Introduction
Endometrial carcinoma (EC) is a common malignant
tumor of the female genital tract, which has notably increased in
incidence over recent years (1).
Tumor invasion and migration are characteristic features in the
majority of malignant tumors, including EC (2,3).
Previous studies have identified that abnormalities in the Wnt
signaling pathway contribute to tumorigenesis in favor of tumor
migration, invasion and metastasis (4–8). DKK1,
an inhibitor of the Wnt signaling pathway, has been identified in
the invasion and migration of specific benign and malignant
tissues. β-catenin is a pivotal molecule in the Wnt signaling
pathway and metalloproteinase 14 (MMP14) is a downstream target
gene. In addition, these molecules have been identified as
mediators of tumor invasion and migration. Therefore, in the
current study, β-catenin and MMP14 were targeted using DKK1 siRNA
to identify the effects of DKK1 on the invasion and migration of EC
cells.
Materials and methods
Cell culture
Ishikawa EC cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were maintained in DMEM/F12 medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (GE
Healthcare, Amersham, UK), 100 μg/ml penicillin and 100 μg/ml
streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C. Routine testing confirmed that the cells
were free of mycoplasma and viral contaminants. The cells were
subcultured every 2 days at a ratio of 1:2.
Cell transfection
The following primer sequences for siRNAs targeting
human DKK1 were used: i) (RNA)-AUA GCG UUG GAA UUG AGA ACC
GAG U; ii) (RNA)-ACU CGG UUC UCA AUU CCA ACG CUA U; and iii)
(RNA)-AAU CCU GAG GCA CAG UCU GAU GAC C. Stealth™ RNAi Negative
Control Med GC was used as a negative control for the siRNA (siRNA
sequences were obtained from Invitrogen Life Technologies).
Transfection conditions
The EC cells were transfected with DKK1 siRNA or
negative control siRNA or untransfected (DKK1 RNAi, control and
blank groups, respectively). The EC cells were then seeded in 35-mm
culture dishes at 1×106 cells/well prior to transfection
with DKK1 siRNA or negative control siRNA using Lipofectamine 2000
reagent, according to the manufacturer's instructions.
Lipofectamine 2000 (5 μl) diluted in 250 μl Opti-MEM was prepared.
In addition, 10 μl DKK1 siRNA (20 μM) and 10 μl negative control
siRNA (20 μM) were diluted with 250 μl Opti-MEM and incubated for
20 min. The 500 μl complexes of Lipofectamine 2000 and siRNA plus
1,500 μl DMEM/F12 were introduced to 35-mm culture dishes and
incubated in a humidified atmosphere containing 5% CO2
at 37°C. After 5–6 h, the medium was replaced with 10%
serum-supplemented DMEM/F12 and the cells were incubated for 24–96
h for further use in various procedures (all reagents were obtained
from Invitrogen Life Technologies).
Transfection efficiency
BLOCK iT™ fluorescent oligos (Invitrogen Life
Technologies) were transfected into the cells of the DKK1 RNAi and
control groups to ensure the successful transfection of siRNA into
the cells.
Silencing efficiency
The silencing efficiency was determined by RT-PCR
and western blot analysis using DKK1-specific primers and
antibodies. Subsequent experiments focused on the primer previously
described as primer ii in the cell transfection methods for siRNAs
targeting human DKK1, since it was identified as the most
effective for inhibiting DKK1 expression.
Semi-quantitative RT-PCR analysis
mRNA levels of DKK1, β-catenin, MMP14 and GAPDH
(internal control) were determined by RT-PCR. Following cell
incubation, total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen Life Technologies). The
reverse transcription reaction was set up using RT reaction mix
(Promega Corporation, Madison, WI, USA) and the resultant cDNA was
used for PCR. The following primers for DKK1, β-catenin, MMP14 and
GAPDH were used: i) DKK1 sense, 5′-CTGCATGCGTCACGCTATGT-3′ and
antisense, 5′-TCCTCGGAAATGATTTTGATCA-3′; ii) β-catenin sense,
5′-CGGGATGTTCACAACCGAAT-3′ and antisense,
5′-TTGGATGTTTTCAATGGGAGAA-3′; iii) MMP14 sense,
5′-CAGGGTCTCAAATGGCAACA-3′ and antisense,
5′-TTGCGAATGGCCTCGTATG-3′; and iv) GAPDH sense,
5′-CAGTCAGCCGCATCTTCTTTT-3′ and antisense,
5′-GTGACCAGGCGCCCAATAC-3′. Experiments were performed in
triplicate.
Western blot analysis
Protein levels of intracellular DKK1,
active-β-catenin, MMP14 and β-actin (internal control) were
detected by western blot analysis. When the cells reached 80–90%
confluence, they were lysed in lysis buffer with protease
inhibitors at 4°C. Cell lysates were also collected and protein
concentrations were determined using the Bradford method. The
lysates were cleared by centrifugation and quantified using the DC
protein assay (Bio-Rad, Hercules, CA, USA). The protein samples (50
μg) were boiled for 5 min prior to being loaded onto 10% SDS-PAGE.
Following electrophoresis, proteins were transferred onto a
nitrocellulose membrane (Pall Corp., Washington, NY, USA). The
membranes were blocked with 5% skimmed milk in PBS and probed with
primary antibodies overnight at 4°C. Horseradish
peroxidase-conjugated secondary antibodies (1:5,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were used and the
membranes were developed following an enhanced chemiluminescence
detection protocol (Santa Cruz Biotechnology, Inc.). The following
primary antibodies and dilutions with blocking solution were used:
DKK1 (mouse monoclonal, 1:300; Abnova, Taipai City, Taiwan);
active-β-catenin (mouse monoclonal, 1:500; Upstate Biotechnology,
Lake Placid, NY, USA); MMP14 (rabbit polyclonal, 1:300; Abcam,
Cambridge, UK) and β-actin (mouse monoclonal, 1:1,000; Santa Cruz
Biotechnology, Inc.). All experiments were performed in
triplicate.
Invasion assay
An invasion assay was performed using the Transwell
chamber assay according to previous studies (9–11),
with modifications. Briefly, Matrigel matrix (1:10 v/v, 1 mg/ml; BD
Biosciences, Franklin Lakes, NJ, USA) was diluted in serum-free
DMEM/F12. Then, the diluted Matrigel matrix was addd to the upper
wells of a 24-well transwell plate (polycarbonate membranes with an
8-μm pore size; Millipore, Billerica, MA, USA) and incubated for
5–6 h at room temperature. The cells of the DKK1 RNAi and blank
groups were trypsinized, washed and resuspended. Viable cells were
added to the upper wells at a density of 2×105
cells/well with 300 μl serum-free DMEM/F12, whilst 500 μl
serum-supplemented DMEM/F12 was filled into the lower wells. Plates
were incubated at 37°C for 48 h, then non-invaded cells on top of
the transwell were scraped off with a cotton swab. The cells in the
chambers were maintained in an incubator at 37°C and allowed to
migrate for 48 h. After 48 h, the non-migrated cells in the upper
compartments were scraped carefully with cotton swabs. Migrated
cells adhering to the lower surface of the membranes were fixed
with methanol and stained with hematoxylin and eosin. The membranes
were excised from the insert and mounted onto glass slides for
light microscopic analysis, and migrated cells (penetrating
Matrigel matrix and pores) were counted (magnification, ×20) from
ten randomly selected fields. Each sample was assayed in
triplicate.
Migration assay
A migration assay was performed following the method
for the invasion assay, with the exception of the exclusion of the
Matrigel matrix and the use of polycarbonate membranes only.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed using SAS Version 9 (SAS Institute Inc.,
Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference. Quantitative analyses were
performed using the Student's t-test for RT-PCR, western blot
analysis and invasion and migration assay results among the three
groups.
Results
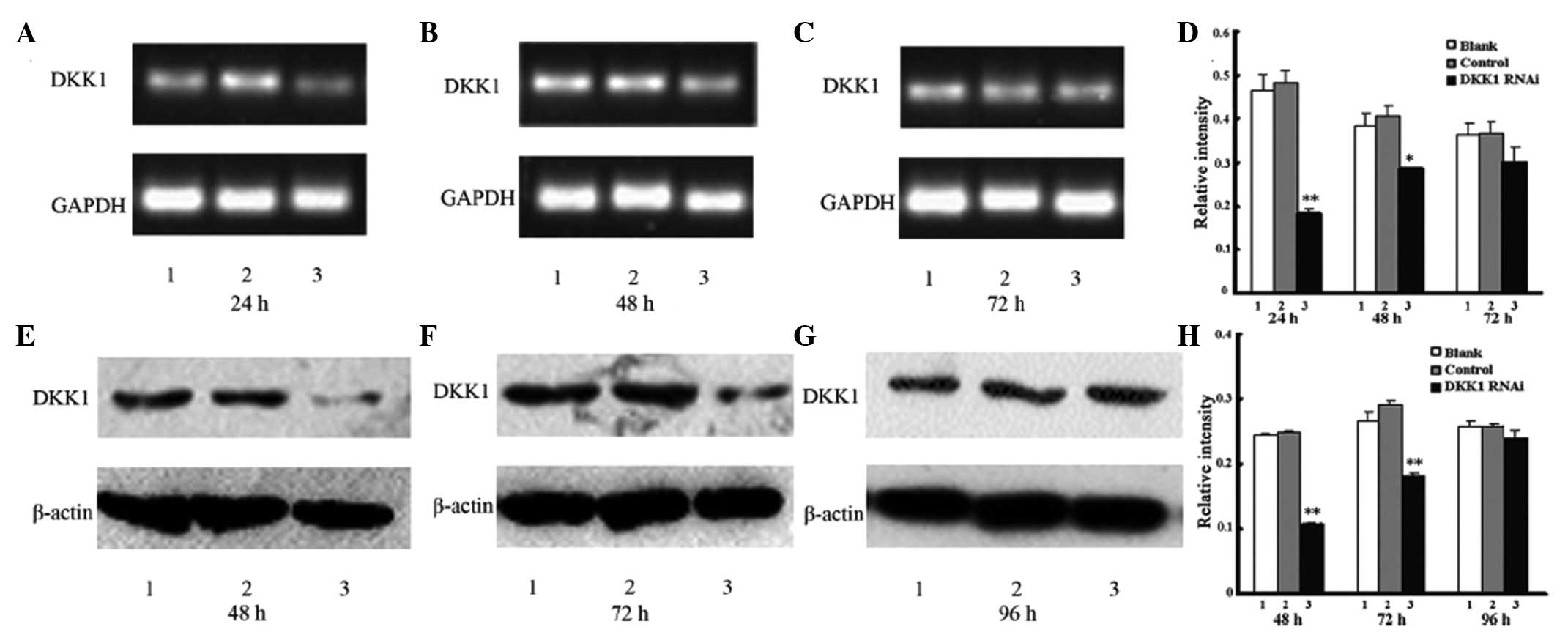
Effect of DKK1 siRNA on DKK1 mRNA
levels
The EC cells were transfected successfully with DKK1
and negative control siRNAs with high efficiency. DKK1 mRNA levels
in total cell extracts were measured by semi-quantitative RT-PCR.
Negligible changes in DKK1 mRNA levels were identified in the blank
and control groups, however, in the DKK1 RNAi group, the DKK1 mRNA
levels showed a decrease between 24, 48 and 72 h (61.47, 25.79 and
17.04%, respectively, vs. blank). In the DKK1 RNAi group, DKK1 gene
expression was significantly inhibited between 24 and 48 h,
however, there was no marked difference after 72 h. mRNA levels of
GAPDH, as a loading control, were also analyzed, however, no
changes were observed (Fig. 1 and
Table I).
 | Table IDKK1 mRNA levels in the three groups
at 24, 48 and 72 h. |
Table I
DKK1 mRNA levels in the three groups
at 24, 48 and 72 h.
| Groups | 24 h | 48 h | 72 h |
|---|
| Blank | 0.465±0.039 | 0.385±0.027 | 0.363±0.029 |
| Control | 0.485±0.027 | 0.408±0.025 | 0.368±0.026 |
| DKK1 RNAi | 0.179±0.013a | 0.286±0.003b | 0.301±0.035 |
Effect of DKK1 siRNA on DKK1
protein level
The levels of DKK1 protein in DKK1 siRNA-treated
cells were identified to significantly decrease between 48, 72 and
96 h (56.74, 31.64 and 7.17%, respectively, vs. blank group) when
compared with the untreated and control-treated cells. Inhibition
was identified as statistically significant between 48 and 72 h,
however, no significant difference was identified after 96 h. No
change in the protein levels of β-actin, which served as a loading
control, were identified. In addition, no significant differences
were identified in protein levels in the blank and control groups
(Fig. 1 and Table II).
 | Table IIDKK1 protein levels in the three
groups at 48, 72 and 96 h. |
Table II
DKK1 protein levels in the three
groups at 48, 72 and 96 h.
| Groups | 48 h | 72 h | 96h |
|---|
| Blank | 0.246±0.002 | 0.266±0.015 | 0.251±0.005 |
| Control | 0.250±0.002 | 0.292±0.006 | 0.258±0.006 |
| DKK1 RNAi | 0.107±0.001a | 0.182±0.003a | 0.233±0.010 |
Effect of DKK1 siRNA on EC cell
invasion
Each field was observed under a light microscope
(magnification, ×20) and a significant difference was identified
between the number of migrated cells in the DKK1 RNAi group
(149.80) when compared with the blank group (122.50), and the
knockdown of DKK1 was identified to result in accelerated EC cell
invasion (Fig. 2).
Effect of DKK1 siRNA on EC cell
migration
Cell migration numbers for DKK1 siRNA-treated and
-untreated cells were 173.90 and 135.80, respectively. The results
indicated that the cells treated with DKK1 RNAi had significantly
accelerated EC cell migration when compared with the blank group
(Fig. 2).
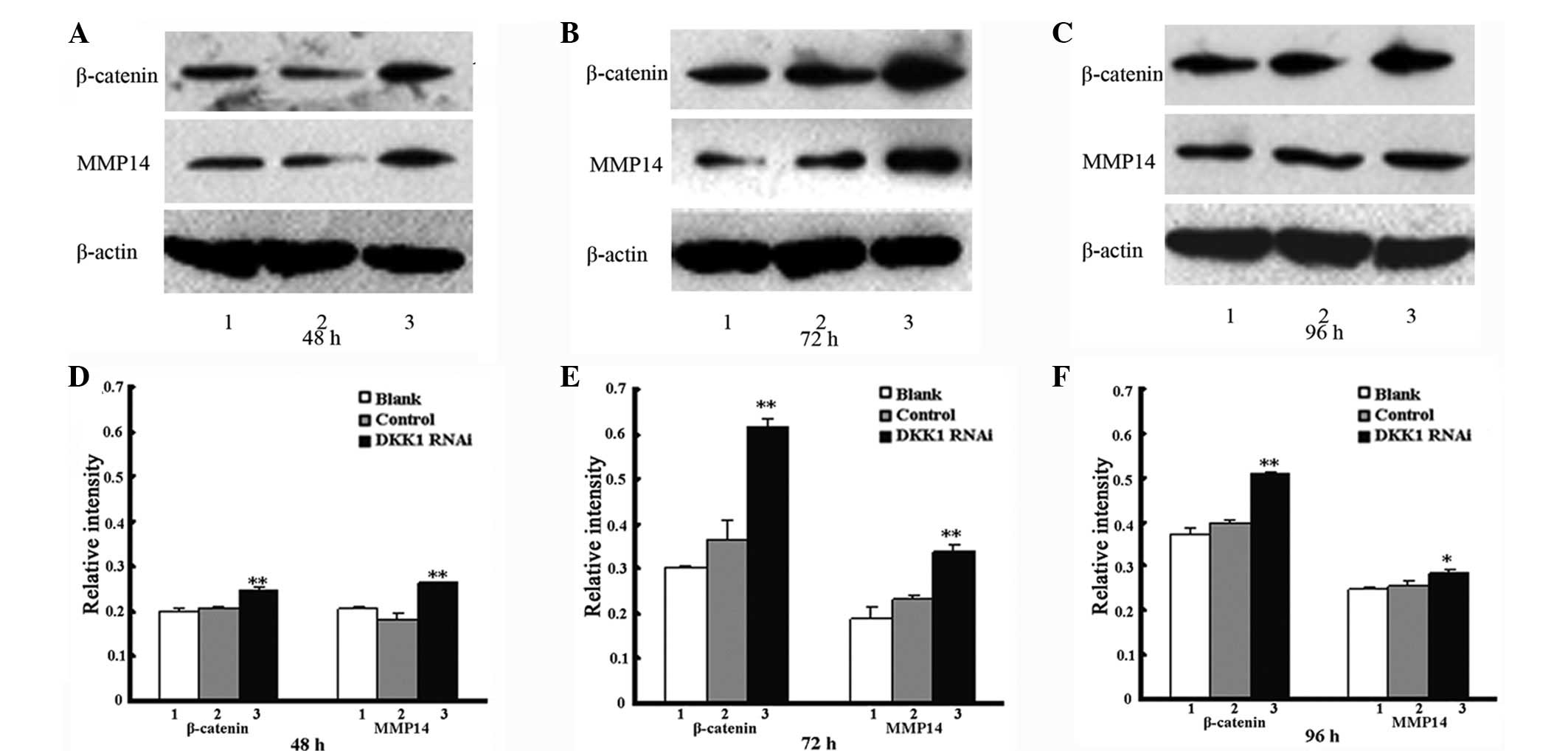
β-catenin and MMP14 mRNA expression
following knockdown of DKK1
The mRNA levels of β-catenin and MMP14 were
increased in the DKK1 siRNA-treated cells when compared with the
untreated and control-treated cells after 72 h. In addition, the
mRNA levels of β-catenin and MMP14 were significantly elevated
following the knockdown of DKK1, however, no significant
differences were identified in the blank and control groups
(Fig. 3 and Tables III and IV).
 | Table IIIActive β-catenin mRNA expression
following knockdown of DKK1 at 24, 48 and 72 h. |
Table III
Active β-catenin mRNA expression
following knockdown of DKK1 at 24, 48 and 72 h.
| Groups | 24 h | 48 h | 72 h |
|---|
| Blank | 0.137±0.006 | 0.147±0.012 | 0.195±0.015 |
| Control | 0.144±0.002 | 0.169±0.006 | 0.182±0.024 |
| DKK1
RNAi | 0.162±0.006a | 0.283±0.016b | 0.325±0.035a |
 | Table IVMMP14 mRNA expression following
knockdown of DKK1 at 24, 48 and 72 h. |
Table IV
MMP14 mRNA expression following
knockdown of DKK1 at 24, 48 and 72 h.
| Groups | 24 h | 48 h | 72 h |
|---|
| Blank | 0.252±0.029 | 0.287±0.005 | 0.296±0.026 |
| Control | 0.303±0.017 | 0.282±0.022 | 0.314±0.015 |
| DKK1
RNAi | 0.162±0.006a | 0.283±0.016a | 0.532±0.044b |
β-catenin and MMP14 protein expression
following DKK1 knockdown
Western blot analyses of cell lysates were performed
to analyze whether protein expression correlated with mRNA
expression following the knockdown of DKK1. The protein levels of
β-catenin and MMP14 in the DKK1 RNAi group were significantly
elevated after 96 h when compared with that of the additional two
groups (Fig. 4 and Tables V and VI), and no significant differences were
identified in the protein levels of the blank and control
groups.
 | Table VActive-β-catenin protein expression
following knockdown of DKK1 at 48, 72 and 96 h. |
Table V
Active-β-catenin protein expression
following knockdown of DKK1 at 48, 72 and 96 h.
| Groups | 48 h | 72 h | 96 h |
|---|
| Blank | 0.200±0.005 | 0.301±0.004 | 0.373±0.014 |
| Control | 0.207±0.003 | 0.366±0.045 | 0.398±0.007 |
| DKK1
RNAi | 0.244±0.007a | 0.618±0.017a | 0.510±0.005a |
 | Table VIMMP14 protein expression following
knockdown of DKK1 at 48, 72 and 96 h. |
Table VI
MMP14 protein expression following
knockdown of DKK1 at 48, 72 and 96 h.
| Groups | 48 h | 72 h | 96 h |
|---|
| Blank | 0.206±0.005 | 0.188±0.026 | 0.247±0.005 |
| Control | 0.181±0.015 | 0.231±0.009 | 0.255±0.010 |
| DKK1
RNAi | 0.262±0.003a | 0.339±0.015a | 0.285±0.006b |
Discussion
EC is the most common malignant tumor in females and
has a high incidence worldwide (1).
The majority of EC cases are metastatic at diagnosis and therefore,
metastasis is the main cause of cancer-related mortality. Tumor
cell invasion is a complex event that involves interactions among
tumor cells, extracellular matrix (ECM) degradation and cell
migration (2). Cell migration and
invasion are early steps in metastasis (3) and therefore it is necessary to
identify specific molecules and proteins that may limit the process
of cell invasion and migration.
Previous studies have identified that abnormalities
in the Wnt signaling transduction pathway contribute to
tumorigenesis involved in cell migration, invasion and metastasis
(4–8). A number of molecules and proteins
involved in the Wnt signaling pathway have been investigated as
targets for the diagnosis and treatment of malignant tumors. The
present study focused on DKK1, a negative regulator in the Wnt
signaling pathway (12,13), as a key factor previously identified
to be involved in the invasion and migration of colorectal
(14), neuroblastoma (15) and placental cells (16,17).
β-catenin functions as a significant component in
the Wnt signaling pathway, and interactions with frizzled and
LRP5/6 receptors result in the dephosphorylation of β-catenin
(non-phosphorylated active β-catenin). The dephosphorylated form of
β-catenin has been shown to have significant effects on the Wnt
signaling pathway (18,19). Subsequent to these interactions,
accumulated active-β-catenin molecules translocate to the nucleus,
activating downstream target genes, the majority of which,
including the MMPs, are involved in tumorigenesis (20–23).
The MMP family is comprised of zinc-dependent endopeptidases that
are crucial for various proteolytic events and a number of tumor
malignancy processes, including metastasis (24). MMPs are also involved in ECM
degradation and therefore contribute to tumor progression and
metastasis. Previous studies have shown that during tumorigenesis,
MMPs are involved in tumor migration, metastasis and invasion into
surrounding tissue. MMP14 is a member of the MMP family and has
been identified to be involved in ECM degradation and invasion. In
addition, the human DKK1 (chromosome 10q11.2) gene encodes an
inhibitor involved in the Wnt signaling pathway, binding to and
antagonizing LRP5/6 (25–27). In the present study, the
hypothesized interactions between β-catenin, MMP14 and DKK1 in EC
cell invasion and migration were investigated.
RNAi is a sequence-specific, post-transcriptional
gene-silencing method initiated by double-stranded RNA and
homologous to the gene being suppressed. RNAi is now routinely used
for the transient knockdown of gene expression in a wide range of
organisms for the analysis of gene function (28). In the present study, siRNA with high
specificity and efficiency to DKK1 were used to suppress DKK1 gene
expression in Ishikawa EC cells. In addition, DKK1, β-catenin and
MMP14 were targeted using DKK1-targeting siRNA to identify the
effects of DKK1 on EC cell invasion and migration. Few studies have
analyzed the biology of DKK1 function in tumor invasion and
migration.
In the present study, the transfection of DKK1 siRNA
downregulated the mRNA and protein levels of DKK1 in the DKK1 RNAi
group. DKK1 siRNA was identified to significantly inhibit DKK1 mRNA
levels after 48 h, and protein levels were downregulated after 72
h. However, no marked changes in the levels of mRNA and protein
were identified in the blank and control groups. These results
demonstrated that the mRNA and protein levels of DKK1 were
successfully downregulated by transfection of DKK1 siRNA, however,
no significant decrease in the levels of mRNA and protein were
identified in the DKK1 siRNA-treated cells after 72 and 96 h,
respectively. This is hypothesized to be due to the transient
nature of the DKK1-knockdown by DKK1 siRNA.
The knockdown of DKK1 has been identified to
accelerate EC cell invasion and migration. In the present study, EC
cells transfected with DKK1 siRNA showed increased tumor cell
invasion and migration when compared with the untreated and
control-treated cells, indicating an upregulation of β-catenin and
MMP14. A significant increase in the mRNA and protein levels of
β-catenin and MMP14 and cell invasion and migration were identified
in the DKK1 RNAi group when compared with the additional two
groups. The results of the current study are consistent to that of
previous studies reporting the involvement of the knockdown of DKK1
in tumor cell invasion and migration (15–18).
In addition, these were consistent with the results of our previous
study, which demonstrated that EC cell invasion and migration may
be inhibited by the upregulation of DKK1 (29–31).
Upregulation of β-catenin and MMP14 by the knockdown
of DKK1 interferes with the Wnt signaling pathway and its
downstream signaling events. Intracellular signaling transduction
pathways are often exploited during tumor invasion and migration
and the involvement of β-catenin and MMP14 in the Wnt signaling
transduction pathway has been identified to accelerate tumor
invasion and migration. Therefore, the hypothesized effects of the
DKK1 RNAi-mediated upregulation of β-catenin and MMP14 on EC cell
migration and invasion were investigated in the present study. A
significant increase in the levels of β-catenin mRNA and protein in
the DKK1 RNAi group was identified. The knockdown of DKK1 increased
the levels of β-catenin dephosphorylation into active-β-catenin,
which resulted in elevated activation of the Wnt signaling pathway.
Accumulated active-β-catenin in the cytoplasm was translocated into
the nucleus where it interacted with lymphocyte enhancers and
T-cell transcription factors to activate downstream target genes,
including MMP14. Marked increases in the MMP14 mRNA and protein
levels in the DKK1 RNAi group indicated significant MMP14
activation. The results indicated that the upregulation of
β-catenin and MMP14 the knockdown of DKK1 may result in the
elevated activation of the Wnt signaling pathway and downstream
signaling events involved in tumor migration and invasion.
In summary, the results of the present study
indicate a novel biological function for DKK1 in the inhibition of
EC cell invasion and migration. In addition, targeting DKK1 may
represent an effective anti-invasion and -migration strategy for
the treatment of EC, as DKK1 may contribute directly or indirectly
to anti-tumorigenesis.
Abbreviations:
|
ECM
|
extracellular matrix
|
|
RNAi
|
RNA interference
|
|
MMPs
|
metalloproteinases
|
|
EC
|
endometrial carcinoma
|
References
|
1
|
Akhmedkhanov A, Zeleniuch-Jacquotte A and
Toniolo P: Role of exogenous and endogenous hormones in endometrial
cancer: review of the evidence and research perspectives. Ann NY
Acad Sci. 943:296–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arai Y, Kubota T, Nakagawa T, et al:
Production of urokinase-type plasminogen activator (u-PA) and
plasminogen activator inhibitor-1 (PAI-1) in human brain tumours.
Acta Neurochir (Wien). 140:377–386. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bogenrieder T and Herlyn M: Axis of evil:
molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar
|
|
4
|
Terstappen GC, Gaviraghi G and Caricasole
A: The Wnt signaling pathway as a target for the treatment of
neurodegenerative disorders. IDrugs. 9:35–38. 2006.PubMed/NCBI
|
|
5
|
Bienz M and Clevers H: Linking colorectal
cancer to Wnt signaling. Cell. 103:311–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collavin L and Kirschner MW: The secreted
Frizz1ed-related protein Sizzled functions as a negative feedback
regulator of extreme ventral mesoderm. Development. 130:805–816.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao B, Wu W, Li Y, et al:
LDL-receptor-related protein 6 is a receptor for Dickkopf proteins.
Nature. 411:321–325. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aravind L and Koonin EV: A colipase fold
in the carboxy-terminal domain of the Wnt antagonists - the
Dickkopfs. Curr Biol. 8:R477–R478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller A, Homey H, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001.PubMed/NCBI
|
|
10
|
Staun RE, Goldman S, Gabarin D, et al:
Expression and importance of matrix metalloproteinase 2 and 9
(MMP-2 and −9) in human trophoblast invasion. Reprod Biol
Endocrinol. 2:592004.
|
|
11
|
Staff AC, Ranheim T, Henriksen T, et al:
8-Iso-prostaglandin f(2alpha) reduces trophoblast invasion and
matrix metalloproteinase activity. Hypertension. 35:1307–1313.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fedi P, Bafico A, Nieto SA, et al:
Isolation and biochemical characterization of the human Dkk-1
homologue, a novel inhibitor of mammalian Wnt signaling. J Biol
Chem. 274:19465–19472. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bafico A, Liu G, Goldin L, Harris V and
Aaronson SA: An autocrine mechanism for constitutive Wnt pathway
activation in human cancer cells. Cancer Cell. 6:497–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maehata T, Taniguchi H, Yamamoto H, et al:
Transcriptional silencing of Dickkopf gene family by CpG island
hypermethylation in human gastrointestinal cancer. World J
Gastroenterol. 14:2702–2714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koppen A, Ait-Aissa R, Hopman S, et al:
Dickkopf-1 is down-regulated by MYCN and inhibits neuroblastoma
cell proliferation. Cancer Lett. 256:218–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pollheimer J, Loregger T, Sonderegger S,
et al: Activation of the canonical wingless/T-cell factor signaling
pathway promotes invasive differentiation of human trophoblast. Am
J Pathol. 168:1134–1147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grisaru-Granovsky S, Maoz M, Barzilay O,
et al: Protease activated receptor-1, PAR1, promotes placenta
trophoblast invasion and beta-catenin stabilization. J Cell
Physiol. 218:512–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao J, Wang J, Liu B, et al: Low-density
lipoprotein receptor-related protein-5 binds to Axin and regulates
the canonical Wnt signaling pathway. Mol Cell. 7:801–809. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
20
|
Crawford HC, Fingleton BM, Rudolph-Owen
LA, et al: The metalloproteinase matrilysin is a target of
beta-catenin transactivation in intestinal tumors. Oncogene.
18:2883–2891. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
23
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichikawa Y, Ishikawa T, Tanaka K, Togo S
and Shimada H: Extracellular matrix degradation enzymes: important
factors in liver metastasis of colorectal cancer and good targets
for anticancer metastatic therapy. Nihon Geka Gakkai Zasshi.
102:376–380. 2001.(In Japanese).
|
|
25
|
González-Sancho JM, Aguilera O, García JM,
et al: The Wnt antagonist DICKKOPF-1 gene is a downstream target of
beta-catenin/TCF and is downregulated in human colon cancer.
Oncogene. 24:1098–1103. 2005.PubMed/NCBI
|
|
26
|
Mao B and Niehrs C: Kremen2 modulates
Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 302:179–183.
2003. View Article : Google Scholar
|
|
27
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holen T, Amarzguioui M, Babaie E and Prydz
H: Similar behaviour of single-strand and double-strand siRNAs
suggests they act through a common RNAi pathway. Nucleic Acids Res.
31:2401–2407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi N, Liao QP, Li T and Xiong Y: Novel
expression profiles and invasiveness-related biology function of
DKK1 in endometrial carcinoma. Oncol Rep. 21:1421–1427.
2009.PubMed/NCBI
|
|
30
|
Yi N, Liao QP and Li T: Expression and
influence of Dickkopf1 on invasion ability in endometrial
carcinoma. Xian Dai Fu Chan Ke Jin Zhan Bian Ji Bu. 2:113–116.
2009.
|
|
31
|
Yi N, Liao QP, Xue XO and Liu M:
Expression of Dickkopf-1 in endometrial carcinoma and normal
endometrial tissues and its clinicopathological significance. Zhong
Guo Quan Ke Yi Xue Bian Ji Bu. 9:981–983. 2011.
|