Introduction
Pituitary adenomas are common intracranial tumors
with a rising incidence in China (1). However, the mechanism of occurrence
remains unclear. The alteration of p53 gene function has been shown
to be closely associated with tumorigenesis, tumor progression and
prognosis. p53 gene mutation plays a significant role in malignant
tumors, although the incidence of p53 gene mutation is low in
pituitary adenomas. Therefore, a functional alteration in the
wild-type p53 gene may play a more significant role in pituitary
adenomas. Nucleostemin (2) and
apoptosis-stimulating of p53 protein 2 (ASPP2) (3,4) are
two newly-identified genes that have been shown to correlate with
the regulation of p53 gene function. However, in pituitary adenoma,
the expression of the nucleostemin and ASPP2 genes and their role
in tumor cell proliferation remains unknown. The present study
aimed to investigate the expression of nucleostemin, ASPP2 and
Ki-67 in pituitary adenomas and lay a foundation for the further
research of pituitary tumor occurrence and progression.
Materials and methods
Tissue samples
A total of 71 cases of pituitary adenoma resection
specimens were collected between January 2004 and August 2005.
Associated clinical data were also collected for the present study.
The tissues were collected anonymously and this procedure was
exempted from the consent requirement. The age range of the
patients was 16–72 years (mean, 42.54 years). Among the total
cases, 33 were male was and 38 were female at a ratio of 0.87:1.
The shortest course of the disease was 15 days and the longest was
18 years (mean, 39.58 months). The tumor diameter ranged between
0.8 and 7 cm (mean diameter, 2.61 cm). Among the total cases, eight
were micro adenomas (11.27%), 50 were large adenomas (70.42%) and
13 were huge adenomas (18.31%). Of the 71 patients, 64 cases were
followed up and seven were lost. The rate of follow-up was 90.1%.
The range of follow-up duration was 8–26 months. Five cases of
recurrence were observed during the follow-up period.
The invasive characteristics of the tumor were
judged according to the Wilson modified Hardy classification
(5). Among the total 71 cases,
invasive pituitary adenomas were identified in 36 cases (50.70%)
and non-invasive pituitary adenomas in 35 cases (49.30%).
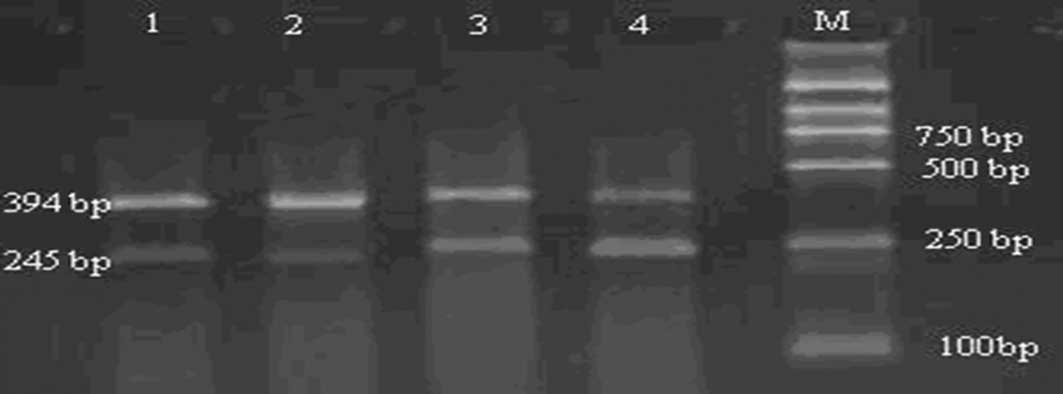
Semi-quantitative PCR
The nucleostemin and ASPP2 mRNA expression levels
were determined using semi-quantitative PCR. The PCR primer was
designed according to the reported gene sequence of nucleostemin,
ASPP2 mRNA and β-actin in GenBank (Table I). Due to the of low amplification
of ASPP2, nest PCR (N-PCR) was used in order to enhance the
sensitivity and specificity of detection.
 | Table IPrimer sequence and amplification
fragment length of nucleostemin and ASPP2 for PCR. |
Table I
Primer sequence and amplification
fragment length of nucleostemin and ASPP2 for PCR.
| Gene | Primer sequence | Product length,
bp | GeneBank no. |
|---|
| Nucleostemin |
| Upstream |
GAAGCCTCCGATGTTGTCCT | 245 | NM014366 |
| Downstream |
CACACGCTTGGTTATCTTCCC | | |
| β-actin
(nucleostemin) |
| Upstream |
CCCAGAGCAAGAGAGGCATCC | 394 | NM001101 |
| Downstream |
AGGTAGTCAGTCAGGTCCCG | | |
| ASPP2 |
| R1 |
TGCCGATGTTTCTTACCGTG | 310 | NM001031685 |
| R2 |
CGAGAGTGATTGCCATTTGG | | |
| R3 |
AATCTGTTGCTGCTGGCGAG | | |
| R4 |
ACTTGTTGCTGTTGTCGCTG | | |
| β-actin (ASPP2) |
| β1 |
GAGACCTTCAACACCCCAGC | 694 | NM001101 |
| β2 |
CCCAGAGCAAGAGAGGCATCC | | |
| β3 |
ACATCTGCTGGAAGGTGGAC | | |
Methods of qPCR
i) Total RNA extraction. The total RNA was extracted
following cell lysis using TRIzol, and the RNA quality was
determined; ii) cDNA compounding. The volume of the reverse
transcription reaction system was 30 μl, and the reaction
conditions were 42°C for 60 min and 95°C for 5 min. The final cDNA
product was conserved at 4°C; and iii) PCR. The compounded cDNA was
used as a template for PCR. Gene fragments of nucleostemin and
ASPP2 were achieved, separately. The PCR amplification product (6
μl) was used to perform 2% agarose gel electrophoresis and the
optical density (OD) was calculated for nucleostemin, ASPP2 and
β-actin. Finally, a ratio between the three was obtained.
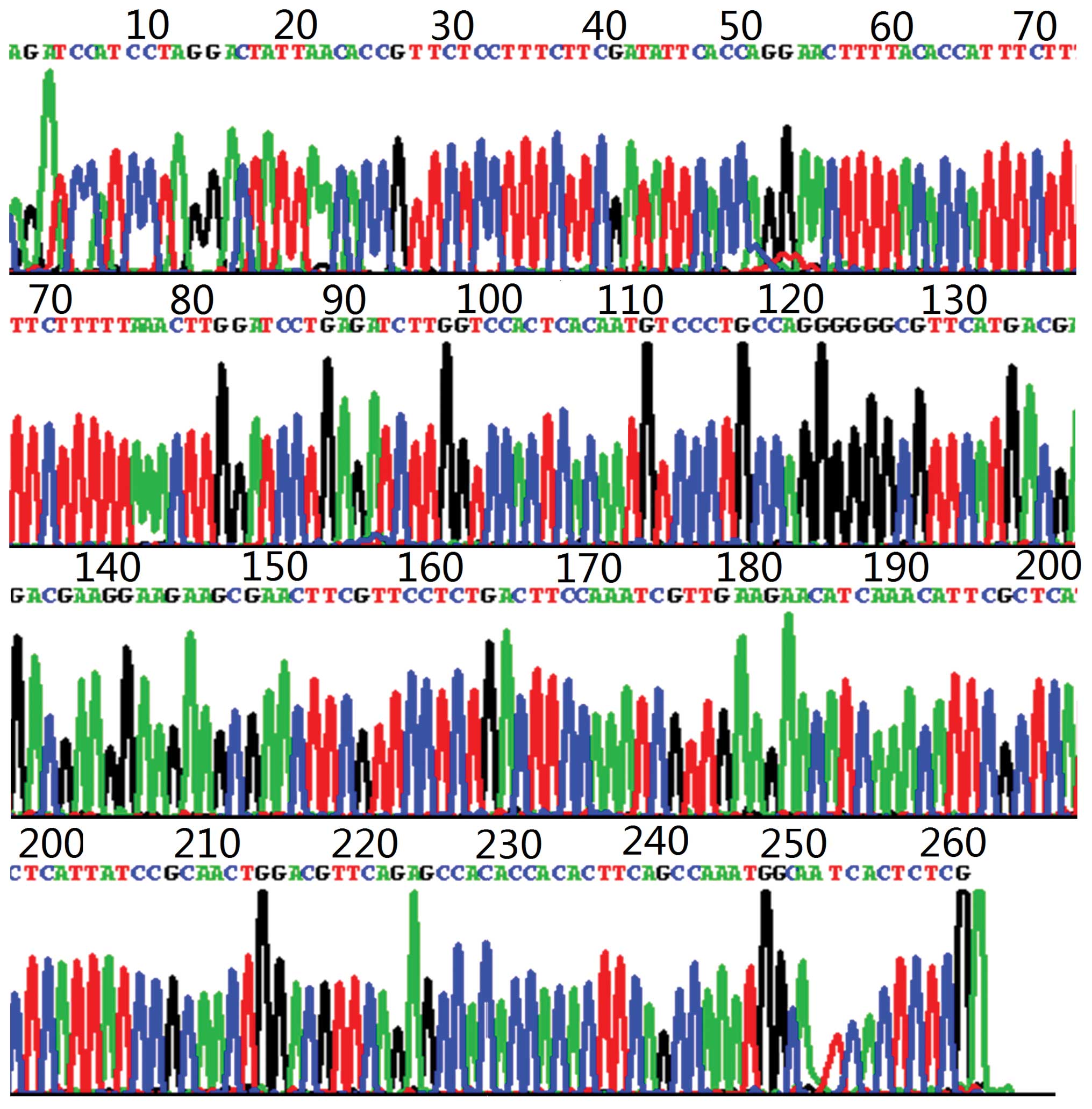
Sequencing
Subsequent to separating and purifying the
amplification banding, sequencing was performed to verify the
sequence. The aforementioned experiment was performed by Shanghai
Sheng-Gong Biological Engineering Technology Service Co., Ltd.
(Shanghai, China) and analyzed using Blast software (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence
of the amplification fragment was confirmed to correspond to the
target gene completely (Figs. 1 and
2).
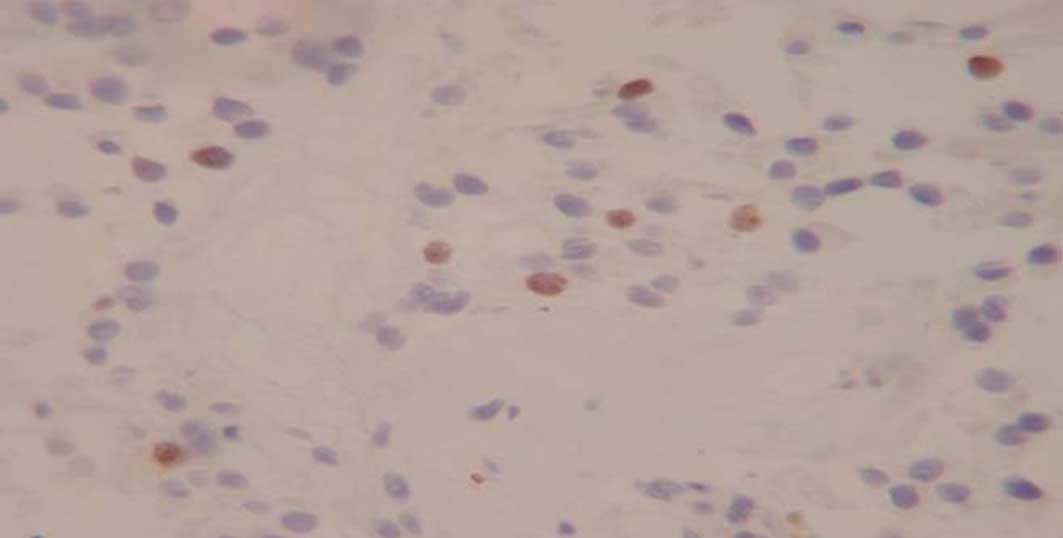
Immunohistochemistry for detecting Ki-67
expression
MIB-1 monoclonal antibody immunohistochemical
staining was used to detect Ki-67 expression. The primary antibody
was rat anti-human Ki-67 monoclonal antibody (1:100), the secondary
antibody was goat anti-rat IgG (1:100) and the tertiary antibody
was horseradish peroxidase marker chain mildew avidin. The reagents
were all obtained from Beijing Zhongshan Biological Technology Co.,
Ltd., (Beijing, China).
The results were defined as positive or negative
according to whether Ki-67 staining was observed in the nucleus. On
each slide, five high-power fields were selected randomly. The
numbers of total and positive cells were counted and the percentage
of positive cells from the total cells was calculated as the Ki-67
mark index. The calculation formula was as follows: Number of
positive cells/total cells × 100.
Statistical analysis
The results are presented as the means ± standard
deviation. The experimental data were analyzed and processed using
SPSS 11.5 statistical software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
ASPP2 mRNA and Ki-67 expression in human
pituitary adenomas
Nucleostemin, ASPP2 mRNA and Ki-67 expression was
observed in all the 71 cases of pituitary adenomas (Figs. 3–5).
The statistical results revealed that a positive correlation
existed between the nucleostemin mRNA levels and Ki-67 (r=0.237;
P<0.05). A negative correlation was observed between the ASPP2
mRNA expression levels and Ki-67 (r=−0.256; P<0.05), and a
negative correlation was observed between nucleostemin and ASPP2
mRNA levels (r=−0.340; P<0.01).
Comparison of the indicators in various
pathological pituitary adenomas
The pathological pattern was classified according to
the immunohistochemical staining of the pituitary hormone. In all
71 cases, there were 14 growth hormone (GH), 13 prolactin (PRL),
five adrenocorticotropic hormone (ACTH), seven gonadotropic, 10
non-functional and 22 plurihormonal adenomas.
The expression of nucleostemin, ASPP2 mRNA and Ki-67
in the various pathological patterns are shown in Table II. The statistics demonstrated no
significant difference between nucleostemin and ASPP2 mRNA
expression in the various pathological patterns (P>0.05).
However, a significant difference existed between the Ki-67 LI
(P<0.05). The expression level of Ki-67 was highest in the PRL
adenomas (Ki-67 LI, 3.63±0.84) and lowest in the non-functional
adenomas (Ki-67 LI, 2.18±1.08). When compared with each other, the
diversity of Ki-67 LI in the PRL and non-functional adenomas was
varied (P<0.01). The Ki-67 LI level between the PRL and
gonadotropic adenomas (P<0.05), the non-functional and GH
adenomas (P<0.05) and the non-functional and plurihormonal
adenomas (P<0.05) differed from each other significantly.
 | Table IIExpression of nucleostemin, ASPP2 mRNA
and Ki-67 in various pathological pituitary adenomas. |
Table II
Expression of nucleostemin, ASPP2 mRNA
and Ki-67 in various pathological pituitary adenomas.
| Pathological
type | Nucleostemin | ASPP2 | Ki-67 LI, % |
|---|
| GH adenomas
(n=14) | 0.47±0.41 | 1.12±0.64 | 3.13±1.12 |
| PRL adenomas
(n=13) | 0.56±0.16 | 0.46±0.22 | 3.63±0.84 |
| ACTH adenomas
(n=5) | 0.39±0.23 | 0.81±0.47 | 2.82±0.85 |
| Gonadotropic adenomas
(n=7) | 0.31±0.47 | 0.84±0.74 | 2.48±0.81 |
| Non-functional
adenomas (n=10) | 0.21±1.81 | 0.73±0.49 | 2.18±1.08 |
| Plurihormonal
adenomas (n=22) | 0.45±0.53 | 0.69±0.64 | 2.99±1.08 |
| Total (n=71) | 0.42±0.40 | 0.76±0.58 | 2.96±1.07 |
Comparison of various indicators in
invasive and non-invasive pituitary adenomas
Nucleostemin expression levels and the Ki-67 LI were
higher in the invasive adenomas compared with the non-invasive
adenomas. However, for ASPP2, the invasive adenomas exhibited lower
levels (Table III). The
difference in nucleostemin and ASPP2 expression between the
invasive and non-invasive adenomas was of notable significance
(P<0.01) and the difference in the Ki-67 LI was also significant
(P<0.05).
 | Table IIIExpression of nucleostemin, ASPP2 mRNA
and Ki-67 in invasive and non-invasive pituitary adenomas. |
Table III
Expression of nucleostemin, ASPP2 mRNA
and Ki-67 in invasive and non-invasive pituitary adenomas.
| Group (n=71) | Nucleostemin | ASPP2 | Ki-67 LI, % |
|---|
| Invasive adenomas
(n=35) | 0.55±0.49a | 0.55±0.46a | 3.28±1.09b |
| Non-invasive adenomas
(n=36) | 0.30±0.22 | 0.97±0.62 | 2.65±0.97 |
Comparison of various indicators in
recurrent and non-recurrent pituitary adenomas
Among the 64 cases that were followed up, five
experienced recurrence (7.8%). In the recurrence group, the
expression of nucleostemin and Ki-67 LI was higher compared with
the non-recurrence group. However, the expression of ASPP2 was
lower compared with the non-recurrence group (Table IV). The statistical results
revealed that for the Ki-67 LI, significant differences existed
between the two groups (P<0.05). However, the nucleostemin and
ASPP2 expression levels were not significantly different
(P>0.05).
 | Table IVExpression of nucleostemin, ASPP2 mRNA
and Ki-67 in recurrent and non-recurrent pituitary adenomas. |
Table IV
Expression of nucleostemin, ASPP2 mRNA
and Ki-67 in recurrent and non-recurrent pituitary adenomas.
| Recurrent | Nucleostemin | ASPP2 | Ki-67 LI, % |
|---|
| Yes (n=5) | 0.56±0.23 | 0.39±0.17 | 4.11±1.39a |
| No (n=59) | 0.40±0.31 | 0.77±0.59 | 2.87±1.04 |
Discussion
Pituitary adenomas are common intracranial tumors
that account for ~10% of all intracranial tumors. According to
their biological behavior, pituitary adenomas may be divided into
invasive and non-invasive subgroups. Although the pathogenesis of
pituitary adenomas is a multi-step process containing multiple
factors, the exact pathogenesis remains unclear.
Nucleostemin is a relatively new p53 binding protein
that was first reported by Tsai and McKay (2) in 2002. Nucleostemin is located in the
nucleus of stem and tumor cells and is involved in the regulation
of stem and tumor cell proliferation. The protein maintains the
proliferation state of stem cells and inhibits their
differentiation into mature cells. Although the expression of
nucleostemin was highly detected in human tumor cells, the protein
does not exist in terminally differentiated cells.
Previous studies have shown that nucleostemin
combines and reacts with p53 in the nucleoplasm. Based on existing
experimental evidence, a pattern of action for nucleostemin may be
proposed. Nucleostemin exists in the nucleolus prior to binding
with GTP and is then transferred back to the nucleolus afterwards,
where it recombines with p53 protein and inhibits its growth
inhibition function. Cell differentiation leads to a decline in
nucleostemin gene expression, and p53 is released and activated.
Finally, activated p53 is able to induce certain genes to be
expressed, which allow cells to exit the mitotic cycle and
differentiate (2,6).
Tsai and McKay (7)
also reported a dynamic mechanism of nucleostemin moving across the
nucleolus and nucleoplasm. Nucleostemin regulates its combined
state with GTP using its amino terminal, thus adjusting the amount
of nucleostemin protein in the nucleolus and nucleoplasm. Under the
effects of internal and external signals, cells may
bi-directionally and rapidly regulate the amount of nucleostemin
protein in the nucleolus. Therefore, nucleostemin combines with p53
to form a complex, affecting the function of cell cycle regulation
and cell apoptosis induction. The increased expression of
nucleostemin may enhance the percentage of S-phase cells and
increase the rate of tumor growth. When siRNA was used to inhibit
nucleostemin expression in the HeLa cell line, the percentage of
S-phase cells decreased, the percentage of
G0/G1 stage cells increased, cell
proliferation reduced significantly and cell tumorigenicity
decreased (8).
The ASPPs are a recently discovered protein family
containing three members, ASPP1, ASPP2 and iASPP (9). Subsequent to forming complexes with
p53, ASPPs produce various effects on cell apoptosis. ASPP1 and
ASPP2 may spur the combination of the apoptosis gene promoter of
p53 with DNA, thus heightening the cell apoptosis function of p53
(3,4). iASPP combines with the p53 binding
site, competing with ASPP1 and ASPP2, thus reducing the tumor
suppression function of p53 (9).
Numerous studies have investigated how the ASPP
family regulates p53 function. Llanos et al(10) formed the co-expression of ASPP1 or
ASPP2 with p53 through transfection and observed that apoptosis
occurred in 50% of the transfected tumor cell lines. The
co-expression of ASPP1 or ASPP2 with tumor suppressor genes, such
as E2Fi and Bax, resulted in no change in the amount of apoptotic
cells. In addition, ASPP2 expression was observed to be associated
with clinical prognosis (11). A
study by Lossos et al(11)
on diffused large B-cell lymphoma and follicular lymphoma revealed
that the survival time of patients with higher levels of ASPP2 was
longer than those with lower levels of ASPP2. Zhu et
al(12) revealed that the ASPP2
protein level and apoptotic function were regulated by mechanisms
of proteasome degradation, which participated in the p53-ASPP2
apoptotic pathway. In contrast, Mdm and mdmX were shown to block
the p53 apoptotic effect that was induced by ASPP1 and ASPP2
(13). A study suggested that ASPP
is a crucial regulatory protein for p53 in vivo and that
ASPP1 and ASPP2 may enhance p53-dependent apoptosis through
increasing the vitality of the original apoptosis gene (14).
Ki-67 is a nuclear protein that is associated with
cell proliferation. The protein is regarded as a good indicator to
evaluate cell proliferation as it is expressed in each period of
cell proliferation. The ratio of Ki-67-positive cells in all cells
(Ki-67 LI) is often used to evaluate cell proliferation. Previous
studies have shown that the Ki-67 LI is associated with the
invasive ability of pituitary adenomas (15–19).
One study revealed that the levels of Ki-67 LI in various
pathological patterns of pituitary adenomas differed, indicating
that the proliferative activity of the various endocrine types also
differed (20). Another study
identified that the Ki-67 LI was significantly higher in recurrent
pituitary adenomas than in primary tumors, which indicated that the
Ki-67 LI may be used to judge recurrence and prognosis (21).
The present study identified that the expression of
nucleostemin and ASPP2 may be detected in pituitary adenomas.
However, ASPP2 expression was significantly higher in the
non-invasive pituitary adenomas than in the invasive adenomas.
Nucleostemin expression was significantly higher in the invasive
pituitary adenomas than in the non-invasive adenomas. In the
pituitary adenomas, nucleostemin expression was negatively
correlated with ASPP2 expression. ASPP2, but not nucleostemin gene
expression, was positively correlated with the Ki-67 LI. Higher
nucleostemin gene expression, lower ASPP2 gene expression and
higher Ki-67 expression correlated with each other, indicating that
changes in all three factors occurred simultaneously or
concomitantly in pituitary adenoma tumorigenesis and biological
alterations, and that there may be a causal connection. The three
factors may regulate the cell proliferative status through a
specific mechanism. An increase in nucleostemin expression and a
reduction in ASPP2 caused the inhibition of the cell apoptotic
function of p53. Therefore, the tumor cell proliferation ability
was strengthened and the invasive character improved.
In conclusion, nucleostemin and ASPP2 were expressed
in pituitary adenoma tissues and were associated with the
aggressive behavior and proliferation activity of the tumor,
indicating that these factors play a significant role in pituitary
adenoma oncogenesis and tumor progression, and have the potential
to be a target for pituitary adenoma gene therapy. However, the way
the factors are combined to regulate the proliferation state
requires further study.
Acknowledgements
This study was supported by grants from the
Zhengzhou committee of Science and Technology (no.
083SGY2612-9).
References
|
1
|
Kepes JJ, Chen WY, Pang LC and Kepes M:
Tumors of the central nervous system in Taiwan, Republic of China.
Surg Neurol. 22:149–156. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai RY and McKay RD: A nucleolar
mechanism controlling cell proliferation in stem cells and cancer
cells. Genes Dev. 16:2991–3003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riddihough G and Pennisi E: The evolution
of epigenetics. Science. 293:10632001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samuels-Lev Y, O’Connor DJ, Bergamaschi D,
Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T
and Lu X: ASPP proteins specifically stimulate the apoptotic
function of p53. Mol Cell. 8:781–794. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mampalam TJ, Tyrrell JB and Wilson CB:
Transsphenoidal microsurgery for Cushing disease. A report of 216
cases. Ann Intern Med. 109:487–493. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu J and Bishop JM: Nucleostemin maintains
self-renewal of embryonic stem cells and promotes reprogramming of
somatic cells to pluripotency. J Cell Biol. 197:731–745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsai RY and McKay RD: A multistep,
GTP-driven mechanism controlling the dynamic cycling of
nucleostemin. J Cell Biol. 168:179–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sijin L, Ziwei C, Yajun L, Meiyu D,
Hongwei Z, Guofa H, Siguo L, Hong G, Zhihong Z, Xiaolei L, Yingyun
W, Yan X and Weide L: The effect of knocking-down nucleostemin gene
expression on the in vitro proliferation and in vivo tumorigenesis
of HeLa cells. J Exp Clin Cancer Res. 23:529–538. 2004.PubMed/NCBI
|
|
9
|
Bergamaschi D, Samuels Y, O’Neil NJ,
Trigiante G, Crook T, Hsieh JK, O’Connor DJ, Zhong S, Campargue I,
Tomlinson ML, Kuwabara PE and Lu X: iASPP oncoprotein is a key
inhibitor of p53 conserved from worm to human. Nat Genet.
33:162–167. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llanos S, Royer C, Lu M, Bergamaschi D,
Lee WH and Lu X: Inhibitory member of the apoptosis-stimulating
proteins of the p53 family (iASPP) interacts with protein
phosphatase 1 via a noncanonical binding motif. J Biol Chem.
286:43039–43044. 2011. View Article : Google Scholar
|
|
11
|
Lossos IS, Natkunam Y, Levy R and Lopez
CD: Apoptosis stimulating protein of p53 (ASPP2) expression differs
in diffuse large B-cell and follicular center lymphoma: correlation
with clinical outcome. Leuk Lymphoma. 43:2309–2317. 2002.
View Article : Google Scholar
|
|
12
|
Zhu Z, Ramos J, Kampa K, Adimoolam S,
Sirisawad M, Yu Z, Chen D, Naumovski L and Lopez CD: Control of
ASPP2/(53BP2L) protein levels by proteasomal degradation modulates
p53 apoptotic function. J Biol Chem. 280:34473–34480. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergamaschi D, Samuels Y, Zhong S and Lu
X: Mdm2 and mdmX prevent ASPP1 and ASPP2 from stimulating p53
without targeting p53 for degradation. Oncogene. 24:3836–3841.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Godin-Heymann N, Dan Wang X,
Bergamaschi D, Llanos S and Lu X: ASPP1 and ASPP2 bind active RAS,
potentiate RAS signalling and enhance p53 activity in cancer cells.
Cell Death Differ. 20:525–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolfsberger S, Kitz K, Wunderer J, Czech
T, Boecher-Schwarz HG, Hainfellner JA and Knosp E: Multiregional
sampling reveals a homogenous distribution of Ki-67 proliferation
rate in pituitary adenomas. Acta Neurochir (Wien). 146:1323–1328.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honegger J, Prettin C, Feuerhake F,
Petrick M, Schulte-Mönting J and Reincke M: Expression of Ki-67
antigen in nonfunctioning pituitary adenomas: correlation with
growth velocity and invasiveness. J Neurosurg. 99:674–679. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Ma S, Yao Y, Li G, Feng M, Deng K,
Dai C, Cai F, Li Y, Zhang B and Wang R: Differential expression of
folate receptor alpha in pituitary adenomas and its relationship to
tumor behavior. Neurosurgery. 70:1274–1280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saeger W, Lüdecke B and Lüdecke DK:
Clinical tumor growth and comparison with proliferation markers in
non-functioning (inactive) pituitary adenomas. Exp Clin Endocrinol
Diabetes. 116:80–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rishi A, Sharma MC, Sarkar C, Jain D,
Singh M, Mahapatra AK, Mehta VS and Das TK: A clinicopathological
and immunohistochemical study of clinically non-functioning
pituitary adenomas: a single institutional experience. Neurol
India. 58:418–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolfsberger S, Wunderer J, Zachenhofer I,
Czech T, Böcher-Schwarz HG, Hainfellner J and Knosp E: Expression
of cell proliferation markers in pituitary adenomas - correlation
and clinical relevance of MIB-1 and anti-topoisomerase-IIalpha.
Acta Neurochir (Wien). 146:831–839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zada G, Woodmansee WW, Ramkissoon S,
Amadio J, Nose V and Laws ER Jr: Atypical pituitary adenomas:
incidence, clinical characteristics, and implications. J Neurosurg.
114:336–344. 2011. View Article : Google Scholar : PubMed/NCBI
|