Introduction
Lung cancer is the most commonly diagnosed type of
cancer (1.6 million cases each year) and the most common cause of
cancer mortality (1.18 million mortalities each year). The number
of new cases and mortalities is progressively increasing every
year. Lung cancer is not easily diagnosed. Only ~10% of lung cancer
patients are alive 5 years after diagnosis. Lung cancer is the
leading cancer site in males, comprising 17% of total new cancer
cases and 23% of total cancer mortalities (1). Non-small cell lung cancer (NSCLC) is
the most common type of lung cancer. The development and
progression of NSCLC is a complex process, and the tumor
microenvironment plays an important role. The tumor
microenvironment is composed of structural (extracellular matrix),
soluble (cytokines, proteases and hormones) and cellular components
(tumor cells, fibroblasts, inflammatory cells, vascular and
lymphatic endothelial cells, vascular smooth muscle cells and
pericytic cells). In 1993, Takeshita et al(2) identified an 811-amino acid protein,
named osteoblast specific factor-2 (OSF-2), which was secreted by
the mouse MC3T3-E1 osteoblastic cell line. OSF-2 has a typical
signal sequence, followed by a cysteine-rich domain (EMI domain), a
4-fold repeated domain and a C-terminal domain. The 4-fold repeated
domain shows homology with the insect protein, fasciclin I
(2). Each of the 4-fold repeated
domains has 150 amino acids, and 90–100 of these 150 amino acids
are highly conserved, forming an area called the fas domain. As
OSF-2 is also expressed by the periosteum and periodontal ligament,
this protein was renamed periostin (3). In humans, the periostin gene (POSTN)
is located on chromosome 13, at map position 13q13.3, and the
protein is 835 amino acids in size and 90 kDa in molecular weight
(4). Mouse and human periostin
share 89.2% amino acid identity overall and 90.1% identity in their
mature forms.
Periostin is expressed at the mRNA level by the
majority of normal adult tissues, including the aorta, stomach,
lower gastrointestinal tract, placenta, uterus and breast. The
expression of periostin is high in fetal tissue at the mRNA and
protein levels (5). Periostin
protein expression is observed in normal adult tissues, including
the adrenal glands, lung, thyroid, stomach, colon, vagina, ovary,
testis and prostate, by western blot analysis (6). Periostin may be induced by
transforming growth factor-β (TGF-β) (7) and Bmp-2 (8), and is involved in osteoblast
recruitment, attachment and spreading (3). Periostin is considered to be a
regulator of cardiac remodeling and hypertrophy and may be a
suitable pharmacological target to mitigate heart failure (9).
Recently, periostin was identified as a novel factor
in the growth, invasion, angiogenesis and metastasis of numerous
types of tumors. Periostin is overexpressed in various types of
human cancer tissues, including ovarian cancer (5), cholangiocarcinoma (10), breast cancer (11), colon cancer (12), esophageal cancer (13), head and neck cancer (14), and pancreatic ductal adenocarcinoma
(ADC) (15). Notably, periostin
expression is well correlated with malignant behavior, including
growth, invasion, angiogenesis, metastasis and poor survival in
ovarian cancer (5),
cholangiocarcinoma (10), breast
cancer (11), colon cancer
(12), esophageal cancer (14) and pancreatic ductal ADC (15).
Periostin has also been detected in the serum of
NSCLC by chemiluminescence assays. Notably, a previous study
identified no significant difference between NSCLC patients and
normal controls, and there was also no correlation between the
serum periostin level and gender, stage, bone metastasis, lymph
node status or primary tumor status. However, the NSCLC patients
with high periostin levels had significantly poorer survival than
the patients with normal periostin levels (16). Periostin mRNA has been shown to be
upregulated in NSCLC tissue in relation to normal lung tissue, and
also to be correlated with adeno cell subtype and higher tumor
grade (17).
However, little information is available on the
expression of periostin protein in NSCLC cancer tissues, and the
correlation between periostin expression and the
clinicopathological characteristics of NSCLC patients is unknown.
Previously, we identified that serum periostin was elevated in
NSCLC patients compared with normal healthy volunteers, and showed
that periostin promotes the proliferation and migration of A549
cells by inducing vimentin and N-cadherin expression and
downregulating E-cadherin expression (18). In the present study, the mRNA and
protein level of periostin in NSCLC and its correlation with
established biological and prognostic factors were investigated. To
provide evidence that the inactivation of the periostin gene is a
common event in NSCLC, periostin gene expression was examined at
the transcriptional and translational levels in 49 paired
normal/paratumor/cancer tissues, and the correlation between
periostin expression and prognosis in NSCLC was assessed.
Materials and methods
Patients
Lung specimens from cancer tissues and paired
paratumor tissues (with 1–2 cm distance from tumor edge) and normal
tissues (with >5 cm distance from tumor edge) from 49 NSCLC
patients (Table I) and 6 benign
lung tumors (including 3 inflammatory pseudotumors and 3 pulmonary
tuberculosis cases), who underwent pulmonary resection surgery,
were included in this study. The samples were obtained by the
Department of Cardiothoracic Surgery of Jinling Hospital (Nanjing,
Jiangsu, China) between June 2007 and June 2008. All diagnoses were
based on pathological evidence. Patients were grouped according to
the size of the primary tumor (T), nodal involvement (N) and
distant metastasis (M) to TNM stages I–IV according to the World
Health Organization criteria for the TNM system and staged
appropriately. Patients did not receive chemo-, radio- or
immunotherapy prior to surgery. The tissues were snap frozen and
stored at −80°C until use. This study was authorized by the
principle committee of Jinling Hospital. Written informed consent
was obtained from the patients.
 | Table ICharacteristics of the patients with
primary non-small cell lung cancer (western blot analysis and
qPCR). |
Table I
Characteristics of the patients with
primary non-small cell lung cancer (western blot analysis and
qPCR).
| Characteristics | Value |
|---|
| Total patients, n
(%) | 49 (100) |
| Age, yearsa | 57.132±1.743 |
| Gender, n (%) |
| Male | 38 (77) |
| Female | 11 (23) |
| Age, n (%) |
| <60 years | 27 (55) |
| ≥60 years | 22 (45) |
| Histological type, n
(%) |
| ADC | 35 (71) |
| Non-ADC | 14 (29) |
| Stage, n (%) |
| I | 15 |
| II | 10 |
| I+II | 25 (51) |
| III | 23 |
| IV | 1 |
| III+IV | 24 (49) |
| Tumor size and
invasiveness, n (%) |
| T1 | 11 |
| T2 | 28 |
| T1+T2 | 39 (79) |
| T3 | 5 |
| T4 | 5 |
| T3+T4 | 10 (21) |
| Lymph node status,
n (%) |
| + | 25 (51) |
| − | 24 (49) |
| Smoking history, n
(%) |
| ≥20 pack year | 25 (51) |
| <20 pack
year | 24 (49) |
Protein extraction and western blot
analysis
Frozen tissues were washed twice with ice-cold
phosphate-buffered saline (PBS), and homogenized on ice in 10
volumes (w/v) of lysis buffer [0.1% SDS, 50 mM Tris-HCl (pH 7.5) 1%
NP-40, 150 mM NaCl, 1 mM Triton X-100, 1 mM EDTA] containing
complete protease inhibitor (PMSF+P8340). Subsequent to incubation
on ice for 1 h and centrifugation at 12,000 × g for 15 min at 4°C,
the supernatant was collected and stored at −70°C. The protein
concentration was measured by the bicinchoninic acid (BCA) protein
assay (Sigma, St. Louis, MO, USA). A total of 100 μg protein was
separated by 5–10% SDS-PAGE. Proteins were then transferred to
polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA,
USA), which were saturated by incubation for 2 h with 5% skimmed
dry milk in Tris-buffered saline (TBS)/0.05% Tween-20 at 37°C. The
membranes were then incubated with the rabbit polyclonal
anti-periostin antibody (ab14041, 1:1,000; Abcam, Cambridge, MA,
USA) overnight at 4°C in blocking buffer (5% skimmed dry milk in
TBS/0.05% Tween-20). Subsequent to being washed 4 times (5 min
each) with TBS/0.05% Tween-20, the membranes were incubated with
anti-rabbit immunoglobulin labeled with horseradish peroxidase
(ab6721, 1:2,000; Abcam) at 37°C in blocking buffer. After 1 h of
incubation, the membranes were washed 4 times (5 min each) with
TBS/0.1% Tween-20. Chemiluminescence was detected with an ECL
western blot analysis detection kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA), according to the manufacturer’s instructions,
and the results were quantified by densitometry using an Image
System (GelDoc 2000; Bio-Rad). Polyclonal anti-β-actin (a
housekeeping protein used as a loading control to assure equal
amounts of protein in all lanes) antibody was used as a
control.
RNA extraction and cDNA synthesis
Total RNA was isolated from the frozen tissue with
TRIzol (Invitrogen, Carlsbad, CA, USA). Using random hexamer
primers, 2 μg RNA was reverse transcribed to cDNA with a
PrimeScript™ 1st Strand cDNA Synthesis kit (Takara Biotechnology,
Inc., Shiga, Japan).
Quantitative polymerase chain reaction
(qPCR)
Primers for POSTN and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) were designed and synthesized by Takara
Biotechnology, Co., Ltd. (Dalian, China). The basic information of
the primers including gene name, National Center for Biotechnology
Information (NCBI) reference, forward primer, reverse primer and
product size (bp), respectively, were as follows: POSTN, NM_006475,
CATTGATGGAGTGCCTGT GGA, CAATGAATTTGGTGACCTTGGTG and 167; and GAPDH,
NM_002046, GCACCGTCAAGGCTGAGAAC, TGGTGAAGACGCCAGTGGA and 138. qPCR
was performed in triplicate for each sample in a 25-μl reaction
mixture, which consisted of template DNA (2 μl), primers (0.2 μM),
ROX Reference Dye II (1X), dH2O (9.0 μl) and
SYBR® Premix Ex Taq [1X; SYBR Premix Ex Taq (perfect
real-time) kit; Takara]. PCR was performed using a Stratagene
Mx3005P instrument (Stratagene, La Jolla, CA, USA) with the
following thermal settings: 1 cycle of 10 sec at 95°C and 45 cycles
of 5 sec at 95°C and 20 sec at 60°C. According to the method tested
by Tichopad, the relative expression ratio (RR) of the POSTN was
calculated based on amplication efficiencies and the cycle
threshold comparative with a reference gene (GAPDH) in a
sample.
Immunohistochemistry
Six paraffin sections of tumor were selected for
analysis. Immunohistochemical staining was performed on 4-μm
formalin-fixed, paraffin-embedded tissue sections. The slides were
deparaffinized in xylene and dehydrated in a graded ethanol series.
Endogenous peroxidase was blocked with 3%
H2O2 in methanol for 15 min. The primary
antibody that was used was the rabbit polyclonal anti-periostin
antibody (ab14041, 1:200; Abcam). Immunohistochemical staining was
performed using the Envision™ two-step Visualization System
(Envision Detection kit GK500705, Peroxidase/DAB, rabbit/mouse;
DakoCytomation, Glostrup, Denmark). The next steps were performed
according to the manufacturer’s instructions.
Statistical analysis
The statistical analysis was performed using SPSS
19.0 (SPSS, Inc., Chicago, IL, USA). Values are presented as the
mean ± SEM. The significant differences between the tumor, tumor
adjacent and surrounding tissues were assessed by the paired
samples t-test. The correlation between periostin gene expression
and the clinicopathological characteristics of the NSCLC patients
was analyzed by the independent samples t-test. The Kaplan-Meier
method was used to generate survival curves, and survival
differences were analyzed with the log-rank test, based on the
status of periostin expression. Uni- and multivariate analyses were
performed using Cox’s proportional hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of periostin mRNA and protein
in each tissue
At the mRNA level, the difference between the
expression in the cancer tissue and normal tissue was not
significant (Table II). The
western blot analysis showed that the periostin level was elevated
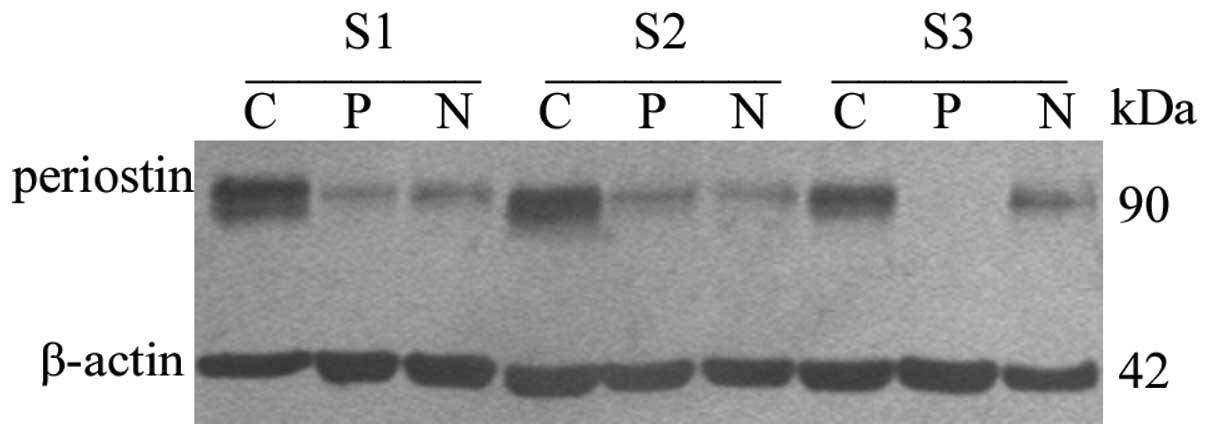
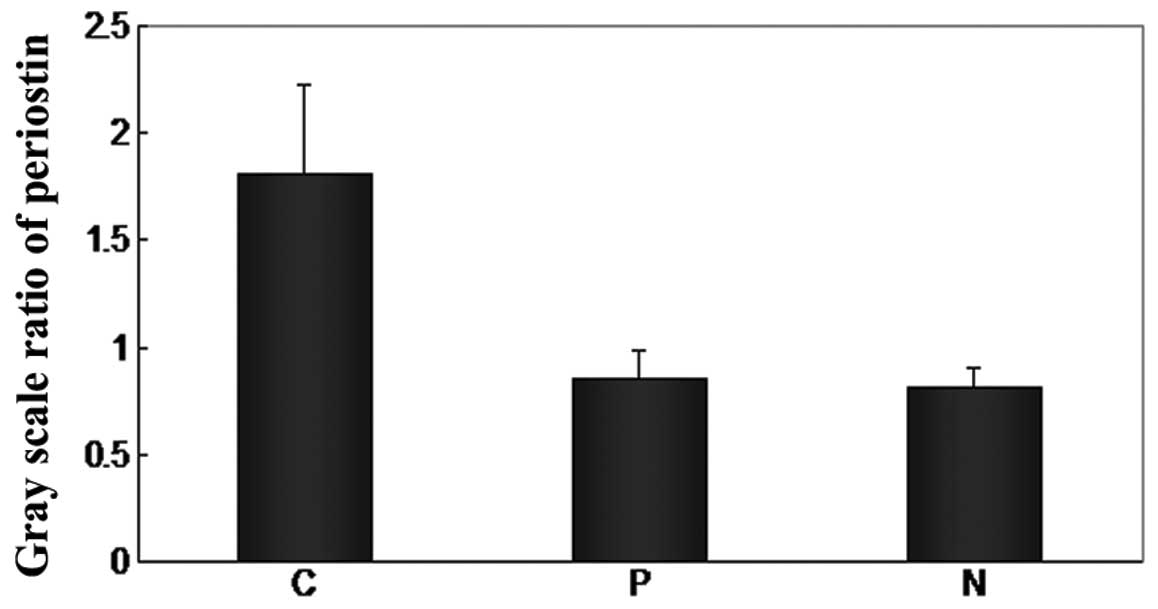
in the cancer tissue of the NSCLC patients (Fig. 1). The periostin protein gray scale
levels of cancer, paratumor and normal tissues were 1.810±0.415,
0.857±0.130 and 0.808±0.100, respectively (Table II and Fig. 2). The protein level of periostin in
the cancer tissues was significantly higher than in the paratumor
(P=0.000) and normal (P=0.017) tissues. However, there were no
differences between the paratumor tissues and normal tissues
(P=0.978). Periostin expression was also analyzed in 6 benign lung
tumors (including 3 inflammatory pseudotumors and 3 pulmonary
tuberculosis), and higher expression was observed at the protein
level in the pseudotumors and tuberculosis than in the adjacent and
surrounding tissues (Table III;
P<0.05). In addition, there was no prominent difference between
the NSCLC patients and the benign lung tumor patients (data not
shown).
 | Table IIExpression of periostin mRNA and
protein in three types of tissues of NSCLC patients. |
Table II
Expression of periostin mRNA and
protein in three types of tissues of NSCLC patients.
| Group | Periostin mRNA | P-value | Periostin
protein | P-value |
|---|
| Cancer tissue | 0.326±0.086 | - | 1.810±0.415 | - |
| Paratumor
tissuea | - | - | 0.857±0.130 | 0.000b |
| Normal tissue | 0.389±0.085 | 0.433c | 0.808±0.100 | 0.017c |
 | Table IIIExpression of periostin mRNA and
protein in three types of tissues of benign lung tumors. |
Table III
Expression of periostin mRNA and
protein in three types of tissues of benign lung tumors.
| Group | Periostin mRNA | P-value | Periostin
protein | P-value |
|---|
| Tumor tissue | 0.237±0.077 | - | 1.178±0.160 | - |
| Adjacent
tissuea | - | - | 0.520±0.161 | 0.016b |
| Surrounding
tissue | 0.460±0.230 | 0.378c | 0.235±0.111 | 0.001c |
Correlation between periostin expression
and the clinicopathological characteristics of NSCLC patients
The correlation between periostin expression and the
clinicopathological characteristics of the NSCLC patients was
analyzed, and the result is shown in Table IV. As indicated in this table, the
expression of periostin had no correlation with age, pathological
type, TNM stage, lymph node status, smoking history, tumor size or
invasiveness. Periostin gene expression (at the mRNA and protein
level) was shown to correlate with the gender of the NSCLC
patients; the value of the mRNA and the gray scale level of protein
in the male group was 1.438±0.427 and 3.915±0.663, respectively,
while those of the female group were 0.449±0.117 and 1.463±0.202,
respectively. Statistical significance was determined by the
independent samples t-test (Table
IV; P<0.05).
 | Table IVExpression of periostin in NSCLC
cancer tissue and its correlation with clinicopathological
characteristics of NSCLC patients. |
Table IV
Expression of periostin in NSCLC
cancer tissue and its correlation with clinicopathological
characteristics of NSCLC patients.
| Parameter | Periostin mRNA | P-value | Periostin
protein | P-value |
|---|
| Gender |
| Male | 1.438±0.427 | 0.010 | 3.915±0.663 | 0.001 |
| Female | 0.449±0.117 | | 1.463±0.202 | |
| Age, years |
| <60 | 0.742±0.104 | 0.085 | 2.468±0.490 | 0.075 |
| ≥60 | 1.913±0.809 | | 4.663±1.060 | |
| Pathological
type |
| ADC | 0.669±0.096 | 0.029 | 3.068±0.665 | 0.566 |
| Non-ADC | 1.939±0.754 | | 3.701±0.897 | |
| TNM stage |
| I+II | 0.982±0.193 | 0.534 | 3.688±0.880 | 0.543 |
| III+IV | 1.403±0.612 | | 3.016±0.649 | |
| Lymph node
status |
| + | 1.347±0.557 | 0.621 | 3.859±0.759 | 0.255 |
| − | 1.008±0.216 | | 2.613±0.708 | |
| Smoking
history |
| ≥20 pack year | 1.159±0.197 | 0.895 | 4.350±0.927 | 0.059 |
| <20 pack
year | 1.249±0.645 | | 2.319±0.446 | |
| Tumor size and
invasiveness |
| T1+T2 | 0.997±0.148 | 0.538 | 3.356±0.684 | 0.948 |
| T3+T4 | 1.784±1.222 | | 3.275±0.723 | |
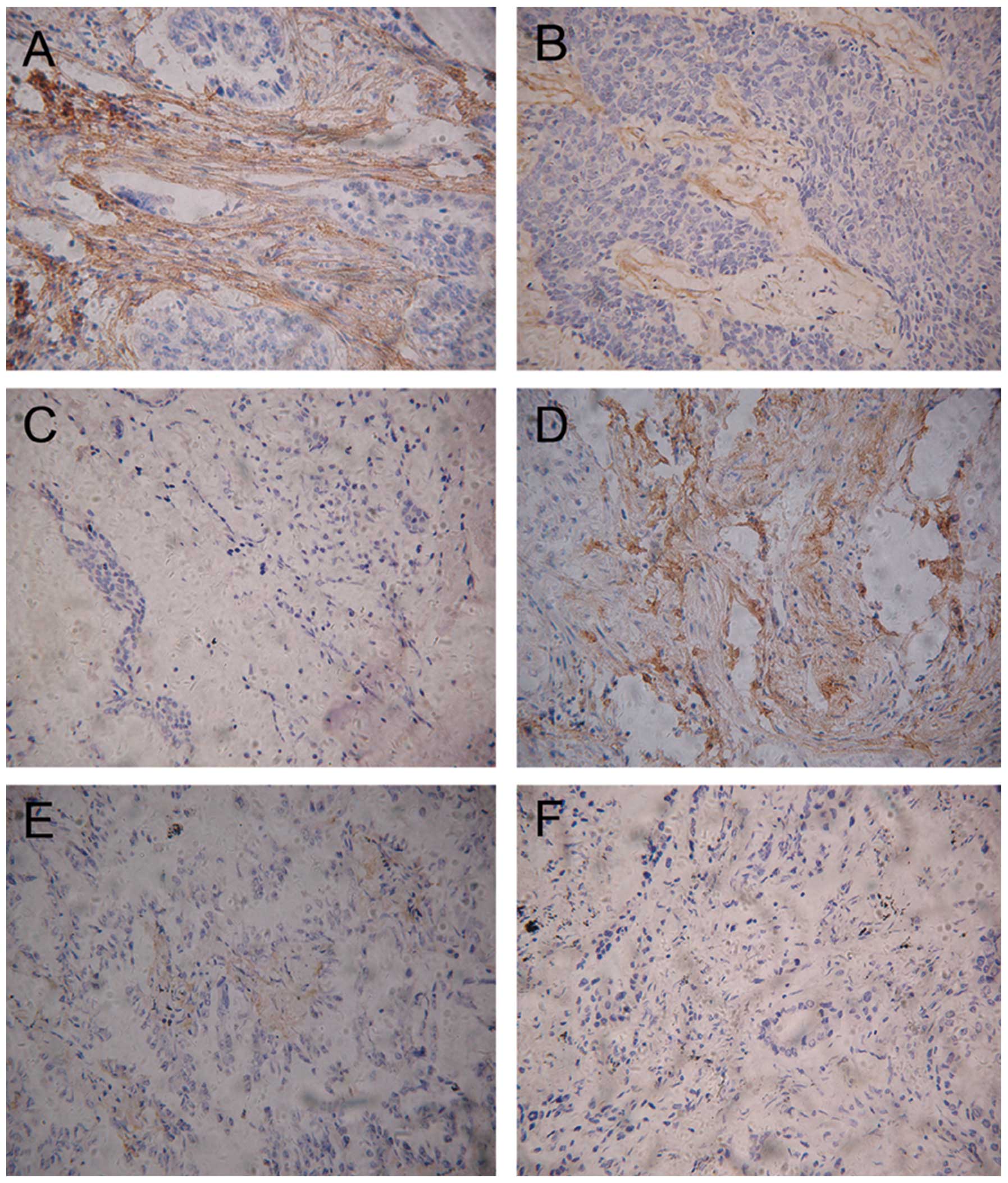
Locating periostin in NSCLC by
immunohistochemistry
To investigate the location of periostin in NSCLC,
immunohistochemistry was carried out on 3 ADC slides and 3 squamous
carcinoma slides. The immunostaining indicated that high levels of
periostin were present in the mesenchymal areas, but not in the
cancer cells themselves. Certain samples were highly stained and
others demonstrated no staining (Fig.
3).
Prognostic significance of periostin
expression
A Kaplan-Meier analysis indicated that the NSCLC
patients whose tumors showed high levels of periostin expression
(periostin-H) had significantly shorter overall survival times
compared with those with low levels of periostin expression
(periostin-L; P=0.036, log-rank test; Fig. 4). The 3-year survival rate was 81.5%
for patients with periostin-L (n=27), and 45.4% for patients with
periostin-H (n=22). A univariate analysis was also performed to
evaluate the associations between patient prognosis and other
factors, including age (<60 vs. ≥60 years), gender (male vs.
female), pT status (T1 vs. T2–4), pathological node (pN) status (N0
vs. N1 and N2), histological type (ADC vs. non-ADC), smoking
history (smoker vs. never-smoker) and periostin expression
(periostin-H vs. periostin-L). Among these parameters, advanced pN
status (P=0.044) and periostin status (P=0.044) were significantly
associated with a poor prognosis. Since the variables shown to have
prognostic affects by univariate analysis may represent covariates,
all significant variables from the univariate analysis were
included in the multivariate regression analysis in order to
identify independent prognostic factors. The resulting data are
presented in Table V. Periostin
expression was identified as an independent prognostic factor
(P=0.011).
 | Table VPrognostic factors in Cox’s
proportional hazards model. |
Table V
Prognostic factors in Cox’s
proportional hazards model.
| Variables | Risk ratio | Univariate 95%
CI | P-value | Risk ratio | Multivariate 95%
CI | P-value |
|---|
| Age, years |
| <60/≥60 | 1.635 | 0.680–3.933 | 0.272 | 2.425 | 0.857–6.859 | 0.095 |
| Gender |
| Male/female | 0.541 | 0.159–1.849 | 0.328 | 0.962 | 0.207–4.460 | 0.960 |
| pT status |
| pT2–4/pT1 | 0.329 | 0.076–1.420 | 0.136 | 0.416 | 0.084–2.061 | 0.282 |
| pN status |
| pN1–2/pN0 | 0.353 | 0.128–0.974 | 0.044a | 0.431 | 0.152–1.224 | 0.114 |
| Histological
type |
| Non-ADC/ADC | 0.448 | 0.185–1.084 | 0.075 | 0.448 | 0.153–1.316 | 0.144 |
| Smoking
history |
|
Smoker/non-smoker | 0.708 | 0.289–1.733 | 0.450 | 0.987 | 0.335–2.905 | 0.981 |
| Periostin
expression |
|
Periostin-H/-L | 0.399 | 0.163–0.977 | 0.036a | 0.251 | 0.087–0.724 | 0.011a |
Discussion
Periostin is homologous with fasciclin I, a protein
expressed on the surface of a subset of axon pathways in the
embryonic central nervous system in insects. Fasciclin I supports
cell aggregation and mediates cell sorting, and disruption of
fasciclin I causes defects in axonogenesis (19). In mammals, another novel protein
that has a similar structure is βig-h3, which was originally cloned
as a molecule induced by TGF-β. βig-h3 promotes adhesion and
spreading of fibroblasts in vitro, and may be associated
with microfibrils in vivo. Periostin is also a TGF-β-induced
extracellular matrix protein involved in cell survival,
angiogenesis, invasion and metastasis (20).
Periostin is overexpressed in the tumor tissue of a
number of human tumors, and a similar result is shown in the serum
of lung cancer (16) and thymoma
(21) patients. The mechanism by
which periostin interacts with tumors has not been completely
elucidated. The majority of analyses have shown that periostin
stimulated tumor growth by connecting with integrins, particularly
αvβ3, αvβ5 and α6β4. Zhu et al demonstrated that recombinant
periostin promoted adhesion and migration of epithelial ovarian
tumor cells, and that this function was inhibited by the αvβ3 or
αvβ5 antibody, indicating that periostin is important in the αvβ3
or αvβ5 integrin-dependent adhesion and migration of epithelial
cells (5). Further studies showed
that periostin is the ligand of αvβ3 and αvβ5 integrins in breast
(11), colon (12) and oral (22) cancer cells. In pancreatic cancer
cells, the α6β4-integrin complex acts as the cell receptor of
periostin, and this interaction promotes migration through
phosphorylation of focal adhesion kinase (FAK) and protein kinase B
(AKT) through activation of the PI3 kinase pathway (15). In a previous study, we demonstrated
that periostin promotes the proliferation and migration of the
human lung ADC cell line (A549) in vitro by the EMT pathway
(18). Malanchi et
al(23) showed that periostin
was required for cancer stem cell maintenance and that blocking its
function prevents metastasis. Periostin recruits Wnt ligands and
thereby increases Wnt signaling in cancer stem cells.
In the present study, periostin expression was
detected in the tumor, paratumor and normal tissues, and the
clinical significance of periostin in the progression and
development of NSCLC was observed. The genome was not exactly the
same in the paratumor tissue and precancerous lesions, but they
were almost identical in histomorphology. When the changes in
molecular biology and gene map in the process of tumor progression
and development were discussed, the paratumor was selected, and the
relative normal tissue from the same patient acted as the
control.
Periostin protein levels of the tumor, paratumor and
normal tissues of 49 NSCLC patients were detected in the present
study. It was demonstrated that the protein level of periostin was
much higher in the tumor tissue than in the other 2 groups, but
that there was no difference between the paratumor and normal
tissues. These findings are similar to the majority of other types
of epithelial cancer, including breast (11) and colon (12) cancer. There was no difference
between the paratumor and normal tissues at the protein level,
therefore periostin was analyzed in the tumor and normal tissue at
the mRNA level. However, there was no significant difference
between the tumor and normal tissues at the mRNA level. It is well
known that mRNA reflects the transcriptional level and protein
reflects the translocational level. In the present study, the mRNA
level was not consistent with the protein level. The
ribosome-loading regulation system and miRNA are likely to play an
important role in the translocation of the periostin gene. There
are several levels of regulation from transcription to
translocation, and mRNAs either degrade or stop translocation in
the process. The protein level of periostin is notable with regard
to illustrating the function of the gene. Periostin is a novel
molecule in the progression and development of NSCLC.
In the present study, the level of periostin was
higher in the tumors from the male and non-ADC groups. These
findings are also supported by the study of Soltermann et
al(24), which showed that the
high expression of periostin in either the stroma or tumor
epithelia was detected in NSCLC tissues by immunohistochemistry,
particularly in the male study group. The majority of non-ADC
tumors in this study were squamous carcinomas. It is well known
that lung squamous cell carcinoma is a male-dominated cancer.
Puglisi et al(25) showed
that periostin was significantly correlated with the expression of
the estrogen and progesterone receptors in breast cancer, thus
providing a reason why periostin is overexpressed in male and
squamous cell carcinoma. However, tobacco smoking may be the most
important reason behind this. In the present data, the periostin
expression of the patients who had a smoking history (4.350±0.927)
was higher than those who did not smoke (2.319±0.446), although
there was no statistically significant difference. Tobacco produces
>4,000 chemical substances, and 1/80 of them are carcinogenic.
Studies have indicated that the polycyclin aromatic and nitroso
compounds in the smoke damage the bronchial epithelial cell DNA
through a variety of mechanisms, and activate oncogenes (Ras) and
deactivate anti-oncogenes (p53). The bronchial epithelial cells
transform into cancer cells via this process (26,27).
Periostin may be a promoter in this process.
In the present study, periostin was highly expressed
in the chronic inflammation patients. It is well known that chronic
inflammation may act as tumor promoter. Examples of this may be
seen in studies of MALT lymphoma (28) and intestinal carcinogenesis
(29). In the present study, there
is no direct evidence to reveal the correlation between chronic
inflammation and NSCLC. A group of proteins, known as the
matricellular proteins, which include thrombospondin (TSP-1 and
TSP-2), SPARC (secreted protein acidic and rich in cysteine),
osteopontin (OPN), the tenascins (TN-C and TN-X) and the CCN family
members (CCN1–6), are known to be expressed at lower levels in
normal adult tissue, but are upregulated during wound healing and
tissue remodeling. The expression of these proteins is also
involved in tumor development and progression. In normal
situations, these proteins are well controlled by various signals.
However, in tumors, the expression of the matricellular proteins is
defined in a deregulated manner as a ‘wound that does not heal’
(30). In the present study,
periostin was upregulated in the NSCLC and chronic inflammation
patients, therefore we hypothesize that periostin may be a member
of the matricellular protein family and that it has an effect on
chronic inflammation and cancer. Periostin may be a suitable target
to block the dangerous loop between cancer and inflammation.
Using immunohistochemistry, the present study showed
that periostin was only located in the mesenchymal tissue
surrounding the tumor cells. This corresponded to the location of
periostin previously identified in other types of cancer cells
(5,6,11,12).
In the present study, the pattern of the localization of periostin
in the tumoral stroma appears typically fibrillar and branched,
suggesting a possible association between periostin and the fibers
of the desmoplastic stroma of NSCLC. Recently, immunoelectron
microscopy analyses of mouse periodontal ligaments has revealed a
close association between periostin and collagen fibers, indicating
that a similar association may take place in NSCLC tissues
(31).
The localization and function of periostin in the
juxtatumoral stroma exhibits similarities to other secreted
proteins, such as the CCN family of proteins. The CCN family is a
group of 6 secreted proteins that are specifically associated with
the extracellular matrix. Similar to periostin, CNN family members
are induced by growth factors and cytokines, such as TGF-β and
endothelin 1, and cellular stress, including hypoxia, and are
overexpressed in pathological conditions that affect connective
tissues, including scarring, fibrosis and cancer. They also
interact with integrins and act as matricellular proteins to
mediate cell adhesion, migration, tissue repair (32), fibrosis (33), cancer and vascular disease (34).
For the present survival analysis, the 3-year
overall survival rate for the patients with periostin-L expression
was much higher than for those with periostin-H expression.
Furthermore, the multivariate analysis revealed that periostin-H
expression was an independent prognostic factor. The results
indicate that periostin is a crucial prognostic factor. These
findings lead us to believe that the overexpression of periostin is
likely to represent an important transformation factor associated
with a more malignant phenotype in NSCLC patients.
In conclusion, although the present study involved
only 49 patients, it may be concluded that periostin is important
in the progression and development of NSCLC. There was an
abnormally high expression level of periostin in the NSCLC and lung
chronic inflammation patients, and the periostin expression level
was much higher in the male and non-ADC groups of NSCLC patients.
We considered that periostin may be pivotal in the pathogenesis and
development of NSCLC, and that chronic inflammation may promote
cancer development using certain molecules, including periostin. It
was demonstrated that periostin was only located in the
juxtatumoral stroma of the NSCLC tissues, along with those proteins
belonging to the CCN family members, which had been shown to be
integrin-dependent. Patients with high level of periostin achieved
a significantly inferior outcome, indicating that it is a malignant
phenotype in NSCLC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81001039), Nanjing
City Medical Technology Development Project (grant no. QYK10138 ),
Engineering Training the Young Medical Talents in Nanjing City and
the Six Talent Peaks in Jiangsu Province to Dr L. Hong.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Takeshita S, Kikuno R, Tezuka K and Amann
E: Osteoblast-specific factor 2: cloning of a putative bone
adhesion protein with homology with the insect protein fasciclin I.
Biochem J. 294:271–278. 1993.PubMed/NCBI
|
|
3
|
Kruzynska-Frejtag A, Wang J, Maeda M,
Rogers R, Krug E, Hoffman S, Markwald RR and Conway SJ: Periostin
is expressed within the developing teeth at the sites of
epithelial-mesenchymal interaction. Dev Dyn. 229:857–868. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo Y, Siriwardena BS, Hatano H, Ogawa I
and Takata T: Periostin: novel diagnostic and therapeutic target
for cancer. Histol Histopathol. 22:1167–1174. 2007.PubMed/NCBI
|
|
5
|
Zhu M, Fejzo MS, Anderson L, Dering J,
Ginther C, Ramos L, Gasson JC, Karlan BY and Slamon DJ: Periostin
promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol.
119:337–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tai IT, Dai M and Chen LB: Periostin
induction in tumor cell line explants and inhibition of in vitro
cell growth by anti-periostin antibodies. Carcinogenesis.
26:908–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji X, Chen D, Xu C, Harris SE, Mundy GR
and Yoneda T: Patterns of gene expression associated with
BMP-2-induced osteoblast and adipocyte differentiation of
mesenchymal progenitor cell 3T3-F442A. J Bone Miner Metab.
18:132–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lorts A, Schwanekamp JA, Elrod JW, Sargent
MA and Molkentin JD: Genetic manipulation of periostin expression
in the heart does not affect myocyte content, cell cycle activity,
or cardiac repair. Circ Res. 104:e1–e7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Utispan K, Thuwajit P, Abiko Y, Charngkaew
K, Paupairoj A, Chau-in S and Thuwajit C: Gene expression profiling
of cholangiocarcinoma derived fibroblast reveals alterations
related to tumor progression and indicates periostin as a poor
prognostic marker. Mol Cancer. 9:132010. View Article : Google Scholar
|
|
11
|
Zhang Y, Zhang G, Li J, Tao Q and Tang W:
The expression analysis of periostin in human breast cancer. J Surg
Res. 160:102–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michaylira CZ, Wong GS, Miller CG,
Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim
SB, Herlyn M, Diehl JA, Gimotty P and Rustgi AK: Periostin, a cell
adhesion molecule, facilitates invasion in the tumor
microenvironment and annotates a novel tumor-invasive signature in
esophageal cancer. Cancer Res. 70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki H, Dai M, Auclair D, Fukai I,
Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, as a
prognostic marker in nonsmall cell lung carcinomas. Cancer.
92:843–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morra L, Rechsteiner M, Casagranda S,
Teichman A, Schraml P, Moch H and Soltermann A: Characterization of
periostin isoform pattern in non-small cell lung cancer. Lung
Cancer. 76:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong LZ, Sun HM, Lv XJ, Yang D, Zhang JN
and Shi Y: Expression of periostin in the serum of NSCLC and its
function on proliferation and migration of human lung
adenocarcinoma cell line (A549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang WC, Zinn K and Bjorkman PJ:
Expression and structural studies of fasciclin I, an insect cell
adhesion molecule. J Biol Chem. 268:1448–1455. 1993.PubMed/NCBI
|
|
20
|
Kim JE, Kim SJ, Lee BH, Park RW, Kim K and
Kim IS: Identification of motifs for cell adhesion within the
repeated domains of transforming growth factor-beta-induced gene,
betaig-h3. J Biol Chem. 275:30907–30915. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasaki H, Dai M, Auclair D, Kaji M, Fukai
I, Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, in
thymoma patients. Cancer Lett. 172:37–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo Y, Takata T, Ogawa I, Kaneda T, Sato
S, Takekochi T, Zhao M, Miyauchi M and Nikai H: p27Kip1
accumulation by inhibition of proteasome function induces apoptosis
in oral squamous cell carcinoma cells. Clin Cancer Res. 6:916–923.
2000.PubMed/NCBI
|
|
23
|
Malanchi I, Santamaria-Martínez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soltermann A, Tischler V, Arbogast S,
Braun J, Probst-Hensch N, Weder W, Moch H and Kristiansen G:
Prognostic significance of epithelial-mesenchymal and
mesenchymal-epithelial transition protein expression in non-small
cell lung cancer. Clin Cancer Res. 14:7430–7437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Puglisi F, Puppin C, Pegolo E, Andreetta
C, Pascoletti G, D’Aurizio F, Pandolfi M, Fasola G, Piga A, Damante
G and Loreto CD: Expression of periostin in human breast cancer. J
Clin Pathol. 61:494–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vineis P, Alavanja M, Buffler P, Fontham
E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J,
Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH,
Zaridze D, Peto R and Doll R: Tobacco and cancer: recent
epidemiological evidence. J Natl Cancer Inst. 96:99–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
International Agency for Research on
Cancer. Tobacco smoking. IARC monographs on the evaluation of
carcinogenic risks to humans. 38. IARC; Lyon: 1986
|
|
28
|
Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR
and Isaacson PG: Helicobacter pylori-associated gastritis and
primary B-cell gastric lymphoma. Lancet. 338:1175–1176. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang WH, Huang JQ, Zheng GF, Lam SK,
Karlberg J and Wong BC: Non-steroidal anti-inflammatory drug use
and the risk of gastric cancer: a systematic review and
meta-analysis. J Natl Cancer Inst. 95:1784–1791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dvorak HF: Tumors: wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rakian A, Yang WC, Gluhak-Heinrich J, Cui
Y, Harris MA, Villarreal D, Feng JQ, Macdougall M and Harris SE:
Bone morphogenetic protein-2 gene controls tooth root development
in coordination with formation of the periodontium. Int J Oral Sci.
5:75–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM
and Lau LF: Integrin-dependent functions of the angiogenic inducer
NOV (CCN3): implication in wound healing. J Biol Chem.
280:8229–8237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leask A and Abraham DJ: The role of
connective tissue growth factor, a multifunctional matricellular
protein, in fibroblast biology. Biochem Cell Biol. 81:355–363.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holloway SE, Beck AW, Girard L, Jaber MR,
Barnett CC Jr, Brekken RA and Fleming JB: Increased expression of
Cyr61 (CCN1) identified in peritoneal metastases from human
pancreatic cancer. J Am Coll Surg. 200:371–377. 2005. View Article : Google Scholar : PubMed/NCBI
|