Introduction
Human esophageal squamous cell cancer (ESCC) is one
of the most prevalent cancers in the world and ~250,000 ESCC cases
are diagnosed each year in China, accounting for half of the
world’s cases (1). However, the
optimal treatment for squamous cell carcinoma of the esophagus
remains unresolved. For the majority of ESCC cases, surgery remains
the first choice of therapy (2,3).
However, the results of surgery at the five-year follow-up period
remain poor, regardless of the type of surgical intervention
(4). Furthermore, a significant
proportion of patients are not eligible for surgery due to a delay
in the diagnosis of the tumor. Others may have an early-stage
cancer but are considered unsuitable for surgery due to comorbid
disease. Chemotherapy and external radiotherapy are suitable for
only a small proportion of patients. Consequently, photodynamic
therapy (PDT), a technique consisting of an application of a light
source following the prior administration of a photosensitizing
drug, which is able to induce necrosis of the targeted tissue
(5,6), may have a role in the management of
patients with esophageal cancer who pose a high surgical risk
(7). Photofrin®-mediated
PDT was first approved by the FDA in 1995 for the palliation of
symptoms and the reduction of obstruction in patients with
completely- or partially-obstructing esophageal cancer. However,
PDT is also associated with prolonged and occasionally severe
cutaneous phototoxicity in patients (8). This limitation has been the major
impetus behind the synthesis of new sensitizers with a higher
efficacy and lower phototoxicity.
As a second-generation chlorin-based compound,
2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH; Fig. 1), has shown favorable photophysical
and pharmacokinetic properties in preclinical studies (9,10).
HPPH has been reported to be an extremely hydrophobic compound that
is the most effective photosensitizer (PS) against murine tumors
amongst a series of homologues with various numbers of methylene
groups on the ether function (10).
The compound strongly absorbs light at 665 nm. Therefore, light
penetration into the tumor tissue is increased compared with
Photofrin (11). In addition, HPPH
may exhibit lower skin phototoxicity since it has been shown to be
rapidly cleared from the skin (12). Clinical phase I and II studies of
HPPH that were conducted in patients with Barrett’s esophagus and
obstructive esophageal carcinoma have indicated excellent response
rates (13). However, to the best
of our knowledge, no prior publication has investigated the effects
of HPPH-mediated PDT on human ESCC.
The present study aimed to investigate the efficacy
of HPPH in PDT of human esophageal squamous cancer cells (Eca109)
in vivo and in vitro, therefore providing additional
evidence for the study of HPPH-mediated PDT on human ESCC.
Materials and methods
PSs
Photofrin (sterile freeze-dried powder, 75 mg/vial)
was purchased from the Axcan Pharma Company (Birmingham, AL, USA)
and freshly prepared in 5% dextrose solution in the dark prior to
use. HPPH freeze-dried powder and HPPH vehicle, provided by
Zhejiang Hisun Pharmaceutical Co., Ltd. (Zhejiang, China) were
freshly diluted using sterile 0.9% normal saline (NS).
In vitro photosensitivity activity
The human Eca109 cell line was purchased from the
Shanghai Institute for Biological Sciences (Chinese Academy of
Sciences, Shanghai, China). The cells were maintained in RPMI-1640
medium (Gibco, Carlsbad, CA, USA) containing 10% FBS, 2.5 mg/ml
glucose, 0.11 mg/ml sodium pyruvate and 1% penicillin-streptomycin,
and cultured at 37°C in a humidified 5% CO2 incubator
(Thermoscientific, Waltham, MA, USA). The cells in the log phase of
growth at 70–90% confluency were inoculated in a 96-well
microplate. Following overnight incubation, the cells were divided
into a light exposure group and a no light exposure group. Each
group contained cells that were incubated with HPPH vehicle or
variable concentrations of HPPH or Photofrin. All groups were
incubated at 37°C for 24 h without exposure to any light. For the
exposure to light, the initial incubation media was replaced with
drug-free complete media prior to the light treatment and then
illuminated with light from an argon-pumped dye laser set at 665 nm
for HPPH or 630 nm for Photofrin at a fluence rate of 20
mW/cm2 for 2 J/cm2 (2.5 cm-diameter
illumination). Following PDT, the cells were cultured for a further
48 h at 37°C in the dark. For the cells that were not exposed to
light, following a replacement of the initial incubation media with
drug-free complete media, the cells were cultured for a further 48
h at 37°C in the dark. Following the 48-h incubation period, a cell
counting kit 8 (CCK8) assay was used to assess the phototoxicity of
HPPH to the cells. Briefly, 10 μl CCK8 solution (Dojindo
Laboratories, Kumamoto, Japan) was added to each well and the
96-well plate was continuously incubated at 37°C for 1 h. The OD
value for each well was read at a 450 nm wavelength to determine
the cell survival rate on a microplate reader (Epoch; Biotek,
Winooski, VT, USA). The assay was repeated three times. An
IC50 value was calculated using Origin 7.5 software
(OriginLab, Northampton, MA, USA).
In vivo photosensitizing activity
The six to eight-week-old BALB/c-nude mice were
provided by Guangdong Laboratory Animal Centre (Guangdong, China)
and housed under specific pathogen-free conditions throughout the
study, at 22–24°C in 50% humidity. This study was approved by the
ethics committee of Sun Yat-Sen University (Guangzhou, China). The
mice were inoculated subcutaneously under the right shoulder with
5×106 Eca109 cells in 200 μl serum-free medium. The
in vivo antitumor photosensitizing efficacy of HPPH-mediated
PDT was evaluated when the volumes of the tumors ranged between 100
and 300 mm3. The mice were injected intravenously with
0.9% sterile NS, which was used as a negative control, HPPH vehicle
(1 mg/kg of body weight; control), varying doses of HPPH (0.15,
0.3, 0.6 and 1 mg/kg of body weight) or Photofrin (10 mg/kg of body
weight; positive control). At 24 h post-injection and without
exposure to light, the mice were irradiated with a laser light from
an argon-pumped dye laser set at 665 nm for HPPH or 630 nm for
Photofrin. The treatment parameters consisted of a light spot of
1.6 cm diameter and a total light dose of 135 J/cm2
delivered at a fluence rate of 75 mW/cm2(14,15).
Following PDT, the tumor dimensions were measured using calipers
every four days. The tumor volume (TV) was calculated with the
following formula: TV = (L × W2) × 0.5, where L is the
longest axis of the tumor and W is the axis that is perpendicular
to L. The relative tumor volume (RTV) of each tumor was defined as
the ratio of the volume at a given time to the volume at the start
of treatment (16). The mean RTV
was calculated for each treatment group. The antitumor activity was
determined by calculating the tumor growth inhibition (TGI) value
using the following equation (16,17):
TGI (%) = T/C × 100, where T is the mean RTV of the treated tumors
at the end of the experiment (three weeks) and C is the mean RTV of
the control group. The xenograft tumors were excised and weighed
subsequent to the mice being humanely sacrificed at the end of the
experiment. The tumors were weighed and the weight inhibition value
was calculated using the following equation: Tumor weight
inhibition (%) = 1 − [mean tumor weight (experiment groups)/mean
tumor weight (HPPH vehicle group)] × 100.
The present study was performed according to the
document Guidance Suggestions for Caring for Laboratory Animals
produced by the Ministry of Science and Technology in 2006.
Toxicity following HPPH-mediated PDT
To evaluate the toxicity following HPPH-mediated
PDT, the body weights of the mice in each group were recorded every
four days subsequent to the treatments. Furthermore, the mortality
rate of the mice in each group was recorded daily over the
three-week treatment period and the percentage of lethality was
defined as the ratio of the total amount of dead animals at the end
of the experiment to the total amount of animals at the start of
the treatment.
Histology following HPPH-mediated
PDT
To gauge the pathological effects of HPPH-mediated
PDT, several animals were selected at random from each group and
sacrificed at the end of the experiment. The tumors were excised
and fixed in formaldehyde-mixing fixative for 24 h, then rehydrated
and embedded in paraffin. Representative sections of tumor were
stained using hematoxylin-eosin (HE). The results were observed
under ×40 or ×400 magnification using a light microscope.
Statistical analysis
The experimental data in each group are presented as
the mean ± SD. An analysis of the variance between groups was
performed with SPSS software (SPSS, Inc., Chicago, IL, USA) for
windows 11.5 using Student’s t-test or a one-way ANOVA. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of the incubation time of drugs
prior to and following light exposure on the phototoxicity of
HPPH-mediated PDT
An intracellular PS has been considered as a factor
to determine the efficacy of PDT. The incubation time of drugs
usually affects the intracellular uptake of the PS. Accordingly, in
order to evaluate the effect of the incubation time on the
phototoxicity of HPPH, the Eca109 cells were incubated with HPPH
for 4 h and 24 h, respectively, prior to the light exposure.
Subsequent to being exposed to light at a dose of 2
J/cm2 (2.5 cm-diameter illumination), the cells were
cultured in the dark for a further 24 h. The cell viability was
then determined using a CCK8 assay. As shown in Fig. 2A, the survival fraction of HPPH
following a 24-h incubation period was higher than that of a 4-h
incubation.
Incubation time following light exposure
may play a role in the phototoxicity of PS
To assess the effect of the incubation time
following light exposure on the phototoxicity of HPPH, the Eca109
cells were incubated with various doses of HPPH for 24 h. Following
light exposure, the cells were cultured for a further 24 h or 48 h
in the dark. As shown in Fig. 2B,
there was no significant difference in the phototoxicity of HPPH
between the 24-h and 48-h incubation periods following light
exposure.
Effect of the dose of the exposed light
on the phototoxicity of HPPH-mediated PDT
The dose of light energy and the rate of energy
delivery have been recognized as pivotal factors to determine the
biological consequences of PDT. To investigate the effect of light
intensity on the phototoxicity of HPPH in vitro, the cells
were illuminated with various light doses (0.1 J/cm2–4
J/cm2) at a fluence rate of 4 mW/cm2 or 20
mW/cm2 following a 24-h incubation period with 0.192
μg/ml HPPH in the dark. Following illumination, the cells were
cultured for a further 48 h in the dark and the cell viability was
assessed using a CCK8 assay. As shown in Fig. 3, the inhibition rate of
HPPH-mediated PDT was increased in a light dose-dependent manner.
However, there was no significant difference between the two dose
rates.
In vitro photosensitivity activity
In order to evaluate the efficacy of HPPH- and
Photofrin-mediated PDT in human ESCC cells, the Eca109 cells were
incubated with various concentrations of HPPH (0.005–1 μg/ml) or
Photofrin (0.5–15 μg/ml) for 24 h in the dark prior to being
exposed to a laser light at 665 nm or 630 nm (20 mW/cm2,
2 J/cm2). The cells were then cultured for 48 h and the
in vitro photosensitizing efficacy was determined using a
CCK8 assay. As shown in Fig. 4, the
cells with no light exposure displayed no significant toxicity with
up to 1 μg/ml HPPH and 15 μg/ml Photofrin, which is similar to the
results previously demonstrated in other kinds of ESCC cells
(26). However, subsequent to being
activated by light, HPPH and Photofrin were able to significantly
inhibit cell survival in a concentration-dependent manner. The
IC50 of HPPH-mediated PDT was 0.07 μg/ml, whereas the
IC50 of Photofrin was 3.94 μg/ml. Compared with
Photofrin, HPPH had a higher efficacy in the PDT of the Eca109
cells in vitro (Fig. 4).
In vivo photosensitizing activity
The in vivo photosensitizing efficacy was
determined in the BALB/c-nude mice that were transplanted with
Eca109 tumors. When the tumors reached 100–300 mm3, the
mice were injected with HPPH at various drug doses and exposed to
light (665 nm, 135 J/cm2, 75 mW/cm2) at 24 h
post-injection. Photofrin (10 mg/kg) was used as a positive control
and mice were exposed to light (630 nm, 135 J/cm2, 75
mW/cm2) at 24 h post-injection. The tumor growth was
monitored by measuring the TV every four days for three weeks and
the mean RTV was calculated for each treatment group. As shown in
Table I, HPPH was able to inhibit
the tumor growth in a dose-dependent manner. Doses of 0.6 mg/kg and
1 mg/kg HPPH-mediated PDT were highly effective in controlling the
tumor growth from day 5, which was similar to the Photofrin-PDT
dose of 10 mg/kg.
 | Table IRelative TV in mice following HPPH
and Photofrin®-mediated PDT over the course of the
experiment. |
Table I
Relative TV in mice following HPPH
and Photofrin®-mediated PDT over the course of the
experiment.
| | RTV following
treatment |
|---|
| |
|
|---|
| Drug | Dosage, mg/kg | Day 1 | Day 5 | Day 9 | Day 13 | Day 17 | Day 21 |
|---|
| NS | / | 1.51±0.33 | 2.09±0.43 | 2.91±0.56 | 3.65±0.51 | 4.47±0.61 | 5.50±0.64 |
| Vehicle | 1.00 | 1.60±0.51 | 2.11±0.74 | 2.99±0.94 | 4.11±0.91 | 4.86±1.18 | 5.92±1.56 |
| HPPH | 0.15 | 1.64±0.48 | 1.62±0.35ab | 1.97±0.36ab | 2.44±0.60ab | 3.59±1.37ab | 4.41±1.74ab |
| 0.30 | 1.29±0.43 | 0.49±0.19ac | 0.52±0.25ac | 0.69±0.30ac | 1.24±0.59abc | 1.79±1.00abc |
| 0.60 | 1.60±0.50 | 0.31±0.14ac | 0.29±0.14ac | 0.23±0.26ac | 0.30±0.37acd | 0.07±0.09acd |
| 1.00 | 1.41±0.38 | 0.29±0.12ac | 0.24±0.11ac | 0.19±0.11acd | 0.07±0.16acd | 0.08±0.15acd |
| Photofrin | 10.00 | 1.32±0.49 | 0.40±0.11a | 0.35±0.02a | 0.31±0.05a | 0.16±0.15a | 0.07±0.08a |
The PDT efficacy was estimated by the TGI at three
weeks post-treatment, which was calculated by the formula described
in the methodology section. The National Cancer Institute standard
for the minimal level of antitumor activity (TGI ≤42%) was adopted.
As shown by Table II, at day 21,
the TGI values of the NS and 0.15 mg/kg HPPH groups were 92.91 and
74.49% respectively, which was higher than the 42% minimal level.
The TGI values of the mice that were treated with HPPH (0.3 mg/kg,
0.6mg/kg or 1mg/kg) and Photofrin (10 mg/kg) were 30.24, 1.18, 1.35
and 1.18% respectively, which were also lower than the 42% minimal
level. This data demonstrated that a HPPH dose of 0.6–1 mg/kg has a
comparable PDT efficacy in vivo with that of 10 mg/kg
Photofrin, which is currently used in clinics.
 | Table IITGI following HPPH- and
Photofrin®-mediated PDT. |
Table II
TGI following HPPH- and
Photofrin®-mediated PDT.
| Drug | Dosage, mg/kg | TGI, % |
|---|
| NS | / | 92.91 |
| Vehicle | 1.00 | |
| HPPH | 0.15 | 74.49 |
| 0.30 | 30.24 |
| 0.60 | 1.18 |
| 1.00 | 1.35 |
| Photofrin | 10.00 | 1.18 |
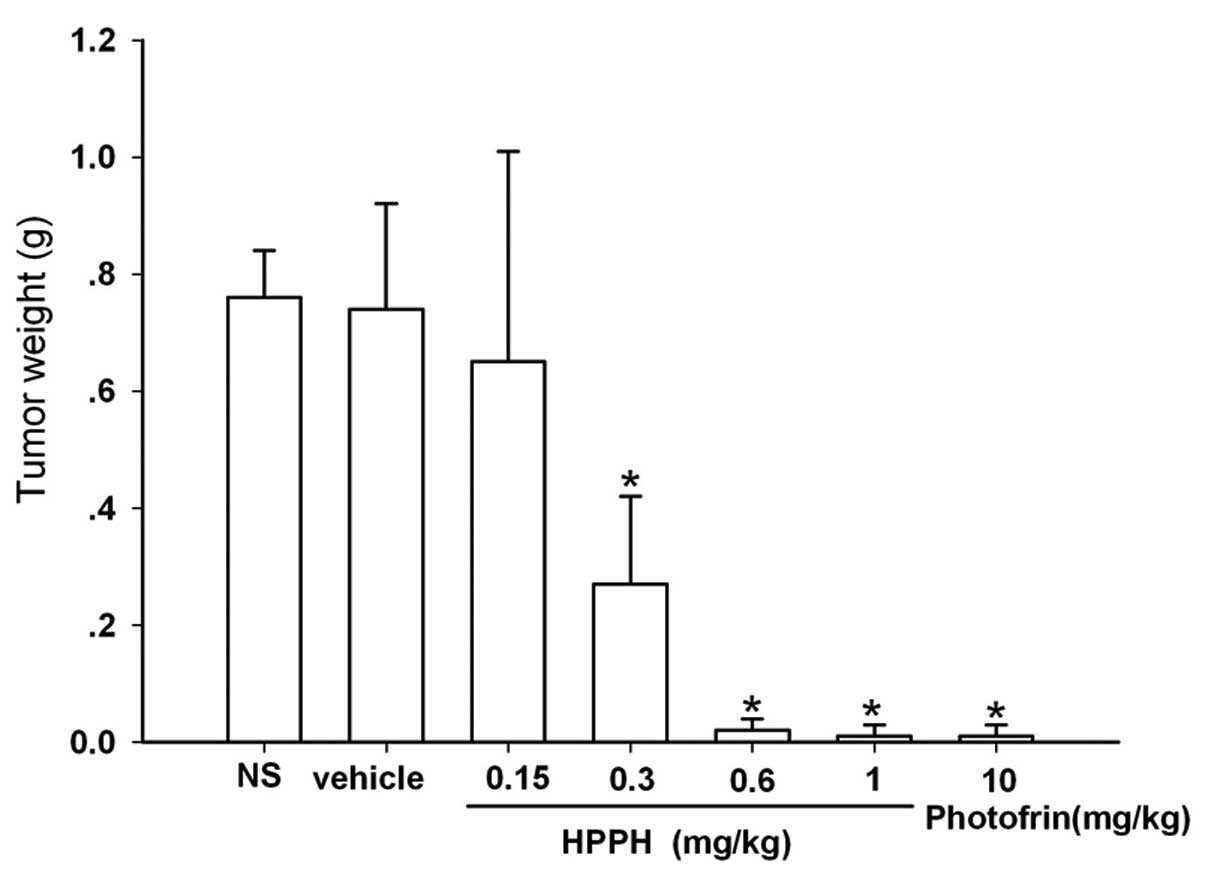
At the end of the treatment period, the tumor
weights of the mice treated with PDT and HPPH doses ranging from
0.3–1 mg/kg or 10 mg/kg Photofrin had remarkably decreased compared
with those of the vehicle group (Fig.
5). The inhibition rates of the tumor weights following 0.6
mg/kg and 1 mg/kg HPPH- and 10 mg/kg Photofrin-mediated PDT reached
97.3, 98.65 and 98.65% respectively (Table III). However, the tumor weights in
the mice following PDT with NS or 0.15 mg/kg HPPH did not show
significant differences compared with those of the vehicle group
(Fig. 5).
 | Table IIITumor weight inhibition following
HPPH- and Photofrin®-mediated PDT. |
Table III
Tumor weight inhibition following
HPPH- and Photofrin®-mediated PDT.
| Drug | Dosage, mg/kg | Tumor weight
inhibition, % |
|---|
| NS | / | −2.70 |
| Vehicle | 1.00 | |
| HPPH | 0.15 | 98.65 |
| 0.30 | 12.16 |
| 0.60 | 63.51 |
| 1.00 | 97.30 |
| Photofrin | 10.00 | 98.65 |
Effects of PDT with various doses of HPPH
on mouse lethality and body weight
The mean body weights of the mice decreased rapidly
following the administration of HPPH- and Photofrin-mediated PDT
(Table IV). In addition, as shown
in Table V, the percentage of
lethality induced by 0.15, 0.3 and 0.6 mg/kg HPPH-mediated PDT was
37.5%. Notably, high lethality was observed during the experimental
period in mice following 1 mg/kg HPPH-mediated PDT (62.5%) and 10
mg/kg Photofrin-PDT (50%; Table
V).
 | Table IVEffect of HPPH and
Photofrin®-mediated PDT on body weights of mice. |
Table IV
Effect of HPPH and
Photofrin®-mediated PDT on body weights of mice.
| | Body weight
following treatment |
|---|
| |
|
|---|
| Drug | Dosage, mg/kg | Day 1 | Day 5 | Day 9 | Day 13 | Day 17 | Day 21 |
|---|
| NS | / | 21.38±0.97 | 21.53±1.36 | 21.93±0.58 | 22.21±0.57 | 22.30±0.48 | 22.46±0.46 |
| Vehicle | 1.00 | 21.38±1.17 | 21.59±1.29 | 21.64±1.44 | 22.22±1.19 | 22.38±1.05 | 22.52±1.15 |
| HPPH | 0.15 | 20.88±0.81 | 19.03±1.22a | 18.27±0.75a | 17.90±0.63a | 17.50±0.58a | 16.65±1.09a |
| 0.30 | 20.76±1.16 | 20.86±1.20 | 20.78±1.15 | 20.45±1.22a | 19.85±1.19a | 19.37±1.09a |
| 0.60 | 21.01±0.51 | 20.69±0.73 | 20.15±0.86a | 19.54±0.92a | 18.99±0.81a | 18.62±0.79a |
| 1.00 | 21.18±0.48 | 20.91±0.62 | 20.33±0.68a | 20.14±0.98a | 18.78±0.61a | 18.30±0.50a |
| Photofrin | 10.00 | 20.08±1.02 | 18.91±1.06a | 18.10±0.56a | 17.60±0.47a | 17.34±0.89a | 16.83±0.17a |
 | Table VPercentage of lethality induced by
HPPH- and Photofrin®-mediated PDT. |
Table V
Percentage of lethality induced by
HPPH- and Photofrin®-mediated PDT.
| Drug | Dosage, mg/kg | Lethality, % |
|---|
| NS | / | 12.5 |
| Vehicle | 1.00 | 25.0 |
| HPPH | 0.15 | 37.5 |
| 0.30 | 37.5 |
| 0.60 | 37.5 |
| 1.00 | 62.5 |
| Photofrin | 10.00 | 50.0 |
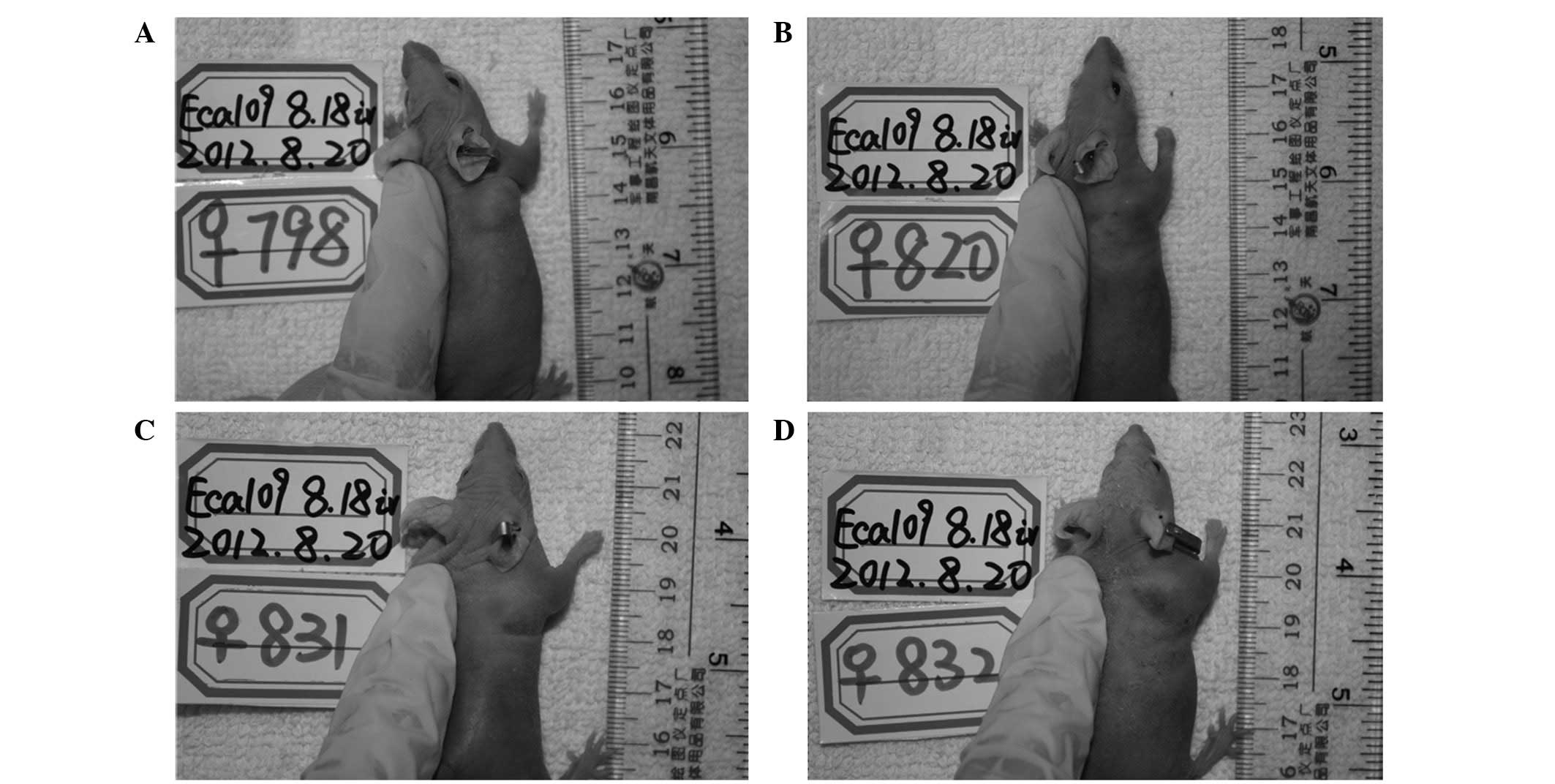
Gross and histological observation of
tumors following PDT
PDT was performed at 24 h post-PS administration and
the reactions of the tumor and mice following PDT were recorded. No
direct injuries, including capillary rupture or hemorrhagic
effusion, were observed at the surface of the tumor nodules or in
the vicinity over the three-week treatment period. In addition,
edema surrounded the tumors in the mice that were injected with PS,
including 0.3–1 mg/kg HPPH and 10 mg/kg Photofrin, at day 1
following PDT. However, there was no edema in the mice subsequent
to PDT with NS, vehicle and 0.15 mg/kg HPPH. In the mice with
subcutaneous edema, the degree was different. As shown in Table VI and Fig. 6, half of the mice exhibited severe
edema following PDT with 1 mg/kg HPPH or 10 mg/kg Photofrin, while
in the groups with PDT and 0.3 or 0.6 mg/kg HPPH, mice with slight
edema accounted for 50%.
 | Table VIEdema arounded the tumors at day 1
post-PDT. |
Table VI
Edema arounded the tumors at day 1
post-PDT.
| Drug | Dosage, mg/kg | No. of mice | Edema, % |
|---|
|
|---|
| None | Slight | Moderate | Severe |
|---|
| NS | / | 8 | 100.0 | | | |
| vehicle | 1.00 | 8 | 100.0 | | | |
| HPPH | 0.15 | 8 | 100.0 | | | |
| 0.3 | 8 | 25.0 | 50.0 | 25.0 | |
| 0.6 | 8 | 12.5 | 50.0 | 25.0 | 12.5 |
| 1.00 | 8 | | 25.0 | 25.0 | 50.0 |
|
Photofrin® | 10.00 | 8 | | 12.5 | 37.5 | 50.0 |
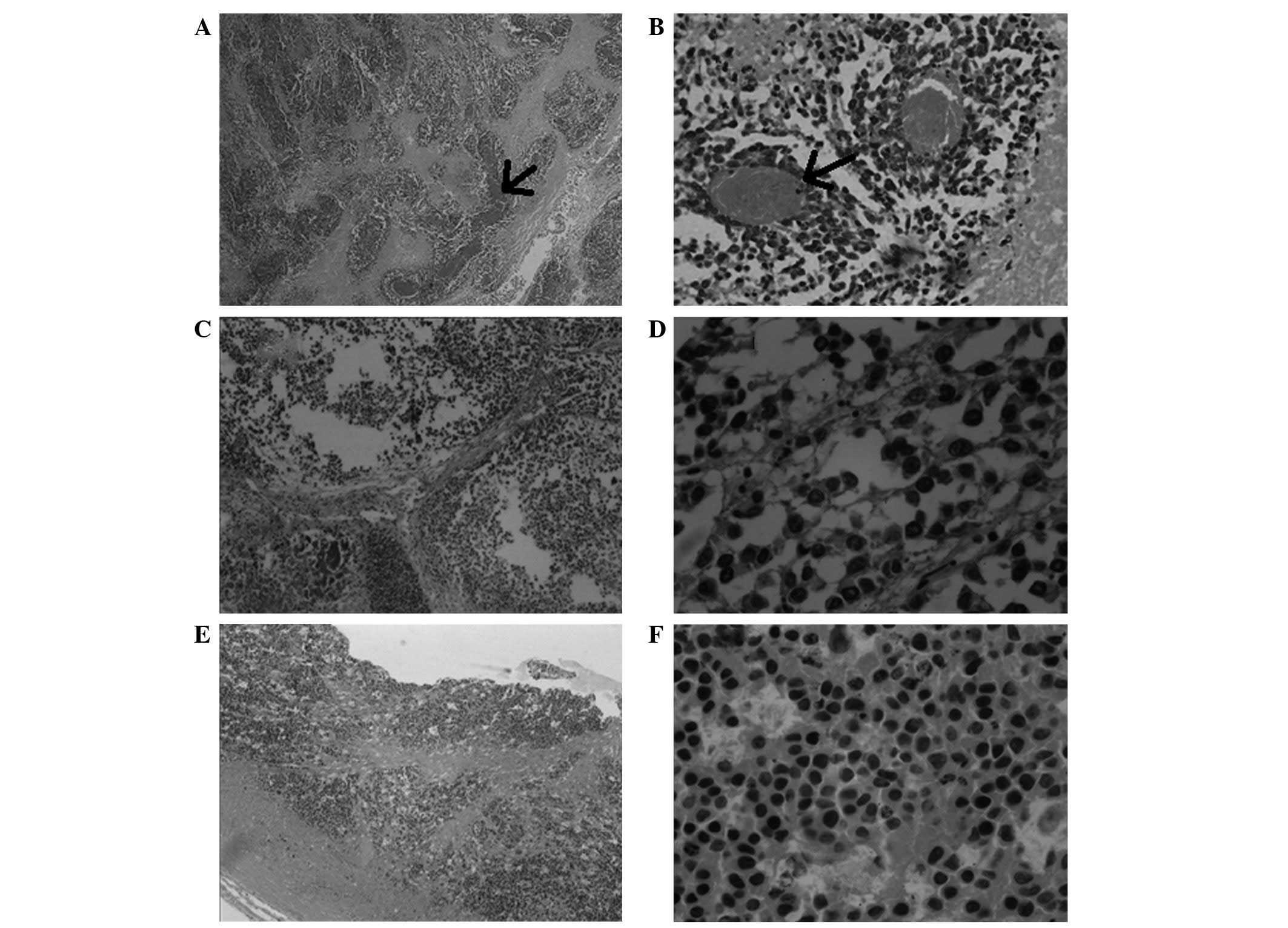
To evaluate the pathological effects of
HPPH-mediated PDT, several animals were selected at random from
each group and sacrificed at the end of the experiment. The tumor
tissue was removed from the mice and the tissue damage induced by
PDT was assessed histologically. The histopathological findings
indicated that the tumor tissues in the PS-treated mice
demonstrated varying degrees of necrosis, while the untreated tumor
tissues were filled with dense tumor cells, in which a basophilic
cytoplasm was observed (Fig. 7A and
B). Furthermore, as illustrated in Fig. 7C and D, less basophilic cytoplasm
was observed in the tumors that were treated with 0.15 or 0.3 mg/kg
HPPH. A low density of tumor cells and eosinophilic cytoplasm was
observed in the tumors following PDT with 0.6 or 1 mg/kg HPPH or 10
mg/kg Photofrin. The histopathological results also demonstrated
that tumor vessels (arrows, Fig. 7A and
B) were observed in the control and negative control groups
(mice injected with vehicle or NS). Tumor vessels were not observed
in the PS-treated tumor tissues (treated with 0.6 or 1 mg/kg HPPH
or 10 mg/kg Photofrin; Fig. 7E and
F).
Discussion
Surgery remains the first choice of therapy for
patients with ESCC, even though the five-year follow-up prognosis
remains poor. HPPH treatment, which has an increased penetration
and lower skin phototoxicity compared with Photofrin (11,12),
has been conducted in patients with Barrett’s esophagus and
obstructive esophageal carcinoma and has indicated excellent
response rates (13). However, the
effect of HPPH-mediated PDT on ESCC remains unknown. The present
study aimed to investigate the effects of HPPH-mediated PDT on the
survival of Eca109 cells and the growth of xenograft tumors derived
from Eca109 cells in mice.
Photofrin cellular concentrations have been reported
to decrease exponentially in certain cell lines on a time-dependent
basis (18), which may result in a
decreased cytotoxicity of PS. However, in another study, following
a 24-h incubation period, the intracellular concentrations of HPPH
were higher than after 4 h of incubation in colon26 cells (19). The present study revealed that the
survival fraction of HPPH-treated cells following 24 h of
incubation was higher than for 4 h of incubation (Fig. 2A), which may be attributed to the
increased uptake of HPPH.
Cells may be able to initiate a rescue response
and/or undergo cell death in an apoptotic or necrotic fashion
following photodynamic damage (5,20). The
present study showed that there was no significant difference in
the phototoxicity of HPPH between the 24-h and 48-h incubation
periods following light exposure (Fig.
2B), which may be due to the cells initiating a rescue
response, including (de)phosphorylation, changes in second
messengers, such as calcium and cAMP, and the activation of
proteins by proteases (21).
Several studies have demonstrated that high-fluence
rate PDT may lead to treatment-limiting deficits in the available
oxygen if the high rate of 1O2 generation
outpaces the resupply of O2(22,23).
In the present study, no significant differences were observed
between the cells that were exposed to light at doses of 4
mW/cm2 or 20 mW/cm2 (Fig. 3), indicating that HPPH-mediated PDT
at a fluence rate of 20 mW/cm2 may result in
treatment-limiting deficits in Eca109 cells.
Numerous lines of evidence have indicated that
HPPH-mediated PDT has shown significant cytotoxicity in
vitro and antitumor efficacy in a number of tumor xenograft
models (24–27). Furthermore, HPPH has been
demonstrated to possess greater cytotoxicity per drug dose than
Photofrin (24). In the present
study, HPPH and Photofrin-mediated PDT were observed to exhibit a
high cytotoxicity in vitro and antitumor efficacy in
vivo in a concentration-dependent manner (Tables I–III; Fig.
4). In addition, HPPH-mediated PDT had a higher efficacy than
Photofrin-mediated PDT in the Eca109 cells and the xenografted
Eca109 solid tumors. The differences in efficacy between HPPH and
Photofrin are almost certainly due to the differences in their
molar extinction coefficients (~3,000 M/cm for Photofrin at 630 nm;
and ~45,000 M/cm for HPPH at 665 nm).
Lethal toxicity induced by various PSs has been
documented as early as 1911 (28),
and systemic toxicity has been reported following whole body and
abdominal light exposure of porphyrin PDT in mice (29). The mechanism of lethality induced by
PDT is consistent with traumatic shock syndrome. Endogenous
vasoactive mediators of shock include the prostaglandins, the
thromboxanes and histamine (28).
In the present study, high lethality rates of 62.5 and 50% were
observed in the mice that were treated with 1 mg/kg HPPH and 10
mg/kg Photofrin, respectivelym which were lower than that
previously reported in several other animal models, which were
treated with 0.5 mg/kg HPPH (28).
A decrease in the body weights of the mice in the present study was
observed during the experimental period (Tables IV and V), indicating that HPPH possessed lower
toxicity than Photofrin at the dose that achieved the same efficacy
in the mice bearing the Eca109 subcutaneous tumors. A lower
lethality was observed in mice following PDT with 0.15, 0.3 and 0.6
mg/kg HPPH over the course of the treatment. This lethality was
likely to be tumor-related rather than drug-related, since the
control mice exhibited a similar lethality rate (25%; Table V).
PDT efficacy is accompanied by the presence of edema
following laser illumination in clinical use, and treated tissues
become edematous, followed by the presence of degenerative and
necrotic changes. A previous study revealed that the efficacy of
HPPH-mediated PDT was accompanied by the presence of mucosal edema
within 24 h of laser illumination in carcinogen-induced tumors of
the hamster buccal cheek pouch (25). The present study identified that the
severity of the edema in the mice after light illumination was
dose-dependent. Half of the mice exhibited severe edema following
PDT with 1 mg/kg HPPH or 10 mg/kg Photofrin, while half of the mice
in the 0.3 and 0.6 mg/kg HPPH-treated groups exhibited slight edema
(Table VI; Fig. 6).
Photofrin-mediated PDT-induced tumor tissue damage
has been characterized by cell degeneration, with little sign of
vascular damage necrosis, edema or severe hemorrhage (30). The histopathological findings in the
present study indicated that the tumor tissues in the PS-treated
mice demonstrated varying degrees of necrosis (Fig. 7). In addition, a large body of
studies have suggested that vascular damage plays a pivotal role in
governing the tumor response to PDT in mouse models (31,32). A
pioneering study revealed that HPPH-mediated PDT induced an
increase in tumor vascular permeability (33). In the present study, the results of
the histopathological examination revealed that HPPH and Photofrin
exhibited vascular cytotoxicity on the treated tumors (Fig. 7E and F), indicating that vascular
damage induced by HPPH-mediated PDT may be a key factor in
controlling tumor growth.
In conclusion, the present study demonstrated that
the phototoxicity of HPPH-mediated PDT was higher than
Photofrin-mediated PDT at the same concentration (dose) in
vivo and in vitro, and that HPPH possessed lower
toxicity than Photofrin at the dose that achieved the same
efficacy. Thereby, HPPH may be a promising agent for the treatment
of human ESCC.
Acknowledgements
This study was supported by the Department of
Science and Technology of Xinjiang Uygur Autonomous Regions (No.
201233150 ) for the construction of the technique plate for
evaluation of the pharmacodynamics of new drugs in Xinjiang Medical
University.
References
|
1
|
Song C, Xing D, Tan W, Wei Q and Lin D:
Methylenetetrahydrofolate reductase polymorphisms increase risk of
esophageal squamous cell carcinoma in a Chinese population. Cancer
Res. 61:3272–3275. 2001.PubMed/NCBI
|
|
2
|
Fink U, Stein HJ and Siewert JR:
Multimodal therapy of tumors of the upper gastrointestinal tract.
Chirurg. 69:349–359. 1998.(In German).
|
|
3
|
Siewert JR and Hölscher AH: Current
strategy in surgery for esophageal cancer. Ann Ital Chir. 63:13–18.
1992.PubMed/NCBI
|
|
4
|
Li SY, Sun XC and Liu L: Progress of
medical and combined treatment for esophageal carcinoma. Ai Zheng.
25:509–515. 2006.(In Chinese).
|
|
5
|
Dougherty TJ, Gomer CJ, Henderson BW, Jori
G, Kessel D, Korbelik M, Moan J and Peng Q: Photodynamic therapy. J
Natl Cancer Inst. 90:889–905. 1998. View Article : Google Scholar
|
|
6
|
Webber J, Herman M, Kessel D and Fromm D:
Current concepts in gastrointestinal photodynamic therapy. Ann
Surg. 230:12–23. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sibille A, Lambert R, Souquet JC, Sabben G
and Descos F: Long-term survival after photodynamic therapy for
esophageal cancer. Gastroenterology. 108:337–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allison RR, Downie GH, Cuenca R, Hu XH,
Childs Carter JH and Sibata Claudio H: Photosensitizers in clinical
PDT. Photodiagn Photodyn Ther. 1:27–42. 2004. View Article : Google Scholar
|
|
9
|
Pandey RK, Bellnier DA, Smith KM and
Dougherty TJ: Chlorin and porphyrin derivatives as potential
photosensitizers in photodynamic therapy. Photochem Photobiol.
53:65–72. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henderson BW, Bellnier DA, Greco WR,
Sharma A, Pandey RK, Vaughan LA, Weishaupt KR and Dougherty TJ: An
in vivo quantitative structure-activity relationship for a
congeneric series of pyropheophorbide derivatives as
photosensitizers for photodynamic therapy. Cancer Res.
57:4000–4007. 1997.
|
|
11
|
Lee LK, Whitehurst C, Pantelides ML and
Moore JV: In situ comparison of 665 nm and 633 nm wavelength light
penetration in the human prostate gland. Photochem Photobiol.
62:882–886. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellnier DA, Henderson BW, Pandey RK,
Potter WR and Dougherty TJ: Murine pharmacokinetics and antitumor
efficacy of the photodynamic sensitizer
2-T1-hexyloxyethyl]-2-devinyl pyropheophorbide-a. J Photochem
Photobiol B. 20:55–61. 1993.PubMed/NCBI
|
|
13
|
Bellnier DA, Greco WR, Loewen GM, Nava H,
Oseroff AR, Pandey RK, Tsuchida T and Dougherty TJ: Population
pharmacokinetics of the photodynamic therapy agent
2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer
patients. Cancer Res. 63:1806–1813. 2003.
|
|
14
|
Srivatsan A, Ethirajan M, Pandey SK, Dubey
S, Zheng X, Liu TH, Shibata M, Missert J, Morgan J and Pandey RK:
Conjugation of cRGD peptide to chlorophyll a based photosensitizer
(HPPH) alters its pharmacokinetics with enhanced tumor-imaging and
photosensitizing (PDT) efficacy. Mol Pharm. 8:1186–1197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fingar VH, Wieman TJ and Haydon PS: The
effects of thrombocytopenia on vessel stasis and macromolecular
leakage after photodynamic therapy using photofrin. Photochem
Photobiol. 66:513–517. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sancéau J, Poupon MF, Delattre O,
Sastre-Garau X and Wietzerbin J: Strong inhibition of Ewing tumor
xenograft growth by combination of human interferon-alpha or
interferon-beta with ifosfamide. Oncogene. 21:7700–7709. 2002.
|
|
17
|
Bissery MC, Guenard D, Guéritte-Voegelein
F and Lavelle F: Experimental antitumor activity of taxotere (RP
56976, NSC 628503), a taxol analogue. Cancer Res. 51:4845–4852.
1991.PubMed/NCBI
|
|
18
|
Hajri A, Wack S, Meyer C, Smith MK,
Leberquier C, Kedinger M and Aprahamian M: In vitro and in vivo
efficacy of photofrint and pheophorbide a, a bacteriochlorin, in
photodynamic therapy of colonic cancer cells. Photochem Photobiol.
75:140–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng X, Morgan J, Pandey SK, Chen Y,
Tracy E, Baumann H, Missert JR, Batt C, Jackson J, Bellnier DA,
Henderson BW and Pandey RK: Conjugation of
2-(1′-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to
carbohydrates changes its subcellular distribution and enhances
photodynamic activity in vivo. J Med Chem. 52:4306–4318. 2009.
|
|
20
|
Gomer CJ, Ferrario A, Hayashi N, Rucker N,
Szirth BC and Murphree AL: Molecular, cellular, and tissue
responses following photodynamic therapy. Lasers Surg Med.
8:450–463. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moor AC: Signaling pathways in cell death
and survival after photodynamic therapy. J Photochem Photobiol B.
57:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foster TH, Murant RS, Bryant RG, Knox RS,
Gibson SL and Hilf R: Oxygen consumption and diffusion effects in
photodynamic therapy. Radiat Res. 126:296–303. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang KK, Mitra S and Foster TH: A
comprehensive mathematical model of microscopic dose deposition in
photodynamic therapy. Med Phys. 34:282–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furukawa K, Yamamoto H, Crean DH, Kato H
and Mang TS: Localization and treatment of transformed tissues
using the photodynamic sensitizer 2-[1-hexyloxyethyl]-2-devinyl
pyropheophorbide-a. Laser Surg Med. 18:157–166. 1996.PubMed/NCBI
|
|
25
|
Kawazoe K, Isomoto H, Yamaguchi N, Inoue
N, Uehara R, Matsushima K, Ichikawa T, Takeshima F, Nonaka T,
Nanashima A, Nagayasu T, Uehara M, Asahina I and Nakao K: Effects
of photodynamic therapy for superficial esophageal squamous cell
carcinoma in vivo and in vitro. Oncol Lett. 1:877–882.
2010.PubMed/NCBI
|
|
26
|
Yang PW, Hung MC, Hsieh CY, Tung EC, Wang
YH, Tsai JC and Lee JM: The effects of Photofrin-mediated
photodynamic therapy on the modulation of EGFR in esophageal
squamous cell carcinoma cells. Laser Med Sci. 28:605–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lobel J, MacDonald IJ, Ciesielski MJ,
Barone T, Potter WR, Pollina J, Plunkett RJ, Fenstermaker RA and
Dougherty TJ: 2-[1-Hexyloxyethyl]-2-Devinyl Pyropheophorbide-a
(HPPH) in a nude rat glioma model: Implications for photodynamic
therapy. Laser Surg Med. 29:397–405. 2001.
|
|
28
|
Ferrario A and Gomer CJ: Systemic toxicity
in mice induced by localized porphyrin photodynamic therapy. Cancer
Res. 50:539–543. 1990.PubMed/NCBI
|
|
29
|
Dougherty TJ: Photosensitizers: therapy
and detection of malignant tumors. Photochem Photobiol. 45:879–889.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang Z, Chen Q, Shakil A, Chen H, Beckers
J, Shapiro H and Hetzel FW: Hyperoxygenation enhances the tumor
cell killing of photofrin-mediated photodynamic therapy. Photochem
Photobiol. 78:496–502. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Henderson BW, Waldow SM, Mang TS, Potter
WR, Malone PB and Dougherty TJ: Tumor destruction and kinetics of
tumor cell death in two experimental mouse tumors following
photodynamic therapy. Cancer Res. 45:572–576. 1985.PubMed/NCBI
|
|
32
|
Henderson BW and Fingar VH: Oxygen
limitation of direct tumor cell kill during photodynamic treatment
of a murine tumor model. Photochem Photobiol. 49:299–304. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Snyder JW, Greco WR, Bellnier DA, Vaughan
L and Henderson BW: Photodynamic therapy: a means to enhanced drug
delivery to tumors. Cancer Res. 63:8126–8131. 2003.PubMed/NCBI
|