Introduction
Multiple myeloma (MM) is a plasma cell malignancy
that is considered to be the second most common hematological
cancer in the world (1). The
remission rate of MM remains low due to the complex pathogenesis
and multidrug resistance. Newer chemotherapeutic regimens and
high-dose chemotherapy have increased the response rate in myeloma.
However, the unpleasant side-effects, including hepatotoxicity,
cardiotoxicity, hematotoxicity and infection, restrict their
clinical efficacy. In recent studies, investigators have recognized
the potential use of natural products as potent chemotherapeutic
drugs for MM to improve the therapeutic efficacy and also reduce
the side-effects (2,3). For instance, oridonin, an active
diterpenoid compound isolated from Rabdosia rubescens,
simultaneously induces the apoptosis and autophagy of human MM
cells (4).
Asiatic acid (AA), a pentacyclic triterpenoid
derived from the tropical medicinal plant Centella asiatica
(Apiaceae family), has a wide variety of biological activities. For
a long period of time, AA was mainly believed to be responsible for
wound healing, protective activities against UV-induced photo
aging, glutamate- or β-amyloid-induced neurotoxicity and
hepatofibrosis (5–9). Recently, the apoptosis-inducing
activity of AA in various cancer cells has aroused the attention of
investigators (10). For example,
AA has been successively reported to possess strong cell growth
inhibition in hepatoma, breast cancer, melanoma, glioblastoma and
gastrointestinal tumor cells (11–15).
However, the effects of AA on hematological malignant cells remain
unclear. Thus, in the present study, AA was first identified to
inhibit cell proliferation through the arrest of RPMI 8226 cells at
the G2/M phase, whereas little is known about the
mechanism of AA-induced anti-myeloma action. A previous study
determined that celastrol, which is also a triterpene, exerts
antitumor activities accompanied by the reduced phosphorylation of
focal adhesion kinase (FAK) (16).
Previous studies also showed that phosphatase and tensin homolog
deleted on chromosome 10 prevented the metastasis of myeloma cells
by downregulating the activity of the FAK/matrix metalloproteinase
signaling pathway. FAK is a non-receptor tyrosine kinase that
modulates cell adhesion, movement and survival, which may be
associated with disease progression, extramedullary infiltration
and the apoptosis of MM cells (17,18).
Earlier studies have indicated that the suppression of FAK
expression, caused by interrupting the nuclear factor κB pathway,
provided a potential molecular target in MM (19,20).
Hence, in conjunction with these findings, we speculate that the
underlying mechanism of the anti-proliferation function of AA may
be through the inhibition of FAK expression in MM cells.
Materials and methods
Main reagents
AA (molecular formula,
C30H48O5; molecular weight, 488.7
Da), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
(MTT), dimethyl sulfoxide (DMSO) and propidium iodide (PI) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). A 50-mmol/l AA
stock solution was prepared in DMSO, stored at −20°C as small
aliquots and then thawed prior to use. RPMI-1640 media and
phosphate-buffered saline (PBS) were purchased from Invitrogen
(Carlsbad, CA, USA). Fetal bovine serum (FBS) products were
purchased from Hangzhou Sijiqing Biological Engineering Materials
Co., Ltd. (Hangzhou, Zhejiang, China). Lymphoprep Ficoll was
purchased from Axis-Shield (Oslo, Norway). The PI reagent kit was
purchased from Nanjing Key-Gen Biotech Co., Ltd. (Nanjing, Jiangsu,
China).
Cell culture
The RPMI 8226 cells had been stored long-term and
passaged in the Institute of Hematology, Huazhong University of
Science and Technology (Wuhan, China). The RPMI 8226 cell line, a
human factor-independent myeloma cell line, was cultured in
RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified
atmosphere containing 5% CO2. The culture medium was
replaced with fresh medium every 2 to 3 days. The cells in the
mid-log phase were used in the experiment. The collection of blood
samples and the isolation of peripheral blood mononuclear cells
(PBMCs) were performed as previously reported (21). All blood donors provided their
informed consent. Briefly, the cells were used directly after
isolation and stored in RPMI-1640 medium with 10% FBS, 1%
penicillin/streptomycin and 1% L-glutamine (both from Invitrogen)
overnight prior to incubation.
MTT assay
The effects of AA on the proliferation of the RPMI
8226 cells were detected by MTT assay. Briefly, the RPMI 8226 cells
were harvested at mid-log phase and the PBMCs were prepared as a
control group. Subsequently, a 200-μl suspension of cells was
seeded in 96-well plates with or without AA at various
concentrations (10, 20, 30, 40, 50, 60 and 70 μmol/l) at a density
of 3×105 cells/well. Subsequent to incubation for a
designated period of time, 20 μl MTT solution (5 mg/ml) was added,
and the cells were incubated at 37°C for another 4 h. The
supernatant was discarded and 150 μl DMSO was added. The plate was
gently vortexed until the blue formazan crystals were fully
dissolved. The absorbance (A) was read at an optical density of 490
nm using a microplate reader (Tecan Spectra; Tecan Group Ltd.,
Männedorf, Switzerland) and the growth inhibitory rates were
calculated as follows: [1 - (A of experimental sample / A of the
control sample)] × 100.
Flow cytometric analysis
The cells in mid-log phase were divided into the
control and the experimental groups, and the cell concentration of
each group was 1×106/ml. The RPMI 8226 cells were
treated with various concentrations of AA (0, 25, 35 and 40
μmol/l), and the cell cycle was analyzed by flow cytometry (FCM).
The RPMI 8226 cells were collected following treatment using EP
tubes, fixed in 70% cold ethanol for 24 h, washed twice with PBS
and resuspended in 440 μl PBS. A volume of 10 μl RNaseA (5 mg/ml)
was added into the tube and incubated for 30 min. Subsequently, 50
μl PI was added and the cells were incubated at 4°C in the dark for
another 30 min. The fluorescence intensity was detected using a
flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) and the
cell cycle was analyzed using FlowJo software (version 7.6; Tree
Star Inc., Ashland, OR, USA).
Western blot analysis
All the RPMI 8226 cells treated with AA at various
concentrations for 24 h were collected and subjected to western
blot analysis. The cells were lysed in a modified RIPA lysis
buffer, and the protein in the supernatant was quantified using the
Coomassie Brilliant Blue kit (Pierce, Rockford, IL, USA). The
prepared protein samples were stored at −10°C prior to use. Next,
10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (90
μg protein per lane) was performed; the proteins were then
transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked in PBS Tween-20 (5 g/l) containing skimmed
milk (at the concentration of 50 g/l) at 4°C overnight, then washed
and incubated with primary rabbit anti-human FAK polyclonal
antibody. The PVDF membranes were then washed and incubated with
the horseradish peroxidase-conjugated secondary antibody (Sanying
Biotechnology Co., Wuhan, Hubei, China) and exposed for 2 sec using
chemiluminescent autoradiography. The X-ray films were then
developed.
Immunoprecipitation
A volume of 30 μl mouse anti-human PY100 was added
into 200 μg total protein and vortexed at room temperature for 1 h.
Next, 50 μl protein G (Sanying Biotechnology Co.) was added into
the tube for precipitation and the sample was washed. The remaining
steps were in line with the western blot analysis.
Statistical analysis
Each experiment was repeated at least three times.
The data were presented as the mean ± SD and analyzed using SPSS
11.0 Statistical Software for Windows (SPSS, Inc., Chicago, IL,
USA). The comparisons between each group were analyzed by t-test.
Statistically significant differences were indicated by
P<0.05.
Results
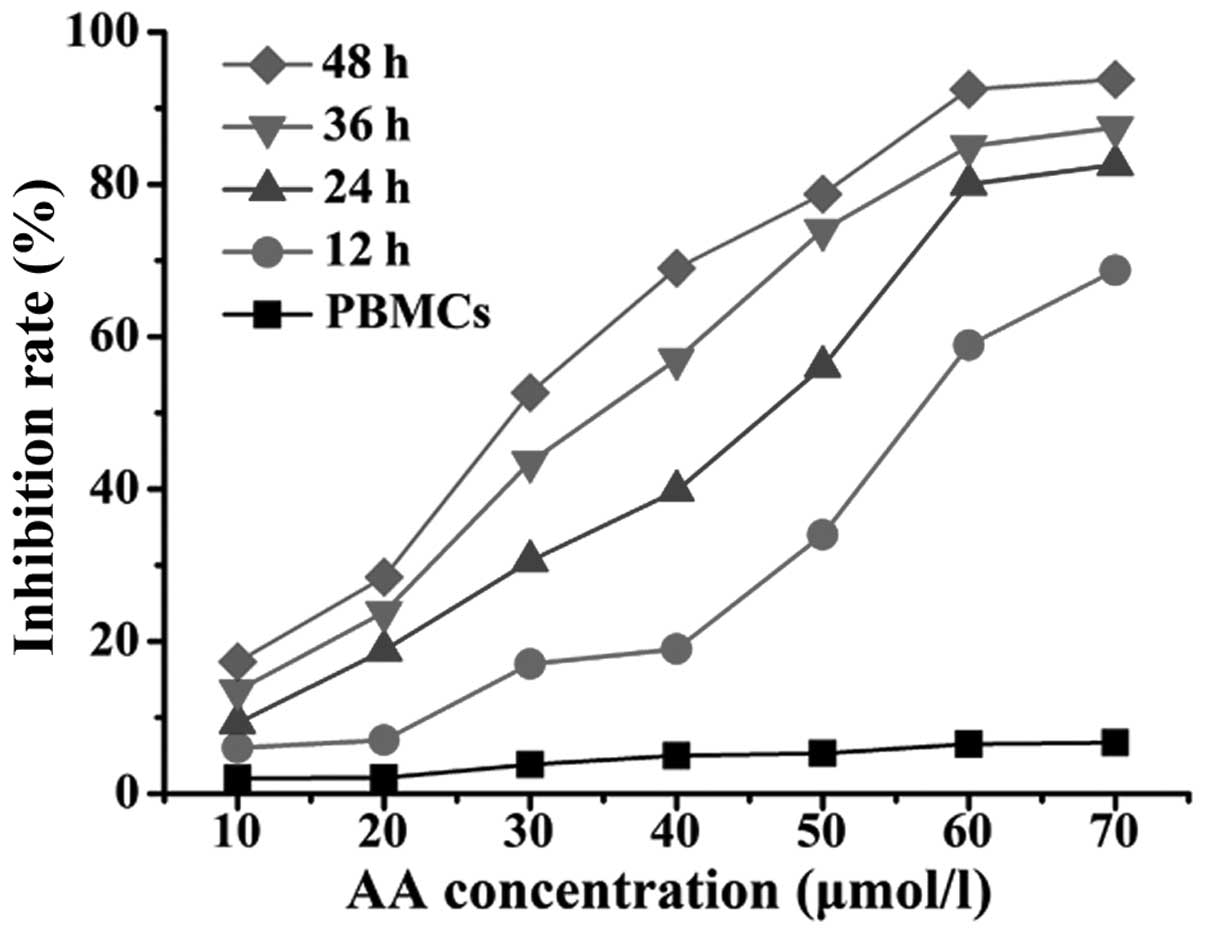
AA inhibits the proliferation of RPMI
8226 cells
To investigate whether AA exerted anti-proliferative
effects on the MM cells, the cytotoxicity of various concentrations
of AA (0, 10, 20, 30, 40, 50, 60 and 70 μmol/l) on RPMI 8226 cells
for 12, 24, 36 and 48 h was detected by MTT assay. As shown in
Fig. 1, the cell viability was
inhibited by AA in a time- and dose-dependent manner in the RPMI
8226 groups. By contrast, rarely detectable changes were exhibited
in the viability of the PBMCs. The rate of proliferative inhibition
for the RPMI 8226 cells increased significantly following
incubation with various concentrations of AA for 12 h (P<0.05).
At the same concentration (40 μmol/l), the rate was also
significantly different at various times (P<0.05). The
IC50 of AA for the RPMI 8226 cells was 53.76±2.88,
42.25±4.57, 32.78±3.25 and 24.88±3.51 μmol/l at 12, 24, 36 and 48
h, respectively.
AA induces cell cycle arrest in RPMI 8226
cells
Subsequent to the treatment of the RPMI 8226 cells
with various concentrations of AA for 24 h, the cell cycle analysis
by FCM showed that the percentage of RPMI 8226 cells in the
G2/M phase had increased significantly at each
time-point tested. The cell cycle distribution of the RPMI 8226
cells measured at various time points is shown in Fig. 2. The cells exposed to 35 and 40
μmol/l AA showed evident cell cycle arrest, with cells
predominantly arrested in the G2/M phase. Notably, no
evident changes of increased G2/M phase cells were noted
in the groups treated with 25 μmol/l AA.
AA decreases the expression of FAK and
p-FAK
The expression levels of FAK and p-FAK were assessed
in the AA-treated RPMI 8226 cells by western blotting and
immunoprecipitation. As shown in Fig.
3, the exposure of the RPMI 8226 cells to 35 and 40 μmol/l AA
for 24 h resulted in a significant inhibition of FAK and p-FAK in a
dose-dependent manner compared with the control group.
Discussion
Despite gradual advancements in the understanding of
drug combinations for MM, the side-effects and relatively low
remission rate of chemotherapy have spurred a number of researchers
to establish more effective treatment regimens by adopting novel
and innovative approaches. The discovery and exploitation of active
medicinal compounds from natural sources have provided alternative
treatment choices for patients (22). For example, AA, a triterpene acid
derived from the traditional medicinal plant C. asiatica,
belongs to the pentacyclic triterpenoids. There have been numerous
studies demonstrating the strong anti-solid tumor efficacy of AA.
AA has been reported to induce apoptosis in human hepatoma, breast
cancer, melanoma, glioblastoma and gastrointestinal tumors
(11–15). The major findings of the present
study were that AA appeared to inhibit the cell proliferation of
the RPMI 8226 cells with the effective concentration being at the
μmol/l level, consistent with the effective concentration level of
AA in solid tumors. In addition, the marked anti-proliferative
activity induced by AA occurred in a time- and dose-dependent
manner, with an IC50 of 53.76±2.88, 42.25±4.57,
32.78±3.25 and 24.88±3.51 μmol/l in the RPMI 8226 cells at 12, 24,
36 and 48 h, respectively. It was also determined that AA had
little impact on normal cells, as the proliferation rate of the
PBMCs was maintained at a steady rate following exposure to various
concentrations of AA. Consequently, these results may aid in the
development of therapeutic agents for MM. However, the specific
mechanism by which AA inhibits cell proliferation remains unknown.
Hsu et al(12) stated that
AA-induced cell growth inhibition in the MCF-7 and MDA-MB-231 cell
lines was mediated by the activation of p38 and extracellular
signal-regulated kinases 1/2. Additionally, it was demonstrated
that a novel mechanism of AA-induced cell death was linked to
disruption of the endoplasmic reticulum, with subsequent calcium
flux into the cytoplasm (23). AA
has been observed to show discrepant anticancer mechanisms in
differing cell types, therefore, further scientific experiments and
sufficient subsequent proofs are required to resolve these
problems.
Tumor cell cycles are closely associated with cell
proliferation, which is mainly regulated at two discrete points,
including the G1/S and G2/M phases. In the
present study, when the concentration of AA fluctuated in the range
of 25–40 μmol/l, the proportion of G2/M-phase cells
increased from 5.21±2.37 to 54.05±5.66% as the drug dosage
increased, indicating that the RPMI 8226 cells were primarily
arrested in the G2/M phase. Other studies have also
reported the AA-induced regulation of tumor cell cycles. For
instance, Hsu et al(12)
stated that AA inhibited cell cycle progression at the
S-G2/M phase through increasing p21/Cdc2 interaction and
decreasing the expression of Cdc2, Cdc25C, cyclin B1 and cyclin
A.
To date, the underlying anti-myeloma mechanism of AA
remains unclear. FAK is a member of the FAK family of non-receptor
protein tyrosine kinases, which resides at sites of integrin
clustering and has an important role in cell proliferation,
survival and migration (24–28).
The increased expression and activity of FAK are frequently
correlated with malignant disease and a poor patient prognosis
(29–31). Recent studies have indicated that
FAK may be a useful therapeutic target for the improved treatment
of acute myeloid leukemia cases with poor prognoses (32), and abnormal expression of FAK in
patients with MM may be associated with clinical stage and
extramedullary infiltration (17).
Furthermore, Schmidmaier et al(18) concluded that LFA-1/FAK/PI3-K/Akt is
a survival pathway in MM and that targeted inhibition may provide
new therapeutic options. Notably, the present study also identified
that the expression levels of FAK and p-FAK were reduced in
AA-treated RPMI 8226 cells. Once again, this study confirmed that
AA may serve as a potent anticancer drug and that its mechanism may
be associated with the downregulation of FAK expression.
Consequently, we speculate that AA may be an adjuvant therapeutic
agent for MM and improve the prognosis of high-risk myeloma
patients through decreasing the expression of FAK and p-FAK.
In conclusion, AA inhibited cell proliferation by
arresting cell cycle progression and downregulating the expression
of FAK in the RPMI 8226 cells. These results strongly indicated
that AA may be a potential candidate for antitumor therapy,
particularly for MM treatment.
Abbreviations:
|
AA
|
asiatic acid
|
|
MM
|
multiple myeloma
|
|
FAK
|
focal adhesion kinase
|
|
PBMCs
|
peripheral blood mononuclear cells
|
References
|
1
|
Sedlarikova L, Kubiczkova L, Sevcikova S
and Hajek R: Mechanism of immunomodulatory drugs in multiple
myeloma. Leuk Res. 36:1218–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harousseau JL: Thalidomide in multiple
myeloma: past, present and future. Future Oncol. 2:577–589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Björkstrand B and Gahrton G: High-dose
treatment with autologous stem cell transplantation in multiple
myeloma: past, present, and future. Semin Hematol. 44:227–233.
2007.PubMed/NCBI
|
|
4
|
Zeng R, Chen Y, Zhao S and Cui GH:
Autophagy counteracts apoptosis in human multiple myeloma cells
exposed to oridonin in vitro via regulating intracellular ROS and
SIRT1. Acta Pharmacol Sin. 33:91–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maquart FX, Chastang F, Simeon A,
Birembaut P, Gillery P and Wegrowski Y: Triterpenes from
Centella asiatica stimulate extracellular matrix
accumulation in rat experimental wounds. Eur J Dermatol. 9:289–296.
1999.
|
|
6
|
Soo Lee Y, Jin DQ, Beak SM, Lee ES and Kim
JA: Inhibition of ultraviolet-A-modulated signaling pathways by
asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur J
Pharmacol. 476:173–178. 2003.PubMed/NCBI
|
|
7
|
Xu MF, Xiong YY, Liu JK, Qian JJ, Zhu L
and Gao J: Asiatic acid, a pentacyclic triterpene in Centella
asiatica, attenuates glutamate-induced cognitive deficits in
mice and apoptosis in SH-SY5Y cells. Acta Pharmacol Sin.
33:578–587. 2012.PubMed/NCBI
|
|
8
|
Patil SP, Maki S, Khedkar SA, Rigby AC and
Chan C: Withanolide A and asiatic acid modulate multiple targets
associated with amyloid-beta precursor protein processing and
amyloid-beta protein clearance. J Nat Prod. 73:1196–1202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang LX, He RH, Yang G, et al: Asiatic
acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in
vivo and in vitro. PLoS One. 7:e313502012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park BC, Paek SH, Lee YS, et al:
Inhibitory effects of asiatic acid on
7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol
13-acetate-induced tumor promotion in mice. Biol Pharm Bull.
30:176–179. 2007.PubMed/NCBI
|
|
11
|
Lee YS, Jin DQ, Kwon EJ, et al: Asiatic
acid, a triterpene, induces apoptosis through intracellular
Ca2+ release and enhanced expression of p53 in HepG2
human hepatoma cells. Cancer Lett. 186:83–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu YL, Kuo PL, Lin LT and Lin CC: Asiatic
acid, a triterpene, induces apoptosis and cell cycle arrest through
activation of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase pathways in human breast cancer
cells. J Pharmacol Exp Ther. 313:333–344. 2005. View Article : Google Scholar
|
|
13
|
Park BC, Bosire KO, Lee ES, Lee YS and Kim
JA: Asiatic acid induces apoptosis in SK-MEL-2 human melanoma
cells. Cancer Lett. 218:81–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho CW, Choi DS, Cardone MH, Kim CW,
Sinskey AJ and Rha C: Glioblastoma cell death induced by asiatic
acid. Cell Biol Toxicol. 22:393–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang XL, Yang XY, Jung HJ, et al: Asiatic
acid induces colon cancer cell growth inhibition and apoptosis
through mitochondrial death cascade. Biol Pharm Bull. 32:1399–1405.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu H, Liu XW, Cai TY, et al: Celastrol
acts as a potent antimetastatic agent targeting beta1 integrin and
inhibiting cell-extracellular matrix adhesion, in part via the p38
mitogen-activated protein kinase pathway. J Pharmacol Exp Ther.
334:489–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang SY, Hao HL, Deng K, et al: Expression
levels of phosphatase and tensin homolog deleted on chromosome 10
(PTEN) and focal adhesion kinase in patients with multiple myeloma
and their relationship to clinical stage and extramedullary
infiltration. Leuk Lymphoma. 53:1162–1168. 2012. View Article : Google Scholar
|
|
18
|
Schmidmaier R, Mandl-Weber S, Gaul L, et
al: Inhibition of lymphocyte function associated antigen 1 by
LFA878 induces apoptosis in multiple myeloma cells and is
associated with downregulation of the focal adhesion
kinase/phosphatidylinositol 3 kinase/Akt pathway. Int J Oncol.
31:969–976. 2007.
|
|
19
|
Ko BS, Chang TC and Liou JY: Focal
adhesion kinase as a therapeutic target of bortezomib. Anticancer
Agents Med Chem. 10:747–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko BS, Chang TC, Chen CH, et al:
Bortezomib suppresses focal adhesion kinase expression via
interrupting nuclear factor-kappa B. Life Sci. 86:199–206. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hofmann T, Klenow S, Borowicki A, Gill CI,
Pool-Zobel BL and Glei M: Gene expression profiles in human
peripheral blood mononuclear cells as biomarkers for nutritional in
vitro and in vivo investigations. Genes Nutr. 5:309–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith M and Boon HS: Counseling cancer
patients about herbal medicine. Patient Educ Couns. 38:109–120.
1999. View Article : Google Scholar
|
|
23
|
Gurfinkel DM, Chow S, Hurren R, et al:
Disruption of the endoplasmic reticulum and increases in
cytoplasmic calcium are early events in cell death induced by the
natural triterpenoid Asiatic acid. Apoptosis. 11:1463–1471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michael KE, Dumbauld DW, Burns KL, Hanks
SK and García AJ: Focal adhesion kinase modulates cell adhesion
strengthening via integrin activation. Mol Biol Cell. 20:2508–2519.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parsons JT, Martin KH, Slack JK, Taylor JM
and Weed SA: Focal adhesion kinase: a regulator of focal adhesion
dynamics and cell movement. Oncogene. 19:5606–5613. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: in command and control of cell motility. Nat
Rev Mol Cel Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomar A and Schlaepfer DD: Focal adhesion
kinase: switching between GAPs and GEFs in the regulation of cell
motility. Curr Opin Cell Biol. 21:676–683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cance WG, Harris JE, Iacocca MV, et al:
Immunohistochemical analyses of focal adhesion kinase expression in
benign and malignant human breast and colon tissues: correlation
with preinvasive and invasive phenotypes. Clin Cancer Res.
6:2417–2423. 2000.
|
|
30
|
Recher C, Ysebaert L, Beyne-Rauzy O, et
al: Expression of focal adhesion kinase in acute myeloid leukemia
is associated with enhanced blast migration, increased cellularity,
and poor prognosis. Cancer Res. 64:3191–3197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlaepfer DD, Mitra SK and Ilic D:
Control of motile and invasive cell phenotypes by focal adhesion
kinase. Biochim Biophys Acta. 1692:77–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Despeaux M, Chicanne G, Rouer E, et al:
Focal adhesion kinase splice variants maintain primitive acute
myeloid leukemia cells through altered Wnt signaling. Stem Cells.
30:1597–1610. 2012. View Article : Google Scholar : PubMed/NCBI
|