude mice by ∼40% compared with either empty vector
(PCI) or untreated controls (26).
Thus, this method of gene transfer may be
appropriate for human gene therapy. In the present study, it was
observed that liposome:DNA complexes administered intravenously
decreased the growth of subcutaneously-inoculated tumors,
demonstrating that DLC-1 had an effect on tumorigenicity in
vivo, which is a result supported by other studies (16,17).
In summary, in the present study, the overexpression
of the DLC-1 gene in the PANC-1 cells inhibited cell proliferation,
suggesting that the DLC-1 gene maybe be a tumor suppressor for
pancreatic cancer. The results of this experiment suggested that
the DLC-1 gene may be a promising target in gene therapy for
pancreatic cancer. The present study also provides experimental
evidence for further research into gene therapy for pancreatic
cancer.
References
|
1.
|
Yuan BZ, Miller MJ, Keck CL, Zimonjic DB,
Thorgeirsson SS and Popescu NC: Cloning, characterization and
chromosomal localization of a gene frequently deleted in human
liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res.
58:2196–2199. 1998.PubMed/NCBI
|
|
2.
|
Ching YP, Wong CM, Chan SF, et al: Deleted
in liver cancer (DLC) 2 encodes a RhoGAP protein with growth
suppressor function and is underexpressed in hepatocellular
carcinoma. J Biol Chem. 278:10824–10830. 2003. View Article : Google Scholar
|
|
3.
|
Kawai K, Kiyota M, Seike J, Deki Y and
Yagisawa H: STARTGAP3/DLC3 is a GAP for RhoA and Cdc42 and is
localized in focal adhesions regulating cell morphology. Biochem
Biophys Res Commun. 364:783–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sekimata M, Kabuyama Y, Emori Y and Homma
Y: Morphological changes and detachment of adherent cells induced
by p122, a GTPase-activating protein for Rho. J Biol Chem.
274:17757–17762. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wong CM, Yam JW, Ching YP, et al: Rho
GTPase-activating protein deleted in liver cancer suppresses cell
proliferation and invasion in hepatocellular carcinoma. Cancer Res.
65:8861–8868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yam JW, Ko FC, Chan CY, Jin DY and Ng IO:
Interaction of deleted in liver cancer 1 with tensin2 in caveolae
and implications in tumor suppression. Cancer Res. 66:8367–8372.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moon SY and Zheng Y: Rho GTPase activating
proteins in cell regulation. Trends Cell Biol. 13:13–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Peck J, Douglas G 4th, Wu CH and Burbelo
PD: Human RhoGAP domain-containing proteins: structure, function,
and evolutionary relationships. FEBS Lett. 528:27–34. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ng IO, Liang ZD, Cao L and Lee TK: DLC-1
is deleted in primary hepatocellular carcinoma and exerts
inhibitory effects on the proliferation of hepatoma cell lines with
deleted DLC-1. Cancer Res. 60:6581–6584. 2000.PubMed/NCBI
|
|
10.
|
Gatalica Z, Velagaleti G, Kuivaniemi H, et
al: Gene expression profile of an adenomyoepithelioma of the breast
with a reciprocal translocation involving chromosomes 8 and 16.
Cancer Genet Cytogenet. 156:14–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang X, Feng J, Cheng Y, et al:
Characterization of differentially expressed genes in ovarian
cancer by cDNA microarrays. Int J Gynecol Cancer. 15:50–57. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kim TY, Jong HS, Song SH, et al:
Transcriptional silencing of the DLC-1 tumor suppressor gene by
epigenetic mechanism in gastric cancer cells. Oncogene.
22:3943–3951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Healy KD, Kim TY, Shutes AT, Bang YJ,
Juliano RL and Der CJ: RhoGAP DLC-1 tumor suppression and aberrant
Rho GTPase activation in lung cancer. Proc Am Assoc Cancer Res.
47:Abstract. 41252006.
|
|
14.
|
Guan M, Zhou X, Soulitzis N, Spandidos DA
and Popescu NC: Aberrant methylation and deacetylation of deleted
in liver cancer-1 gene in prostate cancer: potential clinical
applications. Clin Cancer Res. 12:1412–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yuan BZ, Jefferson AM, Baldwin KT,
Thorgeirsson SS, Popescu NC and Reynolds SH: DLC-1 operates as a
tumor suppressor gene in human non-small cell lung carcinomas.
Oncogene. 23:1405–1411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yuan BZ, Zhou X, Durkin ME, et al: DLC-1
gene inhibits human breast cancer cell growth and in vivo
tumorigenicity. Oncogene. 22:445–450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhou X, Thorgeirsson SS and Popescu NC:
Restoration of DLC-1 gene expression induces apoptosis and inhibits
both cell growth and tumorigenicity in human hepatocellular
carcinoma cells. Oncogene. 23:1308–1313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer Statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
19.
|
Neoptolemos JP, Stoken DD, Friess H, et
al: A randomized trial of chemoradiotherapy and chemotherapy after
resection of pancreatic cancer. N Engl J Med. 350:1200–1210. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Goodison S, Yuan J, Sloan D, et al: The
RhoGAP protein DLC-1 functions as a metastasis suppressor in breast
cancer cells. Cancer Res. 65:6042–6053. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wong CM, Lee JM, Ching YP, Jin DY and Ng
IO: Genetic and epigenetic alterations of DLC-1 gene in
hepatocellular carcinoma. Cancer Res. 63:7646–7651. 2003.PubMed/NCBI
|
|
22.
|
Healy KD, Hodgson L, Kim TY, et al: DLC-1
suppresses non-small cell lung cancer growth and invasion by
RhoGAP-dependent and independent mechanisms. Mol Carcinog.
47:326–337. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kawai K, Yamaga M, Iwamae Y, et al: A
PLCδ1-binding protein, p122RhoGAP, is localized in focal adhesions.
Biochem Soc Trans. 32:1107–1109. 2004.
|
|
24.
|
Nabel EG, Gordon D, Yang ZY, et al: Gene
transfer in vivo with DNA-liposome complexes: lack of autoimmunity
and gonadal localization. Hum Gene Ther. 3:649–656. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhu N, Liggitt D, Liu Y and Debs R:
Systemic gene expression after intravenous DNA delivery into adult
mice. Science. 261:209–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chen QR, Kumar D, Stass SA and Mixson AJ:
Liposomes complexed to plasmids encoding angiostatin and endostatin
inhibit breast cancer in nude mice. Cancer Res. 59:3308–3312.
1999.
|