Introduction
Several studies have demonstrated that erectile
dysfunction and lower urinary tract symptoms due to prostatic
disorders may share a common pathophysiological pathway (1,2). The
serum level of testosterone (Te) may affect sexual function in
males treated by radical prostatectomy (RP) for clinically
localized prostate cancer (PCa) (3). It has been suggested that Te may also
affect urinary continence and increase bladder compliance following
RP, with a relaxing effect in smooth muscle bladder cells through
the nitric oxide synthase/nitric oxide pathway (4,5).
Moreover, certain authors have reported a significant correlation
between Te levels and adverse pathological features and biochemical
recurrence, even though the prognostic value of pre-operative Te
remains unclear (6–7). Few preliminary studies have evaluated
the changes in the levels of Te and gonadotropins following RP, and
these have produced conflicting results (9–12). In
addition, little is known concerning the possible effect of PCa
cells on the hypothalamic-pituitary hormone axis, although certain
authors have suggested that patients with PCa showed lower
gonadotropin levels compared with similarly aged males without PCa
(12). Furthermore, there is no
data with regard to changes in sex hormones in the early
post-operative period following RP. The aim of the present study
was to prospectively evaluate the changes in the serum levels of
Te, luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
within the first 3 months after RP for clinically localized PCa and
to analyze the correlation between LH and Te at various follow-up
times.
Materials and methods
Study design
A total of 100 male patients with clinically
localized PCa were consecutively included in the study, from
February to July 2010. The levels of Te, LH and FSH were assessed
pre-operatively and at 1 and 3 months post-operatively. This study
was approved by the ethics committee of the University of Florence.
Informed consent was obtained from all patients. This study was
planned as a longitudinal cohort study.
Patients
A total of 100 male patients with newly diagnosed,
biopsy proven, clinically localized PCa [prostate-specific antigen
(PSA) <10 mg/ml, clinical stage <T3, negative computed
tomography (CT) scan or bone scintigraphy] prior to RP were
included in the study. Subjects with a known history of
endocrinological diseases, including hypogonadism, previous Te
substitutive therapy and/or hormonal manipulation (including
5α-reductase inhibitors, androgen receptor blockers and
LH-releasing hormone analogues), were excluded from the study. The
adjunctive exclusion criteria were a classification of Eastern
Cooperative Oncology Group (ECOG) grade >1, previous radiation
therapy and chronic ingestion of alcohol or other drugs, including
steroids, barbiturates, spironolactone and cimetidine, that may
have interfered with the serum hormone levels.
Clinical and serological data
collection
The clinical variables registered for each patient
included age at surgery, corporal features [body mass index(BMI),
weight and height] and sex hormone profiles, including total Te, LH
and FSH. Serum samples were collected by venipuncture between 8:00
and 11:00 a.m., 7 to 14 days prior to RP and at the 1- and 3-month
follow-ups. The serum levels of Te, LH and FSH were quantified
using an electrochemoluminescence method (Modular Roche, Milan,
Italy) at the laboratories of the Sexual Medicine Outpatient
Clinic, University of Florence, Italy.
Surgical procedures
The surgical indications were in accordance with the
European Association of Urology guidelines for clinically localized
PCa (13). RP was performed with an
open retropubic antegrade approach, according to our previously
described surgical procedures (14). All surgical procedures were
performed at the Department of Urology, University of Florence,
Florence, Italy, by the same 3 surgeons (M.G., M.C. and S.S.). Each
patient provided written informed consent prior to their surgical
procedure.
Statistical analysis
The Kolmogorov-Smirnov test was used to test the
distribution of each parameter. Data are expressed as the median
(quartiles) when not normally distributed and as the mean ± SD when
normally distributed. The distributions of LH and FSH were
normalized through logarithmic transformations: logarithmically
transformed LH and FSH were expressed as logLH and logFSH,
respectively.
Comparisons between the serum hormone levels at
baseline and the levels at 1 and 3 months after RP were performed
with paired t-tests for normally distributed parameters. The same
test was applied to the logarithmically transformed data for LH and
FSH. The correlation between the logarithmically transformed LH and
Te values at each visit was calculated using Pearson’s correlation
coefficient. All statistical tests were performed using SPSS
version 17.0 statistical software (SPSS, Inc., Chicago, IL,
USA).
Results
Pre-operative data
The baseline patient characteristics are shown in
Table I. Subsequent to 3 months,
complete hormonal data (baseline, 1 and 3 months) were available
for 92/100 patients. In total, 4 patients voluntarily withdrew from
the study and were excluded from the results, while 4 were not
analyzed due to adjuvant radiation and/or hormonal treatment for
persistent PCa (R+, N+) or as they were lost to follow-up.
 | Table I.Pre-operative clinical and hormonal
characteristics of 100 male patients with PCa. |
Table I.
Pre-operative clinical and hormonal
characteristics of 100 male patients with PCa.
| Characteristic | Value |
|---|
| Pre-operative
clinical data | |
| Age | |
| Mean ± SD
(years) | 64.8±6.5 |
| Range
(years) | 48–75 |
| <65 (n) | 52 |
| ≥65 (n) | 48 |
| BMI
(kg/m2) | |
| Mean ± SD | 25.8±3.1 |
| Range | 19.5–34.6 |
| PSA (ng/ml) | |
| Mean ± SD | 7.3±4.0 |
| 0–2.4 | 6 |
| 2.5–3.9 | 8 |
| 4–10 | 69 |
| 10.1–19.9 | 17 |
| Pathological
outcome | |
| Pathological Stage
(n) | |
| pT2 | 54 |
| pT3 | 46 |
| pT4 | 0 |
| Gleason Score of
surgical specimens (n) | |
| <6 | 0 |
| 6 | 55 |
| 7 | 29 |
| 8–10 | 16 |
| Lymph nodes status
(n) | |
| N0 | 98 |
| N+ | 2 |
| Surgical margins
(n) | |
| Positive | 2 |
| Negative | 98 |
| Hormone levels | |
| Testosterone
(nmol/ml) | |
| Mean ± SD | 15.3±5.9 |
| Range | 5.7–37.8 |
| LH (mIU/ml) | |
| Median | 3.5 |
| Quartiles | 2.5–3.5–5.1 |
| FSH (mIU/ml) | |
| Median | 5.0 |
| Quartiles | 3.3–5.0–8.1 |
Changes in sex hormone levels following
RP
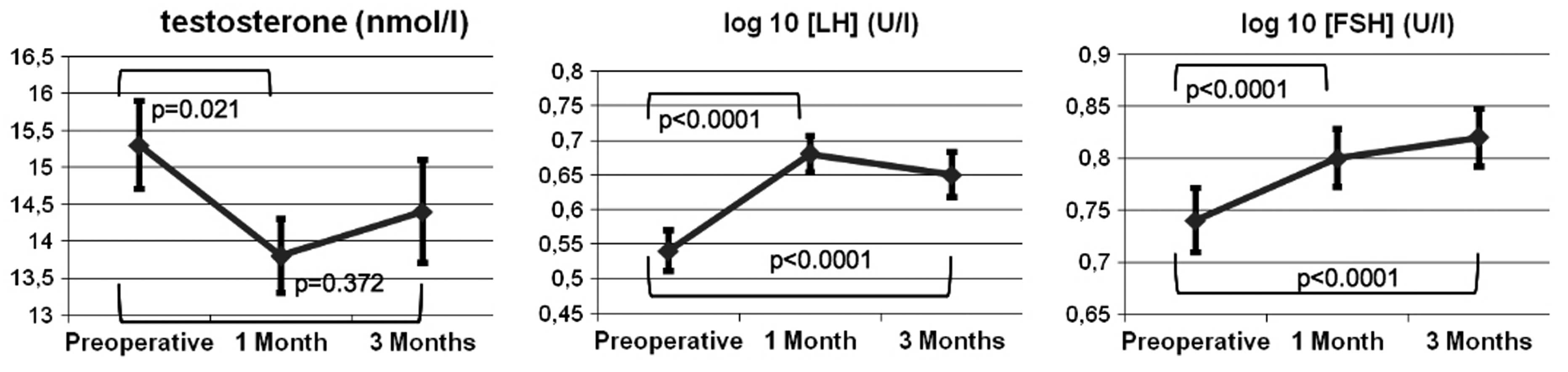
The results of the laboratory analyses of Te, logLH
and logFSH are shown in Fig. 1. A
significant reduction in Te levels was observed 1 month after RP,
as compared with the baseline (baseline vs. 1 month, 15.3 vs. 13.8
nmol/l; P=0.021), with an increase at 3 months that nearly reached
the pre-operative baseline value (baseline vs. 3 months, 15.3 vs.
14.4 nmol/l; P=0.372). In contrast, the logLH level was
significantly increased at 1 and 3 months post-surgery, compared
with the baseline (baseline vs. 1 month logLH, 0.54 vs. 0.68
mIU/ml; P<0.0001; and baseline vs. 3 months logLH, 0.54 vs. 0.65
mIU/ml; P<0.0001). Furthermore, the logFSH level was
significantly increased at 1 month after RP (baseline vs. 1 month
logFSH, 0.74 vs. 0.80 mIU/ml; P<0.0001) and reached the highest
value at the 3-month follow-up (baseline vs. 3 months logFSH, 0.74
vs. 0.82 mIU/ml; P<0.0001). The correlation between the LH and
Te serum levels was then evaluated at various follow-up times
(Fig. 2). A marked positive
correlation was observed between Te and LH pre-operatively
(pre-operative logLH-Te, r=0.370; P<0.0001). This correlation
was not apparent 1 month after RP (one month logLH-Te, r=0.109;
P=0.303). However, the LH level measured at 1 month was markedly
correlated with the Te level at 3 months (one month logLH-3 month
Te, r=0.258; P=0,053). At 3 months post-surgery, the correlation
between Te and LH was completely recovered (three month logLH-Te,
r=0.273; P=0.054).
Discussion
In the early months after RP, the present study
observed a significant decline and subsequent recovery of Te,
associated with increased LH and FSH, which persisted at higher
levels at 3 months compared with the pre-operative value.
Therefore, the removal of the whole prostate gland was followed by
the development of compensated hypergonadotropic hypogonadism. To
date, only a few studies have evaluated the changes in sex hormone
levels following prostatic surgery, and little is known with regard
to the effect of healthy prostatic tissue or PCa on the
hypothalamic-pituitary hormone axis (15).
Certain studies have shown that there are no
significant changes in these hormones following surgical treatment
for benign prostatic hyperplasia (BPH) (12,16,17).
Further investigations are required, but on the basis of these
studies it appears that BPH does not significantly affect the
hypothalamic-pituitary axis. In contrast, other studies have
previously presented changes in the levels of sex hormones after RP
for PCa, but there is no consensus with regard to the existence and
degree of these changes. Olsson et al reported a significant
increase in LH and FSH levels in 55 males following RP in a pilot
study (10). These findings have
been supported by Madersbacher et al, who observed that LH
and FSH were increased by 71 and 63%, respectively, in 49 patients
12 months after RP, without any evident changes to the Te level
(12). In a slightly different
manner, Miller et al showed a significant increase in Te, as
well as LH and FSH, in 63 patients 1 year after RP, when compared
with the pre-operative value (9).
There are two main differences between the present and previous
studies. First, the first post-operative evaluation was subsequent
to only 1 month, whereas in the previous studies, it was at 3 or 6
months. This enabled us to make the new observation, not reported
by previous studies, that Te was transiently decreased 1 month
after RP. The second difference was the correlation analysis with
LH. The decrease in Te levels at 1 month was unassociated with LH,
while at 3 months, the Te level recovered the correlation with LH,
as well as its baseline value. A possible explanation for these
data is that the surgical intervention caused a reduction in the
testicular production of Te, which stimulated the increased
production of gonadotropins by negative feedback, thus normalizing
the Te level after a few weeks. The reduction in Te levels that was
identified at the 1-month follow-up may be a significant
etiological factor of the increased gonadotropins, which have
previously been observed even at 12 months after RP (9,10,12).
In the present study, we were not able to dismiss other potential
explanations, nor identify the cause of the reduced testicular
production of Te following RP. However, we suggest that there may
be numerous factors, including psychological and organic
considerations. The psychological stress of facing a cancer
diagnosis or the fear of surgery have already been considered as
potential causes of the suppression of LH/FSH prior to RP (12). This may also cause a temporary
reduction in testicular endocrine function. Organic factors, e.g.,
the ligature of Santorini’s venous plexus or of prostatic vascular
pedicles and the Trendelenburg position, may theoretically cause
transient ischemic or hypoxic damage to the testicles. Moreover,
damage to the cavernous nerves may alter testicular function in
humans, as has been reported in animal models (18). Following a bilateral cavernous
neurotomy in rats, Vignozzi et al observed the onset of
overt hypogonadism, characterized by reduced Te levels and testis
function, including testis weight and number of Leydig cells, with
an inadequate compensatory increase of LH (19). More detailed studies are required to
completely investigate whether and how RP has an effect on the
testicular production of Te, and if this is similar to the effect
that is described after external beam radiation therapy (20).
Previous studies have presented various
interpretations of the increased gonadotropin levels following RP,
alternately attributing them to the removal of the PCa or healthy
prostatic tissue, or to the surgical event itself. In the study by
Madersbacher et al, the authors suggested that the
hypothalamic-pituitary axis is inhibited in patients with PCa and
that this inhibition is removed following RP. The authors presented
evidence for a possible direct inhibitory effect of tumor cells, as
there was a correlation between higher Gleason scores and lower
pre-operative Te, and significantly lower LH and FSH levels were
observed in the PCa group compared with the BPH group (12). However, Miller et al
suggested that it was the healthy prostatic tissue, producing
dihydrotestosterone (DHT) and inhibin-B, that caused the
pre-operative inhibition of the hypothalamic-pituitary axis
(9). Furthermore, DHT induces
negative feedback for LH and FSH, and decreases in its levels in
serum and urine following RP have also been observed in other
studies (10,12). By contrast, Olsson et al did
not report any changes to the serum inhibin-B levels in 44 patients
following RP, nor any difference between the PCa and BPH patient
groups (10). The present study
does not disagree with any previous justifications, although it
does provide a new observation (the early reduction of Te) which
may present a new interpretation. Further studies are required to
confirm whether RP affects testicular production of Te. In the
present study, this premise is suggested by the levels of LH and
FSH, which remained high at 3 months compared with the baseline
value, despite a normal Te value. This premise is also supported by
the correlation analysis between Te and LH, which showed that the
correlation was lost at 1 month and recovered at 3 months. This
explanation would also justify the increased gonadotropin levels
observed with the normal Te levels in previous studies at 6 and 12
months following RP (9,12). In addition to possessing
significance in endocrinological terms, the fluctuation of the Te
levels in the first 3 months following RP may be important from a
clinical perspective, since this period of time has critical
relevance in the recovery of urinary continence and potency
(21), and the resultant Te levels
are significantly involved in the recovery of these functions
(4,5). Although the present study did not
record data on the functional recovery of these patients, we
suggest that future investigations into the correlation between
functional recovery and sex hormones should focus on the first
months after prostatectomy. In our opinion, the strengths of the
present study are inherent in its prospective nature and the high
significance of the statistical results; conversely, the limits
include the lack of sex hormone-binding globulin (SHBG) and
estrogens results in the analysis, which were excluded due to their
cost. However, not all previous studies on this issue have included
SHBG and estrogens in the evaluation, and no remarkable adjunctive
outcomes have resulted from those that did (9–12). In
conclusion, in the present series, RP induced an early significant
decline in the Te serum levels and a significant increase in the LH
and FSH levels. At 3 months after RP, the full recovery of the Te
level, with concomitant high levels of gonadotropins, appears to
delineate the features of compensated hypergonadotropic
hypogonadism. Studies evaluating the effect of these hormonal
changes on continence and potency are currently in progress.
References
|
1.
|
Gacci M, Eardley I, Giuliano F,
Hatzichristou D, Kaplan SA, Maggi M, McVary KT, Mirone V, Porst H
and Roehrborn CG: Critical analysis of the relationship between
sexual dysfunctions and lower urinary tract symptoms due to benign
prostatic hyperplasia. Eur Urol. 60:809–825. 2011. View Article : Google Scholar
|
|
2.
|
Lowe FC: Treatment of lower urinary tract
symptoms suggestive of benign prostatic hyperplasia: sexual
function. BJU Int. 95(Suppl 4): 12–18. 2005. View Article : Google Scholar
|
|
3.
|
Khera M: Androgens and erectile function:
a case for early androgen use in postprostatectomy hypogonadal men.
J Sex Med. 6(Suppl 3): 234–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gacci M, Corona G, Apolone G, Lanciotti M,
Tosi N, Giancane S, Masieri L, Serni S, Maggi M and Carini M:
Influence of serum testosterone on urinary continence and sexual
activity in patients undergoing radical prostatectomy for
clinically localized prostate cancer. Prostate Cancer Prostatic
Dis. 13:168–172. 2010. View Article : Google Scholar
|
|
5.
|
Gacci M, Ierardi A, Rose AD, Tazzioli S,
Scapaticci E, Filippi S, Maggi M, Nicita G, Carini M and Montorsi
F: Vardenafil can improve continence recovery after bilateral nerve
sparing prostatectomy: results of a randomized, double blind,
placebo-controlled pilot study. J Sex Med. 7:234–243. 2010.
View Article : Google Scholar
|
|
6.
|
Yamamoto S, Yonese J, Kawakami S, Ohkubo
Y, Tatokoro M, Komai Y, Takeshita H, Ishikawa Y and Fukui I:
Preoperative serum testosterone level as an independent predictor
of treatment failure following radical prostatectomy. Eur Urol.
52:696–701. 2007. View Article : Google Scholar
|
|
7.
|
Isom-Batz G, Bianco FJ Jr, Kattan MW,
Mulhall JP, Lilja H and Eastham JA: Testosterone as a predictor of
pathological stage in clinically localized prostate cancer. J Urol.
173:1935–1937. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lane BR, Stephenson AJ, Magi-Galluzzi C,
Lakin MM and Klein EA: Low testosterone and risk of biochemical
recurrence and poorly differentiated prostate cancer at radical
prostatectomy. Urology. 72:1240–1245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Miller LR, Partin AW, Chan DW, Bruzek DJ,
Dobs AS, Epstein JI and Walsh PC: Influence of radical
prostatectomy on serum hormone levels. J Urol. 160:449–453. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Olsson M, Ekström L, Schulze J, Kjellman
A, Akre O, Rane A and Gustafsson O: Radical prostatectomy:
influence on serum and urinary androgen levels. Prostate.
70:200–205. 2010.PubMed/NCBI
|
|
11.
|
Lackner JE, Maerk I, Koller A, Bieglmayer
C, Marberger M, Kratzik C and Schatzl G: Serum inhibin - not a
cause of low testosterone levels in hypogonadal prostate cancer?
Urology. 72:1121–1124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Madersbacher S, Schatzl G, Bieglmayer C,
Reiter WJ, Gassner C, Berger P, Zidek T and Marberger M: Impact of
radical prostatectomy and TURP on the
hypothalamic-pituitary-gonadal hormone axis. Urology. 60:869–874.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Heidenreich A, Aus G, Bolla M, Joniau S,
Matveev VB, Schmid HP and Zattoni F: European Association of
Urology: EAU guidelines on prostate cancer. Eur Urol. 53:68–80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Carini M, Masieri L, Minervini A, Lapini A
and Serni S: Oncological and functional results of antegrade
radical retropubic prostatectomy for the treatment of clinically
localised prostate cancer. Eur Urol. 53:554–561. 2008. View Article : Google Scholar
|
|
15.
|
Corona G, Boddi V, Lotti F, Gacci M,
Carini M, De Vita G, Sforza A, Forti G, Mannucci E and Maggi M: The
relationship of testosterone to prostate-specific antigen in men
with sexual dysfunction. J Sex Med. 7:284–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sasagawa I, Nakada T, Suzuki H, Adachi Y
and Adachi M: Effect of transurethral resection of prostate on
plasma hormone levels in benign prostatic hyperplasia. Br J Urol.
72:611–614. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Türkölmez K, Bozlu M, Sarica K, Gemalmaz
H, Ozdiler E and Gögüş O: Effects of transurethral prostate
resection and transurethral laser prostatectomy on plasma hormone
levels. Urol Int. 61:162–167. 1998.PubMed/NCBI
|
|
18.
|
Reyes JG, Farias JG, Henríquez-Olavarrieta
S, Madrid E, Parraga M, Zepeda AB and Moreno RD: The hypoxic
testicle: physiology and pathophysiology. Oxid Med Cell Longev.
2012:9292852012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Vignozzi L, Filippi S, Morelli A, Marini
M, Chavalmane A, Fibbi B, Silvestrini E, Mancina R, Carini M,
Vannelli GB, Forti G and Maggi M: Cavernous neurotomy in the rat is
associated with the onset of an overt condition of hypogonadism. J
Sex Med. 6:1270–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Daniell HW, Clark JC, Pereira SE, Niazi
ZA, Ferguson DW, Dunn SR, Figueroa ML and Stratte PT: Hypogonadism
following prostate-bed radiation therapy for prostate carcinoma.
Cancer. 91:1889–1895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Gacci M, Simonato A, Masieri L, Gore JL,
Lanciotti M, Mantella A, Rossetti MA, Serni S, Varca V, Romagnoli
A, Ambruosi C, Venzano F, Esposito M, Montanaro T, Carmignani G and
Carini M: Urinary and sexual outcomes in long-term (5+ years)
prostate cancer disease free survivors after radical prostatectomy.
Health Qual Life Outcomes. 7:942009.
|