Introduction
Prostate cancer is ranked second among
cancer-related mortalities in American male citizens (1). Radiotherapy, with or without endocrine
therapy, remains the preferred treatment for the majority of
patients with localized prostate cancer. The principle of
radiotherapy is to improve the dose of radioexposure in tumor
tissues. Generally, the external irradiation dose for conventional
radiotherapy is 65–70 Gy, while those of three-dimensional
conformal and intensity modulated radiation therapy are ~80 Gy
(2–4). Brachytherapy, a term used to describe
radiation treatment, exhibits a higher irradiation dose since the
radiation source is put in direct contact with the tumors. Previous
studies have communicated an equivalent treatment efficiency, as
well as reduced trauma and side effects, following brachytherapy
when compared with that of radical surgery and external beam
radiotherapy in patients with prostate cancer (5,6).
At present, low dose rate brachytherapy, for
example, permanent low dose irradiation via transplantation of
seeds, including 125I, is the preferred treatment for
low risk prostate cancer in a number of countries and the outcome
for moderate- and high-risk prostate cancer patients remains
satisfactory (7–9). However, adverse effects, including
bone marrow depression and migration via the blood circulation, are
frequently reported due to transplanted seeds remaining in
vivo permanently (10,11).
32P has been recognized as the ideal
therapeutic radionuclide for its unique characteristics, including
a pure β-particle emitter with a physical half-life of 14.3 days
and a maximum and average energy of 1.71 and 0.695 MeV,
respectively. A number of radioactive drugs, pharmaceuticals in the
form of colloid and microspheres, are hypothesized to represent
promising drugs for the treatment of solid tumors.
32P-chromic phosphate (32P-CP) colloid has
been applied for the treatment of intracavitary cancer (12) and its efficiency has been shown to
be satisfactory following interstitial injection (13). However, a number of studies have
indicated that toxicity of the liver, spleen and bone marrow may be
induced due to the transmigration of the colloid (14). In addition, 32P-CP
colloid has been identified as a comparatively safe and convenient
procedure for the treatment of refractory solid tumors (15–17),
however, solutions to the following obstacles remain to be
identified: i) enhancement of the local biological effects of
32P by increasing the dosage; ii) control of the
distribution of microspheres or colloid outside the tumor mass; and
iii) reduction or even elimination of toxicity and side effects. In
addition, the complexities associated with dose calculation and
clinical practice prevent the development of 32P-CP
colloid for clinical use. Thus, the identification of a novel
vector for the transportation of 32P radionuclide is
crucial for low dose rate brachytherapy.
Poly (L-lactic acid) (PLLA) has been widely used as
a drug delivery system due to its excellent biocompatibility and
biodegradability (18–20). It is a thermoplastic aliphatic
polyester derived from renewable resources and is capable of
biodegradation under specific conditions. In the present study,
32P-CP-PLLA microparticles were produced with these
characteristics and degraded continuously under specific
temperatures and humidities. In addition, a comparative study was
performed in nude mice with human prostate cancer to investigate
the differences in the pharmacokinetic profile and treatment
efficiency in 32P-CP colloid and 32P-CP-PLLA
groups.
Materials and methods
Cell culture
PC-3M human prostate cancer cells (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) were maintained in stationary
monolayer cultures at 37ºC, with 5% CO2 in a humidified
atmosphere, using Roswell Park Memorial Institute medium
supplemented with 10% heat-inactivated fetal bovine serum (Nanjing
KeyGen Biotech Co., Ltd.) and L-glutamine. A total of 90 healthy
male BALB/c nude mice (Shanghai Laboratory Animal Research Center,
Shanghai, China) at 4–6 weeks old and 18–22 g were maintained in
Streamline® cabinets (Streamline Laboratory Products,
Changi, Singapore) at 25–27ºC and a humidity of 40–50%.
Drug administration
32P-CP-PLLA was prepared as described
previously (21). Briefly, 100 mg
PLLA (0.1 μm in diameter) was added to 1 ml sterile
32P-CP colloid (radiochemical purity, >98%; Beijing
Atom High Tech, Beijing, China) and dehydrated alcohol was used as
a dispersant. The affinity between PLLA and the colloid was
modulated by surface-active agents. The mixture was treated by
ultrasonication for 30 min, kept at room temperature for 1 h and
dried in the drying vacuum oven at 60ºC. Pentobarbital (2%; 0.1 ml)
was injected via peritoneal injection following anesthesia. The
paracentesis needle was inserted into the center of the tumor along
the long axis, followed by injection with microparticles. The
radioactivity concentration was 0.39 GBq/ml (10.5 mCi/ml) and the
needle was removed once resistance was felt. Following dilution
with a physiological solution of sodium chloride, the intratumoral
injection activity was 7.4 MBq (0.05 ml) for the colloid.
Preparation of animal models
A subcutaneous inoculation of 2×106 PC-3M
cells on the right upper flank was performed to induce
tumorigenesis. Continuous measurements of tumor dimensions were
conducted using a caliper. Tumor volume was calculated by the
following formula: Tumor volume = length/2 × width2.
Animals were randomly divided into 32P-CP
colloid (7.4 MBq, intratumoral injection), 32P-CP-PLLA
(7.4 MBq, intratumoral injection) and control (equivalent volume of
saline) groups when tumor volume reached 8 mm in diameter. All
animal experiments were carried out according to national laws.
This study was approved by the ethics committee of Changzhou No. 3
People’s Hospital.
Single photon emission computed
tomography (SPECT) imaging
Bremsstrahlung scintigraphy of
32P-CP-PLLA and 32P-CP colloid distribution
was investigated using SPECT fitted with a low-energy,
general-purpose collimator (Siemens, Erlangen, Germany). The single
pinhole SPECT system was operated in a routine manner. In brief, a
cylinder with a diameter of 25 mm, designed to be tight fitting for
mice, was positioned directly and horizontally above the pinhole
aperture. A mechanical support allowed for the precise and manual
adjustment of the cylinder in two directions; the distance of the
cylinder to the pinhole aperture, which equals the radius of
rotation, and along the axis of the cylinder to select the field of
view. The pinhole collimator was connected to an ADAC ARC 3000
scintillation camera (Philips Co., Ltd., Shanghai, China) and had a
focal length of 320 mm and an opening angle of 60º. The energy
window was set at 78 keV, 30% width and 30 min harvest time. SPECT
images were captured at 1 and 12 h and 1, 2, 4 and 8 days
post-administration.
Treatment efficiency
Tumor volume was determined every two days following
treatment. The tumor inhibition rate was calculated on day 14 using
the following formula: tumor inhibition rate = (W1 – W2)/W1 × 100;
where W1 and W2 represent the average weight of tumor volume in the
control and treatment groups, respectively. For the calculation of
the tumor inhibition rate, 5 mice were sacrificed in each group for
conventional histological examination by formalin fixation and
paraffin embedding. Hematoxylin and eosin staining was performed
for the monitoring of sections.
TUNEL assay
Apoptosis was measured using a TUNEL assay kit
(Nanjing KeyGen Biotech Co., Ltd.) at 1, 6, 12, 24 and 72 h (each
group, n=3) following treatment. The procedures were performed
according to the manufacturer’s instructions. The apoptosis index
was calculated as the ratio of apoptotic cells to total cells.
Expression of caspase 3 and 8
Expression of caspase 3 and 8 was evaluated using
caspase 3 and caspase 8 Activity Assay kits according to the
manufacturer’s instructions (Nanjing KeyGen Biotech Co., Ltd.). The
Bio-Rad Microplate 550 Reader (Bio-Rad, Hercules, CA, USA) was used
to monitor the absorbance at 405 nm. The activities of caspase 3
and 8 were evaluated by the ratio of the optical density of the
inducer to the negative control.
Microvessel density
Slides stained with anti-CD34 monoclonal antibody
were examined via microscope. The cells that were positive for CD34
were designated as a vessel and initially observed at ×100
magnification to select the high density areas of microvessels.
Next, the microvessel density (MVD) of the CD34-positive cells was
investigated at ×200 magnification.
Radioactivity determination
A β-radioactivity meter was used to determine
radioactivity and final radioactivity was calculated as described
previously (22).
Statistical analysis
All data are presented as mean ± standard deviation.
Statistical analyses were performed using SPSS 13.0 Software (SPSS,
Inc., Chicago, IL, USA) and statistical significance was detected
by ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
Anorexia was identified in the control group and
skin ulcers were detected in the right lower extremities of 2 mice.
However, no anorexia or skin ulcers were observed in the treatment
groups and tumor growth was reduced significantly compared with the
control group. In the 32P-CP colloid group, irregular
and eccentric growth of the tumor mass and local recurrence was
identified in 1 mouse. No eccentric growth or recurrence was
observed in the 32P-CP-PLLA group.
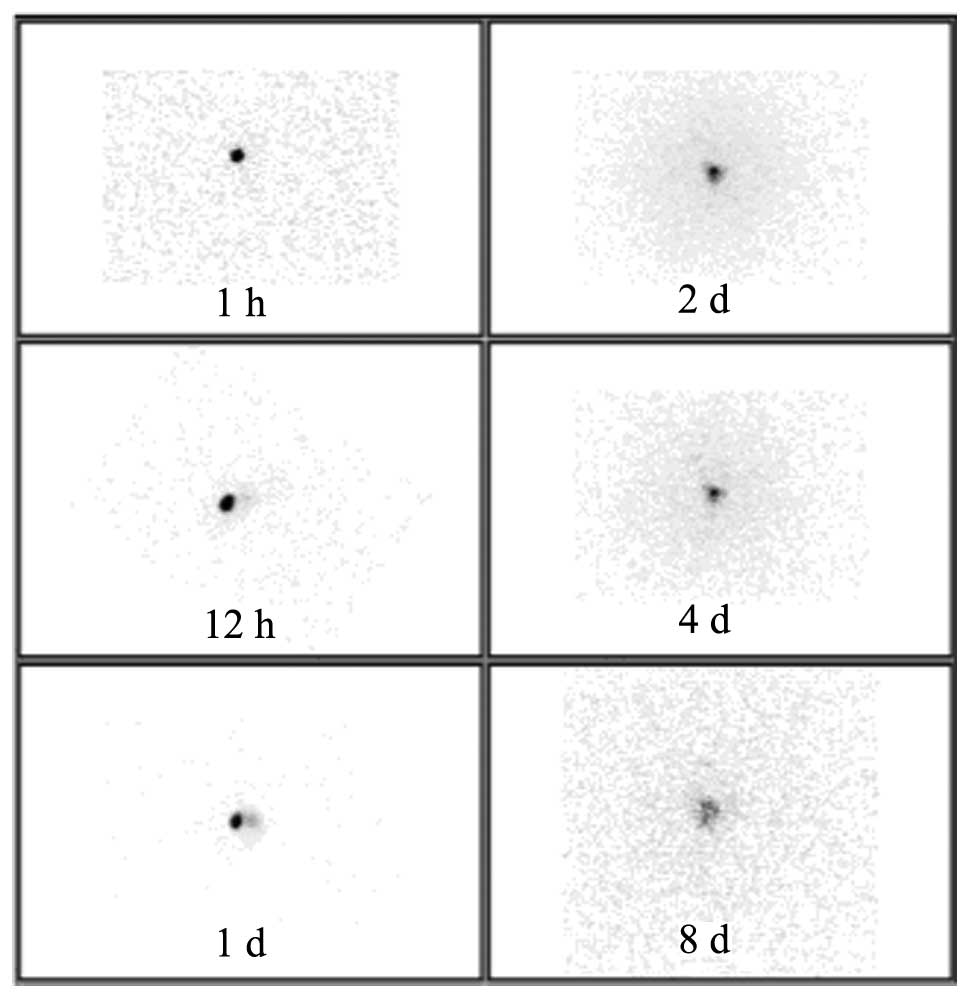
Following implantation of 32P-CP-PLLA
microparticles, biodistribution was detected by SPECT imaging.
During the initial stages, microparticles were concentrated at the
implantation sites, however, over time, a low radioactive uptake
(RAU) of 32P-CP-PLLA was identified in the tumor mass
compared with the baseline levels. In addition, no shifting or loss
of 32P-CP-PLLA was identified (Fig. 1). In the 32P-CP colloid
group, a sharp decrease of 32P-CP colloid was identified
following injection due to the absorption of the colloid by the
peripheral tissues. Thus, a significant increase of RAU was
identified in the peripheral tissues compared with the baseline
levels (Fig. 2).
Delayed growth of the tumor mass was observed in the
32P-CP-PLLA group compared with the
32P-CP-colloid and control groups (Fig. 3). Significant differences were
identified between the 32P-CP-PLLA and 32P-CP
colloid groups and the tumor volume, the tumor inhibition rate and
the ratio of necrocytosis on day 14 (P<0.01; Table I, Fig.
3). The residual activity on day 14 in the
32P-CP-PLLA and 32P-CP colloid groups was
3.02±0.32 and 1.76±0.31 MBq, respectively (Fig. 4).
 | Table IComparison of treatment efficiency in
the control and treatment groups on day 14. |
Table I
Comparison of treatment efficiency in
the control and treatment groups on day 14.
| Group | Radioactivity,
MBq | Tumor mass, g | Tumor-inhibition
rate, % | Necrosis in tumor
mass,% |
|---|
| Control | 0.0 | 1.62±0.21 | - | 4.92±4.25 |
| 32P-CP
colloid | 7.4 | 0.70±0.12a | 55.92±7.65 | 62.58±7.59a |
|
32P-CP-PLLA | 7.4 | 0.53±0.06a,b | 67.24±3.55b | 75.82±3.24a,b |
With regard to pathological detection, various
extents of necrosis were identified in the treatment groups. Tumor
tissues in the control group exhibited karyomegaly and a high
karyoplasmic ratio. Inflammatory cell infiltration was identified
surrounding the 32P-CP-PLLA during the early stages and
coagulation necrosis was observed at later stages. A significant
amount of necrosis was observed in the 32P-CP-PLLA group
compared with the 32P-CP-colloid group (Fig. 5).
The TUNEL assay indicated a large amount of
irregular apoptosis with nuclear debris and apoptotic bodies in the
treatment groups (Fig. 6). The rate
of apoptosis increased progressively with time compared with the
control group (P<0.01; Fig. 7).
In addition, significant differences in caspase expression were
identified in the treatment groups at 6, 12 and 24 h following
administration compared with the control group (P<0.01; Table II), however, no significant
differences were identified at 1 and 72 h.
 | Table IIExpression of caspase 3 and 8 at
specific time intervals. |
Table II
Expression of caspase 3 and 8 at
specific time intervals.
| Caspase 3 | Caspase 8 |
|---|
|
|
|
|---|
| Group | 1 h | 6 h | 12 h | 24 h | 72 h | 1 h | 6 h | 12 h | 24 h | 72 h |
|---|
| Control | 0.96±0.05 | 0.98±0.04 | 1.02±0.06 | 1.06±0.05 | 1.04±0.06 | 1.00±0.04 | 1.02±0.04 | 1.06±0.02 | 1.04±0.05 | 1.06±0.06 |
| 32P-CP
colloid | 1.05±0.12 | 2.56±0.19 | 2.05±0.15 | 1.32±0.11 | 0.91±0.09 | 1.08±0.09 | 2.27±0.21 | 1.45±0.18 | 1.08±0.07 | 1.03±0.07 |
|
32P-CP-PLLA | 1.06±0.08 | 1.53±0.13 | 2.21±0.14 | 1.37±0.11 | 0.93±0.12 | 1.06±0.13 | 1.58±0.21 | 2.12±0.22 | 1.07±0.14 | 1.02±0.05 |
A significant difference in MVD was identified among
the treatment and control groups (P<0.01) with MVD values of
60.71±8.21, 36.15±11.06 and 28.24±10.07 for the control, colloid
and microparticle groups, respectively (Fig. 8).
Discussion
Previously, 32P has been hypothesized to
represent the ideal radionuclide for brachytherapy. In the present
study, PLLA was used as the delivery vector for 32P-CP
to modulate the target orientation and safety of internal radiation
therapy. PLLA has excellent biodegradation and biocompatibility and
was approved as a pharmaceutical adjuvant by the FDA in 1997. PLLA
is now widely used as a drug delivery system (18).
In the present study, once the 32P-CP
colloid was administered by interstitial injection, it was
immediately absorbed by the tumor mass via the capillary vessels,
lymphatic system and tissue space and diffused into the peripheral
tissues. However, the distribution of 32P-CP colloid in
the tumor mass was not uniform due to certain differences,
including the morphous of the injection channel in the tumor mass,
colloid leakage from the injection orifice, tissue density and the
abundance of capillaries. When compared with 32P-CP
colloid, 32P-CP-PLLA with identical radioactivity
carried an equal amount of 32P-CP-colloid and was able
to reduce the amount of 32P-CP colloid passing through
the lymphatic system, capillaries and tissue space by gradual
disintegration. This resulted in improved drug retention and
uniform distribution of 32P-CP-PLLA in the tumor mass.
SPECT imaging identified that the microparticles were limited to
the tumor mass due to the low RAU of the peripheral tissue. A
marked decrease of 32P-CP-colloid was identified as the
colloid was absorbed by the peripheral tissues and the remaining
radioactive intensity of 32P-CP-PLLA was higher compared
with that of the 32P-CP colloid on day 14. Previously,
Yang et al reported that the biodistribution of
32P-CP-PLLA and 32P-CP colloid was 9.3 MBq in
Balb/c nude mice implanted with BxPC-3 human pancreatic tumors,
with concentrations of 32P-CP-PLLA and 32P-CP
colloid at 241.73±131.06 and 170.61±69.01% ID/g, respectively
(21). These observations indicated
that 32P-CP-PLLA shows higher retention effects and is
capable of enhancing the therapeutic effects while decreasing the
blind zone of radiotherapy in addition to 32P-CP
absorption.
It has been previously identified that cell
proliferation and apoptosis are markedly associated with prostate
cancer. In the present study, necrosis and apoptosis were
identified in the tumor mass of the treatment groups, indicating
that 32P-CP has the ability to kill tumor cells and
induce apoptosis by emitting β-rays. A similar radiation effect
(apoptosis rate) was identified between the 32P-CP
colloid and 32P-CP-PLLA groups, therefore, indicating
that 32P-CP-PLLA is likely to maintain its concentration
in the tumor mass using the PLLA delivery system.
A previous study reported that angiogenesis was
associated with lymph node metastasis, recurrence, remote
metastasis and mortality rates due to prostate cancer (23). Currently, CD34 antibody
immunohistochemistry is frequently applied as an indicator of MVD.
The present results showed that MVD in the treatment groups was
lower than that of the control group and that MVD values obtained
from the peripheral tissue were higher than those from the central
tumor mass. This demonstrated that the embolism and necrosis of
blood vessels were induced by β-rays emitted by 32P.
Lower MVD values were identified in the 32P-CP-PLLA
group compared with the colloid group indicating that the
microparticles may inhibit angiogenesis by improving the
radioactive dosage in the tumor mass. Gellman et al reported
that intimal proliferation is likely to be induced by a lower dose
of internal irradiation (24).
However, no intimal proliferation was identified in the present
treatment group, indicating that 32P-CP-PLLA may inhibit
the local recurrence of prostate cancer.
Two major pathways that have been identified as
associated with apoptosis are the extrinsic and intrinsic pathways
(death receptor and mitochondrial pathways, respectively). The
extrinsic pathway is activated by ligand-activated death receptors,
including Fas ligand (FasL). Caspase 8 functions as a significant
initiation factor for apoptosis, with caspase 3 as the concluding
indicator for apoptosis and the core molecule of the Fas/FasL
signaling pathway (24). The
results of the current study identified that the expression of
caspase 8 and 3 showed a gradual increase and reached peak values
at 6 and 12 h following treatment in the colloid and microparticle
groups, respectively. Caspase activity subsequently showed a marked
decrease due to cell necrosis and local radiation effects.
Therefore, this demonstrated that caspase 3 and 8 are involved in
apoptosis induced by β-radiation. In addition, peak levels of
caspase 3 and 8 were obtained later in the
32P-CP-colloid group compared with the
32P-CP-PLLA group (6 vs. 12 h). This is likely to be
associated with the temporarily high local dose in the
32P-CP colloid group during the early stages (~6–12h)
compared with the gradual degradation of
32P-CP-PLLA.
The present results showed that 32P-CP
colloid and 32P-CP-PLLA may kill tumor cells, induce
apoptosis and inhibit angiogenesis. However, the tumor volume was
reduced in the 32P-CP-PLLA group compared with the
32P-CP colloid group at 8 days post-treatment, and the
most significant difference was identified on day 14. This
demonstrated an enhanced retention time of 32P-CP-PLLA
at the target site, and an accumulated yield of radiation was
identified in the 32P-CP-PLLA group following the
gradual delivery of the radioactive source of equivalent
radioactivity levels. Therefore, this indicated that
32P-CP-PLLA functions via low dose and gradual delivery
of the radioactive source.
Overall, the results demonstrated that
32P-CP-PLLA had the following distinct characteristics:
i) enhanced metabolism of the drug at the target site and reduced
dose distribution outside the tumor mass by degradation and delayed
release of the drugs; ii) permanent stagnation and complications
were avoided due to an excellent degradation capacity of the
transplanted microparticles, under specific conditions; iii)
biological metabolism of 32P-CP-PLLA was regulated by
32P-CP-PLLA activity in vivo with specific
dosages convenient for individual therapy; and iv) as a polymer,
modification of the structure of 32P-CP-PLLA was
applicable and may aid the possible development of a delivery
system with increased efficiency, including the synergic
sensitization of chemotherapeutics.
With regard to the application of
32P-CP-PLLA microspheres in the clinical treatment of
prostate cancer, the microspheres may be precisely implanted into
prostate cancer lesions under the three-dimensional radiotherapy
planning system. In addition, as the microspheres are likely to be
degraded gradually over a specific period, repetitive implantation
is possible to prevent the lymph node metastases and
micrometastasis of prostate cancer. Therefore,
32P-CP-PLLA may decrease the recurrence of prostate
cancer and improve the survival rates of patients. In addition, the
range of β-array radiation emitted by 32P was ~4 μm and
thus it may cause slight or no damage to sexual function and
decrease the occurrence of urinary incontinence, urethral stenosis
and rectal complications. It is therefore hypothesized that
32P-CP-PLLA may represent an innovative method for the
treatment of prostate cancer.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 81071185).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar
|
|
2
|
Hummel S, Simpson EL, Hemingway P, et al:
Intensity-modulated radiotherapy for the treatment of prostate
cancer: a systematic review and economic evaluation. Health Technol
Assess. 14:1–108. iii–iv
|
|
3
|
Alicikus ZA, Yamada Y, Zhang Z, et al:
Ten-year outcomes of high-dose, intensity-modulated radiotherapy
for localized prostate cancer. Cancer. 117:1429–1437
|
|
4
|
Michalski JM, Winter K, Purdy JA, et al:
Toxicity after three-dimensional radiotherapy for prostate cancer
on RTOG 9406 dose Level V. Int J Radiat Oncol Biol Phys.
62:706–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zelefsky MJ, Kuban DA, Levy LB, et al:
Multi-institutional analysis of long-term outcome for stages T1–T2
prostate cancer treated with permanent seed implantation. Int J
Radiat Oncol Biol Phys. 67:327–333. 2007.PubMed/NCBI
|
|
6
|
Tward JD, Lee CM, Pappas LM, et al:
Survival of men with clinically localized prostate cancer treated
with prostatectomy, brachytherapy or no definitive treatment:
impact of age at diagnosis. Cancer. 107:2392–2400. 2006. View Article : Google Scholar
|
|
7
|
Chauveinc L, Osseili A, Flam T, et al:
Iodin 125 seed migration after prostate brachytherapy: a study of
170 patients. Cancer Radiother. 8:211–216. 2004.(In French).
|
|
8
|
Moosmayer D, Berndorff D, Chang CH, et al:
Bispecific antibody pretargeting of tumor neovasculature for
improved systemic radiotherapy of solid tumors. Clin Cancer Res.
12:5587–5595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kao J, Stone NN, Lavaf A, et al: (125)I
monotherapy using D90 implant doses of 180 Gy or greater. Int J
Radiat Oncol Biol Phys. 70:96–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taira AV, Merrick GS, Butler WM, et al:
Long-term outcome for clinically localized prostate cancer treated
with permanent interstitial brachytherapy. Int J Radiat Oncol Biol
Phys. 79:1336–1342. 2011.PubMed/NCBI
|
|
11
|
Stone NN, Potters L, Davis BJ, et al:
Multicenter analysis of effect of high biologic effective dose on
biochemical failure and survival outcomes in patients with Gleason
score 7–10 prostate cancer treated with permanent prostate
brachytherapy. Int J Radiat Oncol Biol Phys. 73:341–346.
2009.PubMed/NCBI
|
|
12
|
Mortazavi SM, Asadollahi S, Farzan M, et
al: (32)P colloid radiosynovectomy in treatment of chronic
haemophilic synovitis: Iran experience. Haemophilia. 13:182–188.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao W, Liu L, Liu ZY, et al: Intratumoral
injection of 32P-chromic phosphate in the treatment of implanted
pancreatic carcinoma. Cancer Biother Radiopharm. 25:215–224. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zubillaga MB, Boccio JR, Nicolini JO, et
al: Use of colloids of chromic [32P] phosphate in treatment of
solid tumors. Nucl Med Biol. 23:907–910. 1996.
|
|
15
|
Alimi KA, Firusian N and Dempke W: Effects
of intralesional 32-P chromic phosphate in refractory patients with
head and neck tumours. Anticancer Res. 27:2997–3000.
2007.PubMed/NCBI
|
|
16
|
Zubillaga MB, Boccio JR, Nicolini JO, et
al: Great particles [32P]chromic phosphate for treatment of solid
tumors. Acta Physiol Pharmacol Ther Latinoam. 46:103–110. 1996.
|
|
17
|
Zubillaga M, Boccio J, Nicolini J, et al:
Brachytherapy of solid tumors. Use of chromic phosphate colloid.
Acta Physiol Pharmacol Ther Latinoam. 47:179–185. 1997.(In
Spanish).
|
|
18
|
Kang Y, Yin G, Ouyang P, et al:
Preparation of PLLA/PLGA microparticles using solution enhanced
dispersion by supercritical fluids (SEDS). J Colloid Interface Sci.
322:87–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teupe C, Meffert R, Winckler S, et al:
Ciprofloxacin-impregnated poly-L-lactic acid drug carrier. New
aspects of a resorbable drug delivery system in local antimicrobial
treatment of bone infections. Arch Orthop Trauma Surg. 112:33–35.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pandey A and Aswath P: Indentation creep
reservoirs for drug-eluting poly(L-lactic acid) scaffolds. J
Biomater Sci Polym Ed. 22:1591–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang M, Xu Y, Pan D, et al: Bioevaluation
of a novel [32P]-CP-PLLA microparticle for pancreatic cancer
treatment. Drug Dev Res. 71:364–370. 2010.
|
|
22
|
Finucane CM, Murray I, Sosabowski JK, et
al: Quantitative accuracy of low-count SPECT imaging in phantom and
in vivo mouse studies. Int J Mol Imaging. 2011:1973812011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng L, Jones TD, Lin H, et al:
Lymphovascular invasion is an independent prognostic factor in
prostate adenocarcinoma. J Urol. 174:2181–2185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gellman G, Healey G and Qingsheng C: The
effect of very low dose irradiation on restenosis following balloon
angioplasty. A study in the atherosclerotic rabbit. Circulation.
84(Suppl II): 46A–59A. 1991.
|