Introduction
Ovarian cancer is a common gynecological malignancy
that occurs in females worldwide. Annually, >230,000 new cases
of ovarian cancer are reported, causing >140,000 mortalities
(1). Chemotherapy is the most
effective primary therapy for the treatment of ovarian carcinoma,
with initial response rates varying between 40 and 80% (2). However, numerous patients with ovarian
cancer who initially respond to chemotherapy eventually relapse
with a drug-resistant form of the disease (3). Thus, acquired resistance represents
the major limitation to successful treatment. The molecular genetic
basis of resistance to cancer treatment is complex and involves
multiple processes, including drug transport and metabolism, DNA
repair and apoptosis (4).
Currently, the factors that regulate the development of
chemoresistance in ovarian cancer remain poorly understood.
Paclitaxel is commonly used in the treatment of
several types of cancer, including ovarian, breast and non-small
cell lung cancer. It has also been used in pediatric patients with
refractory malignancies and has been proposed as a potential agent
against high-risk hepatoblastoma (5–7).
Paclitaxel primarily kills cancer cells via microtubule
stabilization; however, other mechanisms have been reported to
mediate paclitaxel-induced cell death. It has been demonstrated
that paclitaxel is able to induce mitochondrion stress through the
activation of p38 (8). Several
major mechanisms have been demonstrated to be important in the
development of drug resistance to chemotherapy, including increased
levels of repair to DNA damage, reduced apoptosis, altered drug
metabolism and the overexpression of ATP-binding cassette (ABC)
transporters (9,10). P-glycoprotein (P-gp) belongs to the
ABC transporter family and its overexpression is considered to
contribute to the development of drug resistance in numerous types
of tumors, including ovarian cancer (11,12);
however, the mechanism by which P-gp is overexpressed has yet to be
elucidated.
The present study examined the role of miR-21 in the
development of drug resistance in human ovarian cancer cells. The
results demonstrated that aberrant miR-21 expression may be
involved in the modulation of hypoxia-inducible factor-1α (HIF-1α)
expression and the resistance of A2780 cells to paclitaxel.
Materials and methods
Cell lines and culture
Human ovarian cancer A2780 cell lines were purchased
from the China Center for Type Culture Collection (Shaghai, China).
The cell lines were cultured in RPMI-1640 medium (Gibco-BRL, Grand
Island, NY, USA) supplemented with 10% FBS (Gibco-BRL, Melbourne,
Australia) and 1% penicillin-streptomycin (Invitrogen Life
Technologies, Carlsbad, CA, USA) and maintained at 37ºC in a
humidified atmosphere of 5% CO2. The cells were passaged
every 2–3 days. The establishment of paclitaxel-resistant ovarian
cancer (A2780/taxol) cell lines was performed as described
previously (13).
miRNA transfection
The mimics and inhibitors of miR-21 were chemically
synthesized by GenePharma Co., Ltd. (Shanghai, China). A2780 and
A2780/taxol cells were seeded in 6-well plates at 3×105
cells/well and cultured for 18 h. The cells were then transfected
with 100 pmol of the miR-21 mimics, inhibitors or negative control
(NC) RNA using Lipofectamine 2000 and Opti-MEM I reduced serum
medium (Invitrogen Life Technologies), according to the
manufacturer’s instructions.
Stem-loop RT-PCR for the detection of
miR-21 expression levels
To validate the differential expression levels of
miR-21 in the A2780 and A2780/taxol cells, real-time RT-PCR
analysis was performed. Stem-loop primers were used for the reverse
transcription of miRNAs as described previously (14). The complementary DNA (cDNA)
underwent 35 rounds of amplification (Bio-Rad S1000; Bio-Rad,
Hercules, CA, USA) as follows: 35 cycles of a 2-step PCR (95ºC for
15 sec and 60ºC for 30 sec) following an initial denaturation (95ºC
for 10 min) with 2 μl cDNA solution and 1X SYBR-Green Premix PCR
reaction buffer (Takara Bio, Inc., Dalian, China). The sequence of
primers used for the amplification was as described previously
(14). Levels of miRNA were
normalized using U6 RNA as an internal reference gene and compared
with parent cells. The relative amount of miRNA to U6 RNA was
examined using the 2−ΔΔCt method (15).
Cell viability assay
The cells were seeded into 96-well culture plates at
a 5×103 cell density. Following cellular adhesion, the
A2780/taxol cells were exposed to 0.04, 0.2, 1.0, 5 and 25 μM doses
of paclitaxel and the A2780 cells were exposed to 0.01, 0.05, 0.25,
1.25 and 6.25 μM doses of paclitaxel for 48 h. Following
incubation, 20 μl of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma, St. Louis, MO, USA) was added to each well. Following
further incubation for 4 h at 37ºC, the medium was gently aspirated
and replaced by 150 μl DMSO. The absorbance of each well was
detected at a wavelength of 570 nm using a microplate reader
(Bio-Rad). The experiments were conducted in triplicate.
Small interfering RNA (siRNA)
transfection
HIF-1α siRNA and a non-targeting control were
purchased from Ambion (Applied Biosystems, Foster City, CA, USA)
and the transfection was performed according to the manufacturer’s
instructions. The cells were prepared for further analysis 48 h
after the transfection. The transfection efficiency was evaluated
by flow cytometry by calculating the percentage of
fluorescein-labeled cells. The transfection efficiency was
~75%.
Immunoblot analysis
The cells were harvested and washed with ice-cold
phosphate-buffered saline. Cell lysates were obtained by
re-suspending the cells in RIPA buffer [10 mM Tris (pH 7.4), 150 mM
NaCl, 1% Triton X-100, 1% Na-deoxycholate (Kanto Chemical, Tokyo,
Japan)] and 5 mM EDTA supplemented with protease inhibitor cocktail
(Sigma). The protein concentration of the cell lysates was
determined by BSA assay using the BSA kit (Beyotime, Shanghai,
China). Equal amounts of protein were separated by SDS-PAGE and
electrotransferred onto a PVDF membrane (Millipore, Billerica, MA,
USA). The membranes were blocked and incubated overnight with P-gp,
HIF-1α or GAPDH antibodies (Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA), according to the manufacturer’s instructions.
Signals present on the membrane were developed using the ECL
reagent (Amersham, San Francisco, CA, USA) and were imaged using a
polaroid imaging system (Amersham).
Statistical analysis
Each experiment was repeated at least three times.
Numerical data are expressed as the mean ± SD. Statistical analyses
were performed using SPSS 12.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of P-gp and miR-21 in
A2780 and A2780/taxol cells
The overexpression of P-gp has been shown to
contribute to the development of drug resistance in numerous types
of cancer cell (16). In the
present study, flow cytometry was used to measure the expression
levels of P-gp in the A2780 and A2780/taxol cells. The expression
levels of P-gp were increased in the A2780/taxol cell line compared
with the parental A2780 cell line (Fig.
1A). Bourguignon et al demonstrated that high levels of
P-gp were associated with high levels of miR-21 in drug-resistant
breast cancer cells (17). In the
present study, the expression levels of miR-21 in the A2780 and
A2780/taxol cell lines were then detected using stem-loop real-time
PCR. It was shown that the expression levels of miR-21 were on
average 3.1-fold higher in the A2780/taxol cells compared with the
A2780 cells (P<0.05; Fig.
1B).
miR-21 modulates sensitivity to
paclitaxel
To further investigate whether miR-21 is capable of
modulating the sensitivity of A2780/taxol and A2780 cells to
paclitaxel, the A2780 and A2780/taxol cells were transfected with
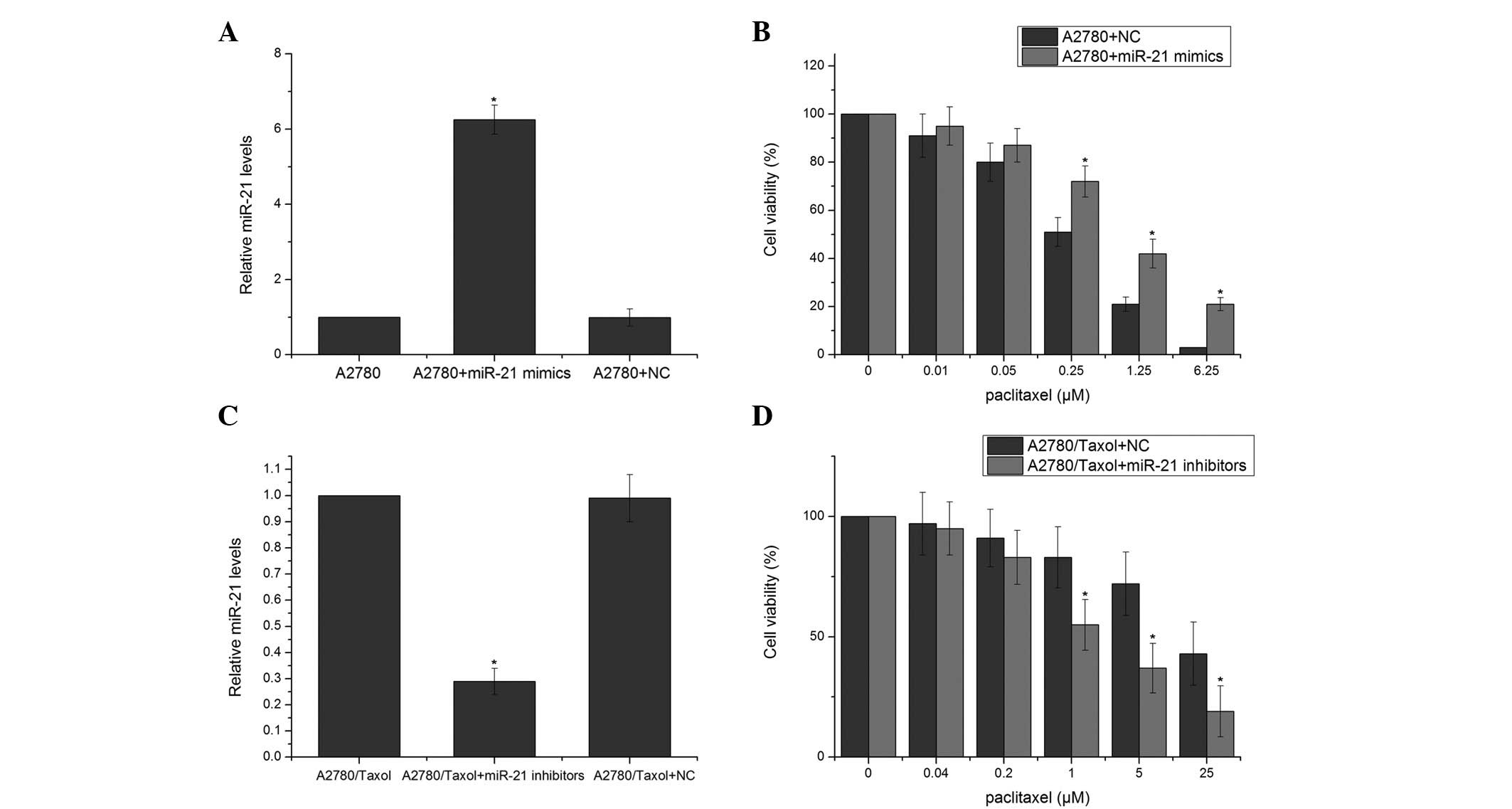
hsa-miR-21 and miR-21 inhibitors, respectively. In the A2780 cells,
miR-21 mimics significantly increased the levels of miR-21
(Fig. 2A). The expression levels of
miR-21 were decreased in the A2780/taxol cells transfected with
miR-21 inhibitors (Fig. 2C). The
MTT assay revealed that the cells transfected with miR-21 mimics
exhibited a significantly increased resistance to paclitaxel
compared with the negative control (NC) RNA-transfected cells
(Fig. 2B). The A2780/taxol cells
transfected with miR-21 inhibitors exhibited a significantly
increased sensitivity to paclitaxel compared with cells transfected
with NC RNA (Fig. 2D). These
results suggested that miR-21 may modulate the sensitivity of A2780
cells to paclitaxel.
Effect of miR-21 on the expression of
multidrug resistance 1 (MDR1) and P-gp
To determine whether miR-21 is capable of regulating
the expression of MDR1/P-gp, the A2780 and A2780/taxol cells were
transfected with mimics and inhibitors of miR-21, respectively, and
the expression levels of P-gp were determined by western blot
analysis. The transfection with miR-21 mimics resulted in increased
expression levels of P-gp, whereas the transfection with NC RNA
demonstrated no changes in the expression of MDR1/P-gp in the A2780
cells (Fig. 3A and B). To further
test the effect of miR-21 on the expression of MDR1, the
A2780/taxol cells were transfected with miR-21 inhibitors or NC
RNA. The transfection with miR-21 inhibitors resulted in decreased
levels of MDR1 mRNA (Fig. 2A) and
P-gp expression (Fig. 3C and
D).
Regulation of HIF-1α expression by
miR-21
HIF-1 is a heterodimeric transcription factor
composed of two subunits: HIF-1α and HIF-1β. HIF-1α is induced by
hypoxia, growth factors and oncogenes, whereas HIF-1β is
constitutively expressed in cells (18). To test whether miR-21 affects HIF-1
expression, the A2780 and A2780/taxol cells were transfected with
miR-21 mimics and inhibitors, respectively. It was demonstrated
that the overexpression of miR-21 significantly increased the
expression of HIF-1α in the A2780 cells (Fig. 4A and B). The treatment with miR-21
inhibitors decreased the expression levels of HIF-1α in the
A2780/taxol cells (Fig. 4C and
D).
HIF-1α is important in paclitaxel
resistance
A previous study demonstrated that HIF-1α is
involved in the development of drug resistance in several types of
cancer (16), however, its role in
paclitaxel sensitivity in the A2780 cell line remains unclear. To
examine the correlation between HIF-1α and paclitaxel-induced
cytotoxicity, HIF-1α siRNA or a scrambled siRNA was transfected
into the A2780/taxol cells, followed by treatment with various
doses of paclitaxel. HIF-1α siRNA significantly decreased the
protein levels of HIF-1α (Fig. 5A and
B). The protein levels of P-gp were decreased in the
A2780/taxol cells transfected with HIF-1α siRNA (Fig. 5C and D). Furthermore, the
A2780/taxol cells treated with HIF-1α siRNA exhibited a decreased
survival rate compared with the control group (Fig. 5E).
Discussion
Although chemotherapeutic agents, including
paclitaxel, are widely used for the treatment of ovarian cancer,
chemoresistance remains a major therapeutic obstacle (19). In the present study, cells from the
human ovarian cancer A2780 cell line were used as targets to
examine the effects of mRNA on reverse MDR of ovarian cancer cells
in an attempt to identify novel treatment targets for ovarian
cancer therapy. The results demonstrated that the knockdown of
HIF-1α expression, in addition to decreased levels of miR-21, was
capable of re-establishing the susceptibility of cancer cells to
paclitaxel through the inhibition of P-gp expression, indicating
that overexpression of miR-21/HIF-1α is important in the
development of chemoresistance.
A recent study has indicated that miR-21 contributes
to drug resistance in solid tumors and leukemia through several
pathways. The inhibition of miR-21 may decrease cell growth, induce
apoptosis and suppress migration and invasion in numerous types of
cancer cell (20). Furthermore,
several studies have demonstrated that the inhibition of miR-21 may
sensitize leukemia cells to chemotherapy drugs (21,22).
The present study demonstrated that miR-21 was upregulated to a
greater extent in the A2780/taxol cells compared with the A2780
cells, indicating that miR-21 is involved in the development of
paclitaxel resistance in ovarian cancer. Additional experiments
involving the overexpression and underexpression of miR-21 were
performed to confirm the effects of miR-21 on paclitaxel resistance
in the A2780 cells. These demonstrated that the overexpression of
miR-21 attenuated cell death, whereas the knockdown of miR-21
expression stimulated cell death.
HIF-1α is a multifunctional transcription factor,
which has been shown to regulate tumor cell invasion and migration.
Recent studies have demonstrated that HIF-1α contributes to the
development of chemoresistance. Nardinocchi et al(23) demonstrated that HIPK2 is able to
downregulate the expression of HIF-1α, which is overexpressed in
several types of tumor and contributes to the development of
chemoresistance by activating MDR1. In the present study, it was
proposed that the HIPK2-mediated inhibition of HIF-1α correlated
with the suppression of MDR1 gene transcription and the
sensitization of cobalt-treated tumor cells to adriamycin-induced
apoptosis. The results showed that HIF-1α and P-gp protein levels
were significantly decreased in the A2780/taxol cells treated with
inhibitors of miR-21 compared with the control group. The
A2780/taxol cells that were pretreated with HIF-1α siRNA exhibited
a decreased survival rate and decreased P-gp protein levels
compared with the control group. Furthermore, the inhibition of
miR-21 may sensitize A2780/taxol cells to paclitaxel-induced cell
death. The miR-21/HIF-1α/MDR1/P-gp cell signaling pathway provides
a novel insight into the underlying mechanisms responsible for
paclitaxel resistance in A2780 cells.
In conclusion, the present study demonstrated that
miR-21 may regulate the expression of MDR1/P-gp, at least in part,
by targeting HIF-1α, which is involved in the development of drug
resistance in paclitaxel-resistant ovarian cancer A2780/taxol cell
lines. Furthermore, it was demonstrated that the inhibition of
miR-21 may sensitize A2780/taxol cells to paclitaxel. This study
may present a promising future strategy to reverse drug resistance
through the targeting of miRNAs.
References
|
1
|
Hassan MK, Watari H, Christenson L,
Bettuzzi S and Sakuragi N: Intracellular clusterin negatively
regulates ovarian chemoresistance: compromised expression
sensitizes ovarian cancer cells to paclitaxel. Tumour Biol.
32:1031–1047. 2011. View Article : Google Scholar
|
|
2
|
McGuire WP 3rd and Markman M: Primary
ovarian cancer chemotherapy: current standards of care. Br J
Cancer. 89(Suppl 3): S3–S8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ozols RF and Young RC: Chemotherapy of
ovarian cancer. Semin Oncol. 11:251–263. 1984.
|
|
4
|
Dai Z, Huang Y and Sadée W: Growth factor
signaling and resistance to cancer chemotherapy. Curr Top Med Chem.
4:1347–1356. 2004.PubMed/NCBI
|
|
5
|
Fuchs J, Habild G, Leuschner I, Schweinitz
DV, Haindl J and Knop E: Paclitaxel: an effective antineoplastic
agent in the treatment of xenotransplanted hepatoblastoma. Med
Pediatr Oncol. 32:209–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heney M, Alipour M, Vergidis D, et al:
Effectiveness of liposomal paclitaxel against MCF-7 breast cancer
cells. Can J Physiol Pharmacol. 88:1172–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pisters KM, Vallières E, Crowley JJ, et
al: Surgery with or without preoperative paclitaxel and carboplatin
in early-stage non-small-cell lung cancer: Southwest Oncology Group
Trial S9900, an intergroup, randomized, phase III trial. J Clin
Oncol. 28:1843–1849. 2010. View Article : Google Scholar
|
|
8
|
Selimovic D, Hassan M, Haikel Y and Hengge
UR: Taxol-induced mitochondrial stress in melanoma cells is
mediated by activation of c-Jun N-terminal kinase (JNK) and p38
pathways via uncoupling protein 2. Cell Signal. 20:311–322. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fojo T and Menefee M: Mechanisms of
multidrug resistance: the potential role of microtubule-stabilizing
agents. Ann Oncol. 18(Suppl 5): v3–v8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roberti A, La Sala D and Cinti C: Multiple
genetic and epigenetic interacting mechanisms contribute to
clonally selection of drug-resistant tumors: current views and new
therapeutic prospective. J Cell Physiol. 207:571–581. 2006.
View Article : Google Scholar
|
|
11
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: the early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006.PubMed/NCBI
|
|
13
|
Li Z, Hu S, Wang J, et al: MiR-27a
modulates MDR1/P-glycoprotein expression by targeting HIPK2 in
human ovarian cancer cells. Gynecol Oncol. 119:125–130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu MD, Xu JC, Fan Y, Xie QC, Li Q, Zhou
CX, Mao M and Yang Y: Hypoxia-inducible factor 1 promoter-induced
JAB1 overexpression enhances chemotherapeutic sensitivity of lung
cancer cell line A549 in an anoxic environment. Asian Pac J Cancer
Prev. 13:2115–2120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourguignon LY, Spevak CC, Wong G, Xia W
and Gilad E: Hyaluronan-CD44 interaction with protein kinase
C(epsilon) promotes oncogenic signaling by the stem cell marker
Nanog and the Production of microRNA-21, leading to down-regulation
of the tumor suppressor protein PDCD4, anti-apoptosis, and
chemotherapy resistance in breast tumor cells. J Biol Chem.
284:26533–26546. 2009.
|
|
18
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
19
|
Hilpert F, Krause G, Venhoff L, Kuhnle E,
Schem C and Maass N: Epithelial ovarian cancer. Ther Umsch.
64:375–380. 2007.(In German).
|
|
20
|
Li H, Hui L, Xu W, et al: Triptolide
modulates the sensitivity of K562/A02 cells to adriamycin by
regulating miR-21 expression. Pharm Biol. 50:1233–1240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Zhu X, Gu J, et al: Anti-miR-21
oligonucleotide sensitizes leukemic K562 cells to arsenic trioxide
by inducing apoptosis. Cancer Sci. 101:948–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zhu X, Gu J, et al: Anti-miR-21
oligonucleotide enhances chemosensitivity of leukemic HL60 cells to
arabinosylcytosine by inducing apoptosis. Hematology. 15:215–221.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nardinocchi L, Puca R and D’Orazi G:
HIF-1α antagonizes p53-mediated apoptosis by triggering HIPK2
degradation. Aging (Albany NY). 3:33–43. 2011.
|