Introduction
Inflammatory myofibroblastic tumor (IMT) is an
uncommon tumor that was first described in the lung and has now
been identified at multiple extrapulmonary anatomical sites
(1). In the genito-urinary tract,
IMT most commonly occurs in the bladder. However, a small series of
renal IMT cases have been reported in the medical literature, to
date (2–5). The etiology and pathogenesis of IMT
remain uncertain; however, infection, vascular causes, autoimmune
disorders and the anaplastic lymphoma kinase (ALK) gene have been
proposed (7,8). The present study describes a case of a
48-year-old female with an IMT of the kidney, who was treated by
radical nephrectomy and had a history of trauma to the left
hypochondrium 13 years previously. The patient also had a history
of hepatitis B for 20 years, which developed into hepatic
cirrhosis, hypersplenism and coagulation disorders and may improve
the understanding of the etiology and pathogenesis of renal IMT.
Written informed consent was obtained from the patient.
Case report
A 48-year-old female visited the First Hospital,
Jilin University (Jilin, China) for routine check-up for hepatitis
B on July 19, 2012 and presented with no symptoms. The patient had
a history of trauma to the left hypochondrium 13 years previously
and a history of hepatitis B for 20 years. The latter developed
into hepatic cirrhosis, hypersplenism and coagulation disorders.
The physical and basic paraclinical examinations were normal. Blood
tests revealed a leukocyte count of 2850/mm3, a
hemoglobin count of 5.6 g/dl, a platelet count of
6700/mm3, a urine leukocyte count of 20.1/HPF and a
urine erythrocyte count of 2.5/HPF. The thrombin time was 19.4 sec
and the prothrombin time was 14.0 sec. The international normalized
ratio was 1.21, the prothrombin ratio was 1.22 and the prothrombin
activity was 69%. Clinical laboratory measurments revealed the
following levels: Serum fibrinogen, 0.55 g/l; hepatitis B virus
surface antigen (HBsAg), 197.260 IU/Ml; hepatitis B virus e
antigen, 0.299S/CO; hepatitis B virus e antibody, 0.110S/CO; and
hepatitis B virus core antibody, 18.210S/CO. An abdominal
ultrasonography revealed a 1.4×1.4-cm-sized mass with an obscure
boundary in the upper pole of the kidney, which protruded through
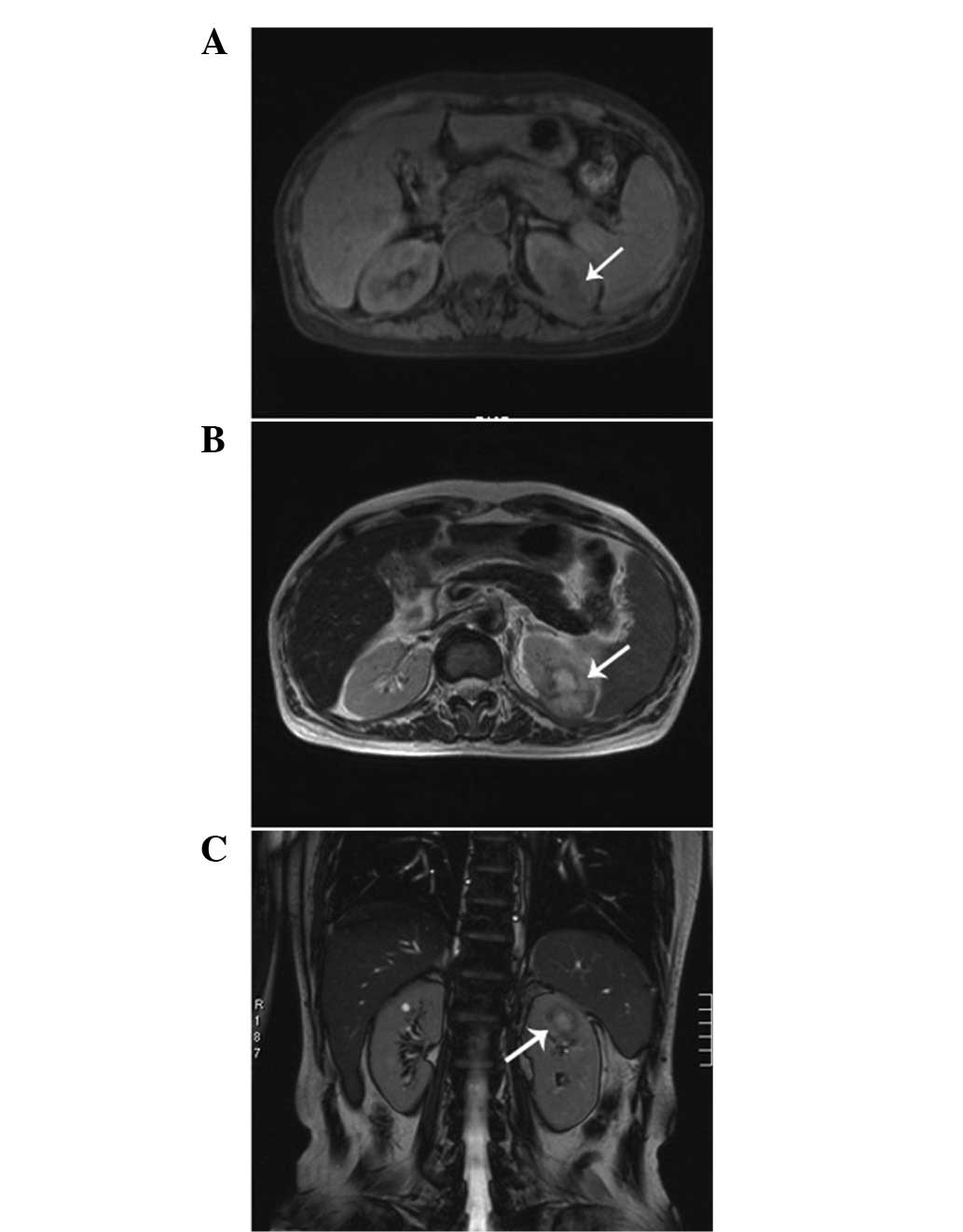
the surface. The computed tomography (CT) scan revealed a
1.6×2.9×2.0-cm lesion in the upper pole of the kidney. The CT was
slightly enhanced with contrast (Fig.
1). The magnetic resonance imaging revealed a heterogeneous
mass measuring 2.6 cm, showing low intensity on the T1-weighted
images and high intensity on the T2-weighted images, which was
accompanied with hypointensity that surrounded the center of the
lesion (Fig. 2). A radical
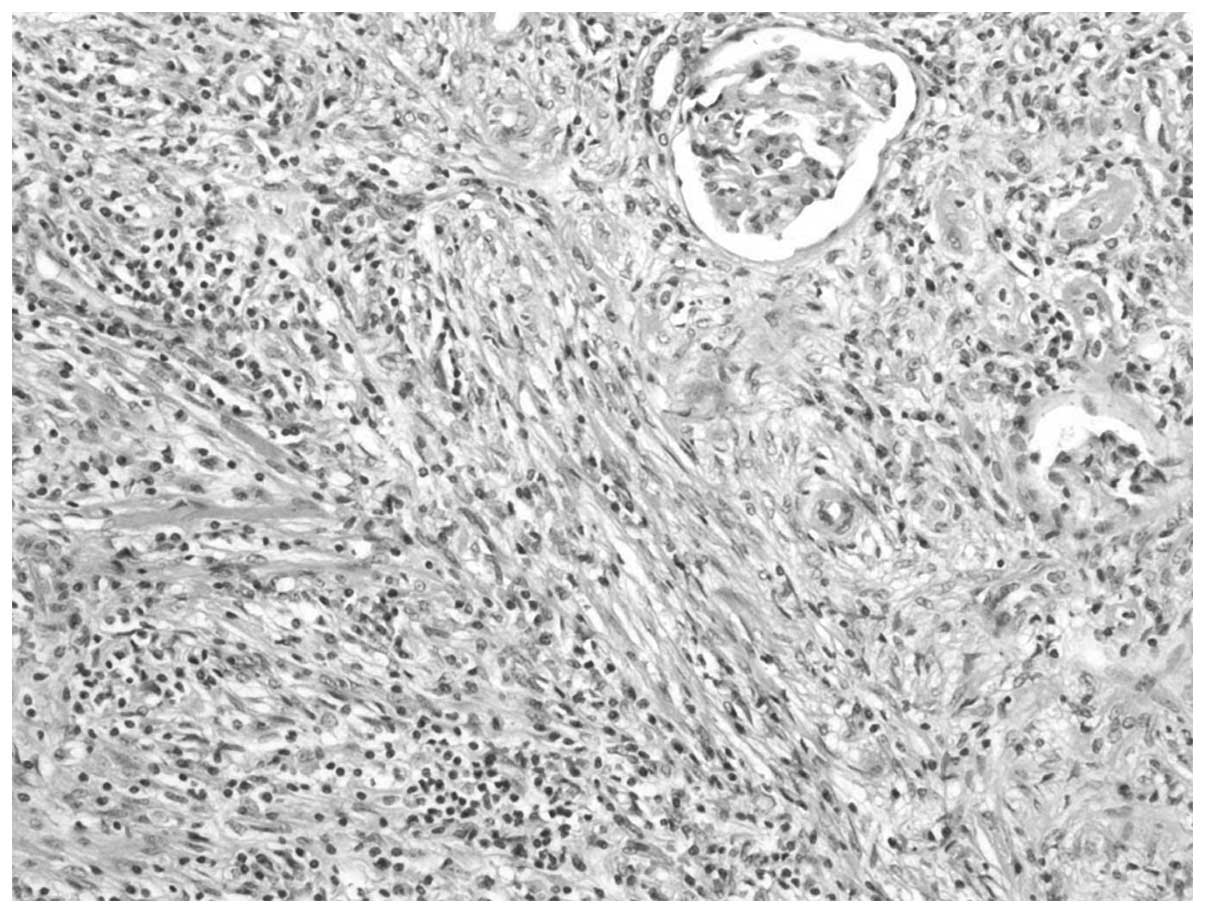
nephrectomy was performed. The histopathological examination
resulted in the lesion being diagnosed as an IMT, in which spindle
cells were admixed with variable amounts of extracellular collagen,
lymphocytes and plasma cells (Fig.
3). Immunostaining was positive for vimentin and focally
positive for smooth muscle actin, desmin and Ki-67 (Fig. 4). There was no evidence of
recurrence during a follow-up period of six months.
Discussion
IMT has been recognized as an inflammatory
pseudotumor and has been considered to be a reparative
post-inflammatory condition rather than a neoplastic process
(6). However, rearrangements
involving ALK have been documented in pulmonary and extrapulmonary
IMTs, supporting the contention that IMT is a neoplasm (7). No definitive cause of the condition
has been identified and the etiology is likely to be
multifactorial, including infection, vascular causes and autoimmune
disorders (8). However, the
identification of the ALK gene fusion has allowed an improved
recognition and understanding of the pathogenesis of IMT with
regard to the genetic aspect (7).
Granulomas and giant cells have been observed in the tumor tissue,
suggesting that infection is associated with IMT (9). Numerous studies have indicated that
IMT may be due to an intraparenchymal hemorrhage that is secondary
to trauma or coagulopathy (10).
Cotelingam and Jaffe have suggested that the initial event may be a
focal parenchymal necrosis with hemorrhage (11). In the present study, the
inflammatory reaction was suspected to have occurred as a
consequence of the sedimentation of HBsAg or an antigen-antibody
complex in the kidney. Furthermore, the intraparenchymal hemorrhage
of the kidney may have been due to the left hypochondrium trauma
history and the coagulation disorders that were caused by hepatic
cirrhosis.
An IMT patient may be clinically asymptomatic or may
present with flank pain, painless hematuria and ureteropelvic
junction stenosis with hydronephrosis (12). Histologically, IMTs are
characterized by a mix of inflammatory cells, including plasma
cells, lymphocytes, eosinophils and bland spindle cells without
nuclear atypia. The tumors may exhibit necrosis, hemorrhage, focal
calcification and mitotic activity (6). Confirming a pre-operative diagnosis is
difficult and the tumor may be misdiagnosed as renal cell
carcinoma, as the symptoms and imaging findings are not specific
and are particularly difficult to distinguish from renal cell
carcinoma. Therefore, a nephrectomy is usually selected as a
treatment strategy (13).
Histological examination is of particular significance to obtain a
clear diagnosis. However, an inadequate amount of tissue is often
obtained by CT-guided fine needle aspiration. Therefore, the
definitive diagnosis is often by histopathological examination of
the resected specimen following surgery (14). IMT exhibits a good prognosis;
Kapusta et al have reported a series of 12 cases of IMT of
the kidney, of which the follow-up information was available in
eight cases and ranged from 1 to 17 years, with no evidence of
recurrence (12).
In summary, IMT of the kidney is extremely rare and
the etiology and pathogenesis remain unclear. In the present case,
the patient had a history of trauma of the left hypochondrium and a
long-term history of hepatitis B, which developed into hepatic
cirrhosis, hypersplenism and coagulation disorders. The subsequent
conditions may have played a significant role in the development of
IMT of the kidney in the present case, and may also be useful in
increasing the understanding of the etiology and pathogenesis of
IMT of the kidney.
Acknowledgements
The authors would like to thank the teachers at the
Center of Urology and the support provided by the Departments of
Radiology and Pathology at the First Hospital of Jilin
University.
References
|
1
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor): a clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar
|
|
2
|
Harik LR, Merino C, Coindre JM, Amin MB,
Pedeutour F and Weiss SW: Pseudosarcomatous myofibroblastic
proliferations of the bladder: a clinicopathologic study of 42
cases. Am J Surg Pathol. 30:787–794. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harper L, Michel JL, Riviere JP, Alsawhi A
and De Napoli-Cocci S: Inflammatory pseudotumor of the ureter. J
Pediatr Surg. 40:597–599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larbcharoensub N, Chobpradit N, Kijvikai K
and Chalermsanyakorn P: Primary renal inflammatory myofibroblastic
tumor. Urol Int. 76:94–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leroy X, Copin MC, Graziana JP, Wacrenier
A and Gosselin B: Inflammatory pseudotumor of the renal pelvis: A
report of 2 cases with clinicopathologic and immunohistochemical
study. Arch Pathol Lab Med. 124:1209–1212. 2000.PubMed/NCBI
|
|
6
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coffin CM, Patel A, Perkins S,
Elenitoba-Johnson KS, Perlman E and Griffin CA: ALK1 and p80
expression and chromosomal rearrangements involving 2p23 in
inflammatory myofibroblastic tumor. Mod Pathol. 14:569–576. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta P, Dhingra KK, Singhal S, Mandal S,
Khurana N and Saroha V: Inflammatory myofibroblastic tumour of the
kidney with a papillary adenoma. Pathology. 42:193–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oz Puyan F, Bilqi S, Unlu E, Yalcin O,
Altaner S, Demir M and Cakir B: Inflammatory pseudotumour of the
spleen with EBV positivity: report of a case. Eur J Haematol.
72:285–291. 2004.PubMed/NCBI
|
|
10
|
Horiuchi R, Uchida T, Kojima T and Shikata
T: Inflammatory pseudotumor of the liver. Clinicopathologic study
and review of the literature. Cancer. 65:1583–1590. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cotelingam JD and Jaffe ES: Inflammatory
pseudotumour of the spleen. Am J Surg Pathol. 8:375–380. 1984.
View Article : Google Scholar
|
|
12
|
Kapusta LR, Weiss MA, Ramsay J,
Lopez-Beltran A and Sriqley JR: Inflammatory myofibroblastic tumors
of the kidney: a clinicopathologic and immunohistochemical study of
12 cases. Am J Surg Pathol. 27:658–666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryu KH, Im CM, Kim MK, Kwon D, Park K, Ryu
SB and Choi C: Inflammatory myofibroblastic tumor of the kidney
misdiagnosed as renal cell carcinoma. J Korean Med Sci. 25:330–332.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bell ND, Gavras JN, Donnell CA and Rodning
CB: Renal inflammatory pseudotumour. South Med J. 91:1050–1053.
1998. View Article : Google Scholar : PubMed/NCBI
|