Introduction
Breast cancer is one of the most common malignant
tumors among females worldwide. Although mortality rates are
decreasing due to combined therapy, breast cancer remains a leading
cause of cancer-related mortality in females. In India, breast
cancer has overtaken cervical cancer, which was the most common
cancer a decade ago (1).
The studies from the National Cancer Institute (NCI;
National Institutes of Health) indicated that 226,870 females would
be diagnosed with breast cancer and 39,510 would succumb to this
disease during 2012. The data from the Indian population based
cancer registry (PBCR; 2006–2008; Indian Council of Medical
Research) suggest that breast cancer accounts for 28–35% of all
cancers in females within the major cities of India. A total of 130
million Indian females are expected to live beyond the menopause
into old age by 2015 (2). While the
natural age of menopause in developed countries is 51 years, in
India the mean age is ~45 years (3). As the breast cancer risk is high among
post-menopausal women, it is predicted that breast cancer may be a
major cause of mortality in India in the next few decades.
Therefore, it is strongly argued that India should adopt screening
strategies for the early diagnosis of cancer, as it is usually
associated with an improved clinical outcome and the increased
overall survival of patients.
The expression profiles of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor (HER2)/neu have been used for predicting the outcome and
response to the therapy of breast cancer for a number of years.
However, the assessment of these clinical and pathological features
is not sufficient to fully capture the heterogeneous clinical
course of breast cancer, making it necessary to identify new
biomarkers that are associated with growth, angiogenesis and
metastases.
Osteopontin (OPN), a secreted, non-collagenous,
extracellular matrix protein that belongs to the small
integrin-binding ligand N-linked glycoprotein (SIBLING) family,
plays a significant role in determining the oncogenic potential of
various cancers and is recognized as a key marker in the processes
of tumorigenicity and metastasis (4). OPN is involved in normal tissue
remodeling processes, including bone resorption, wound healing and
tissue injuries, in addition to restenosis, atherosclerosis,
tumorigenesis and autoimmune diseases (5,6). OPN
has been shown to play a significant role in tumor invasion and
metastasis in breast, lung, prostate and colon cancers. Due to its
known tumor-associated biological functions, OPN appears to have
the potential to aid in the identification of high-risk tumors.
Therefore, the detection of OPN expression levels in breast cancer
patients may be useful in establishing its role as a diagnostic
marker (7,8).
In breast cancer, high OPN levels in the tumor
tissue are associated with a poor prognosis and disease progression
(9). OPN acts as a clinical
prognostic marker and is a key player in the six hallmarks of
cancer that include self-sufficiency in growth signals,
insensitivity to growth-inhibitory signals, evasion of apoptosis,
limitless replicative potential, sustained angiogenesis and tissue
invasion and metastasis in the model of breast cancer (10). A previous study has shown that a
higher fraction of breast cancer is identified by the detection of
OPN-c compared with ER, PR or HER-2 and that OPN-c may be used as a
diagnostic and prognostic marker. This may be particularly useful
as ER and PR are considered to be weak prognostic markers (11–14).
The cyclooxygenases (COXs) are a family of
myeloperoxidases that are located at the luminal side of the
endoplasmic reticulum and nuclear membrane. COXs catalyze the
rate-limiting step of prostaglandin biosynthesis from arachidonic
acid. To date, three COX isoforms have been identified, COX-1,
COX-2 and COX-3. COX-1 is constitutively expressed in various
tissues and plays a role in tissue homeostasis (15).
COX-2 is an inducible isoform, which is
overexpressed during inflammation, and is regulated by growth
factors and various cytokines, including IL1β, IL6 or tumor
necrosis factor (TNF)-α (16).
COX-3 has been identified as a splice variant of COX-1 and is
present mainly in the brain and spinal cord, but its role is not
clearly understood (17,18). There are various studies with regard
to COX-2 overexpression in invasive breast cancer and ductal
carcinoma in situ, and the overexpression of COX-2 has been
identified to be associated with aggressive histological and
clinical features (19–26).
However, to date, there are no data with regard to
OPN and COX-2 overexpression and their correlation with various
subtypes of breast cancer. The present study was designed to
provide an improved definition of the combined effect of OPN and
COX-2 overexpression in the progression of breast cancer, and to
analyze the correlation between the expression pattern and various
subtypes of breast cancer.
Materials and methods
Study population
Approval for the present study was obtained from the
ethical committee of Ruby Hall Clinic (Pune, Maharashtra, India).
Formalin-fixed paraffin-embedded breast tumor specimens were
obtained from the Department of Histopathology, Ruby Hall Clinic.
Records of 375 breast cancer patients treated between 2006 and 2010
were obtained. Patients were excluded from the study if they were
male, had a metastatic disease at the time of diagnosis or were
administered any kind of chemotherapy or radiation therapy prior to
the surgery. Patients with only carcinoma in situ or with
bilateral breast cancer were also excluded from this study. The
records of the patients were retrieved and the clinical data,
histopathological records and treatment information were all
reviewed. The tumor grades of the invasive carcinomas were
classified according to the Scarff-Bloom-Richardson system
(27). The presence of lymph node
metastases was reviewed for each patient. The tumor-node-metastasis
(TNM) stage was determined according to the American Joint
Committee on Cancer’s Cancer Staging Manual (28). The carcinomas were histologically
divided into ductal, lobular and other tumors. The age of menopause
was decided according to the mean age of menopause in India
(3).
Antibodies and reagents
Mouse monoclonal anti-OPN and goat polyclonal
anti-COX-2 antibodies and horseradish peroxidase (HRP)-conjugated
IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). The Super Sensitive Polymer HRP Immunohistochemistry (IHC)
Detection System was purchased from Biogenex (QD 400,60K; Life
Sciences Pvt Ltd., Hyderabad, AP, India).
IHC staining
The specimens that were embedded in paraffin blocks
were cut into 5-μm sections on poly-L-lysine coated slides. IHC was
performed using the IHC detection system (Biogenex). Briefly, the
sections were deparaffinized and subjected to antigen heat
retrieval in a citrate buffer (pH 6.0) at 90°C for 30 min.
Endogenous peroxidase activity and non-specific binding were
blocked by incubation with a peroxide block and a power block,
respectively, using an IHC kit (BioGenex, Life Sciences Pvt. Ltd.).
The slides were then incubated sequentially with primary antibodies
overnight at 4°C and then with their respective secondary
antibodies for 1 h at room temperature. Diaminobenzidine
hydrochloride (DAB) was used as chromogen. Subsequently, the
sections were counterstained with hematoxylin and mounted using DPX
mounting media.
IHC scoring
IHC scoring was performed as previously described.
Briefly, the tumor staining was semi-quantitatively examined by an
oncopathologist using a double-blinded procedure with the Allred
8-unit IHC scoring system. The cytoplasmic staining of OPN and
COX-2 was scored based on two parameters, staining intensity and
positivity (29). Overall staining
(staining index) was calculated by the sum of the intensity (I) and
positivity (P); I + P = 0–8. A staining index of more than four was
defined as high expression, while less than four was defined as low
expression.
Statistical analysis
The statistical analysis was performed using
standard statistical software SPSS version 18.0 (SPSS, Inc.,
Chicago, IL, USA). The differences in the clinicopathological
characteristics, including the TNM stage, tumor grade and lymph
node status, between the HER2-overexpressing and
non-HER2-overexpressing subtypes of breast cancer were calculated
using the χ2 and Fisher’s exact tests. The associations
between OPN and the HER2-overexpressing and non-HER2-overexpressing
subtypes were evaluated using the Mann-Whitney U Test. The
Kruskal-Wallis test was used to evaluate the association between
the mean score of OPN and the TNM stage, histological subtype and
tumor grade of the patients. All the statistical tests were
two-sided. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between tumor subtypes of
breast carcinomas and clinicopathological parameters
Of the 375 breast cancer patients, 287 patients had
complete information on the ER, PR and HER2 statuses. The baseline
characteristics of the subjects, including the tumor subtypes are
presented in Table I. Of these 287
subjects, 87 (30.3%) were of the luminal A subtype, 110 (38.3%)
were of the luminal B subtype, 46 (16.0%) were of the
HER2-overexpressing subtype and 44 (15.3%) were of the triple
negative subtype. The median age of the patients was 54 years (SD,
12; range, 23–83 years; Table
I).
 | Table IDifferences in the clinicopathological
characteristics between various subtypes of breast cancer. |
Table I
Differences in the clinicopathological
characteristics between various subtypes of breast cancer.
| | Subtype, n | |
|---|
| |
| |
|---|
| Characteristics | n | Luminal A | Luminal B |
HER2-overexpressing | Triple negative | P-value |
|---|
| Age at diagnosis,
years |
| ≤ 45 | 64 | 16 | 29 | 4 | 15 | |
| >45 | 223 | 71 | 81 | 42 | 29 | 0.016 |
| T Stage |
| 1 | 45 | 19 | 15 | 7 | 4 | |
| 2 | 124 | 33 | 42 | 24 | 25 | |
| 3 | 19 | 2 | 7 | 3 | 7 | |
| 4 | 10 | 2 | 5 | 3 | 0 | 0.130 |
| Tumor grade |
| 1 | 26 | 17 | 9 | 0 | 0 | |
| 2 | 190 | 58 | 75 | 27 | 30 | |
| 3 | 53 | 7 | 19 | 14 | 13 | 0.000 |
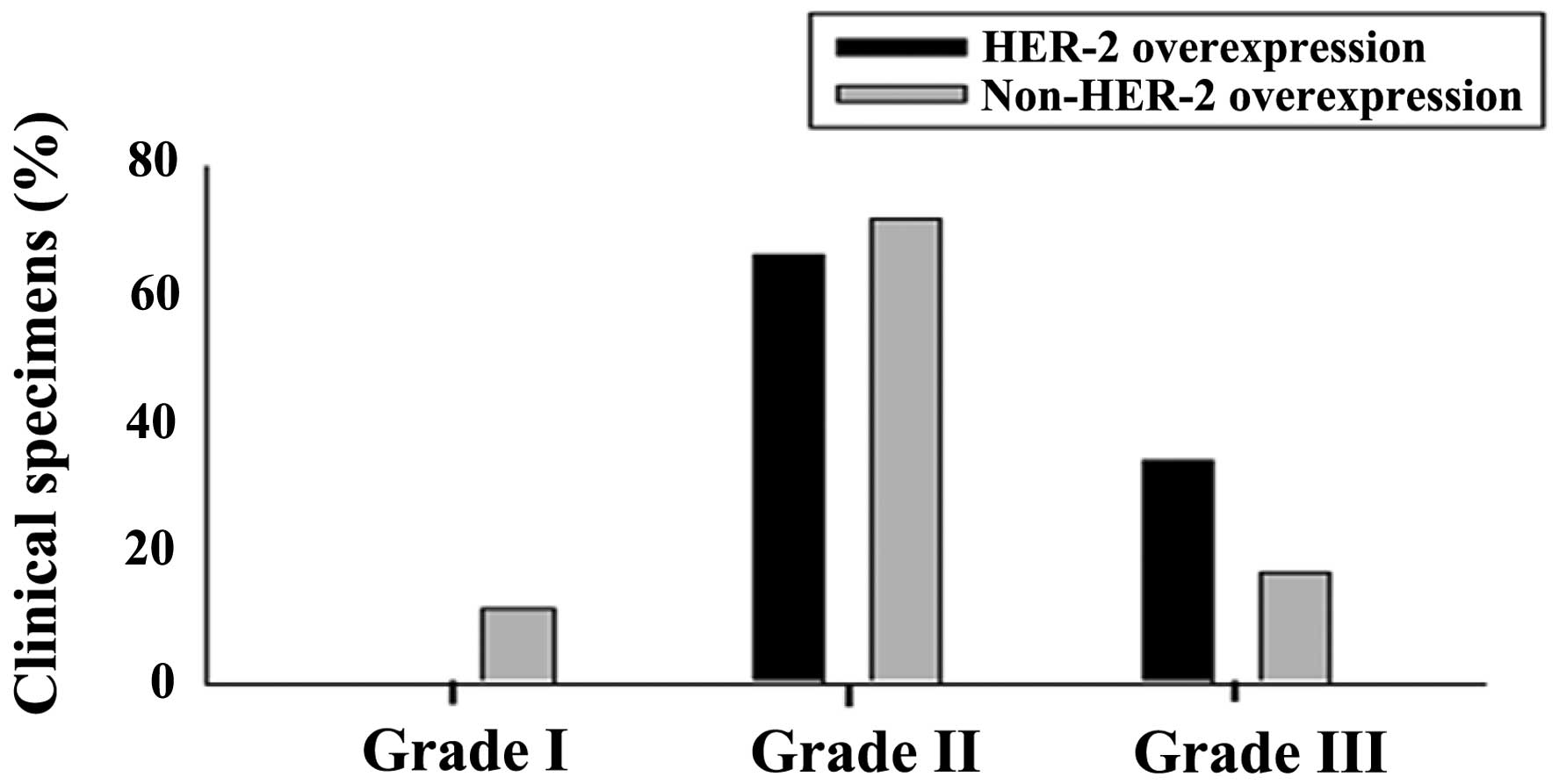
Patients in the HER2-overexpressing and triple
negative groups were more likely to have a higher grade of tumor,
with 32% of these two groups being grade 3 at the time of diagnosis
compared with 14% of the luminal cohort (P=0.000; Table I). There were no grade 1 cases in
either the HER2-overexpressing or triple negative subtypes. The
triple negative subtype was more frequently associated with a
higher T-stage compared with the non-triple negative subtypes
(Tables I and II; Fig,
1). The other tumor subtypes did not significantly correlate
with the tumor grade, stage or lymph node status.
 | Table IITumor grade representation in the
HER2-overexpressing and non-HER2-overexpressing subtypes of breast
cancer. |
Table II
Tumor grade representation in the
HER2-overexpressing and non-HER2-overexpressing subtypes of breast
cancer.
| Type | Grade I, % (n) | Grade II, %
(n) | Grade III, %
(n) | Total no. of
specimens |
|---|
| HER2-overexpressing
(Score, 3+) | 0 | 65.85 (27) | 34.14 (14) | 41 |
|
Non-HER2-overexpressing (Luminal A, B and
triple negative) | 11.4 (26) | 71.49 (163) | 17.10 (39) | 228 |
Correlation between OPN expression and
the tumor subtypes and clinicopathological features
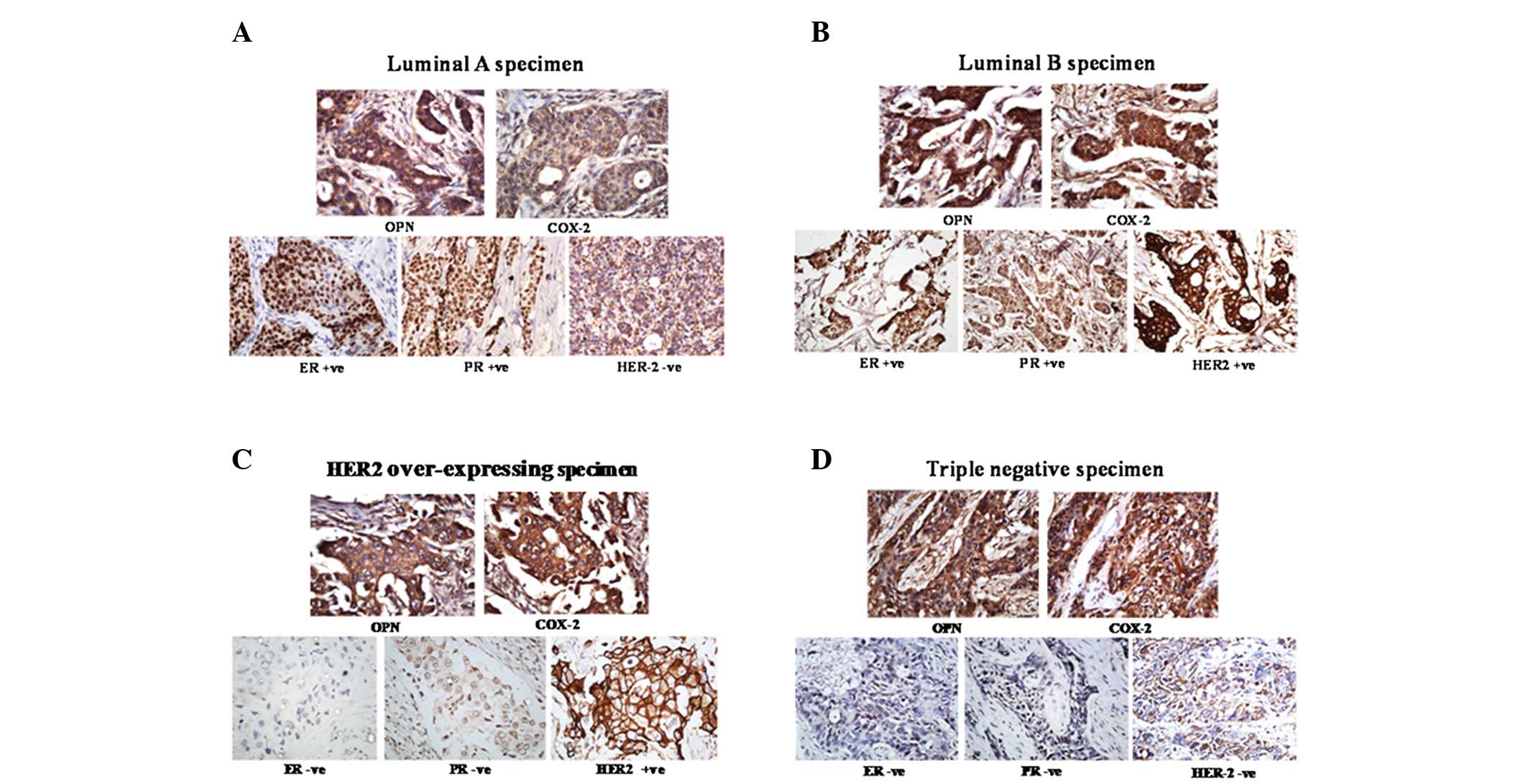
The expression of OPN in the 67 primary tumors (18
luminal A, 17 luminal B, 15 HER2-overexpressing and 17 triple
negative tumors) was analyzed using IHC. The representative images
are shown in Fig. 2. IHC scoring
was performed as described in the materials and methods section.
The results revealed that the mean OPN level was significantly
higher in the HER2-overexpressing subtype than in the
non-HER2-overexpressing subtypes (P=0.043; Table III). However there was no
correlation between OPN expression and the triple negative subtype
of breast cancer. Furthermore, OPN expression did not correlate
with any of the clinicopathological features that were evaluated,
including age, pathological grading, histological subtype, tumor
stage and lymph node metastasis (Table III). The expression of OPN and
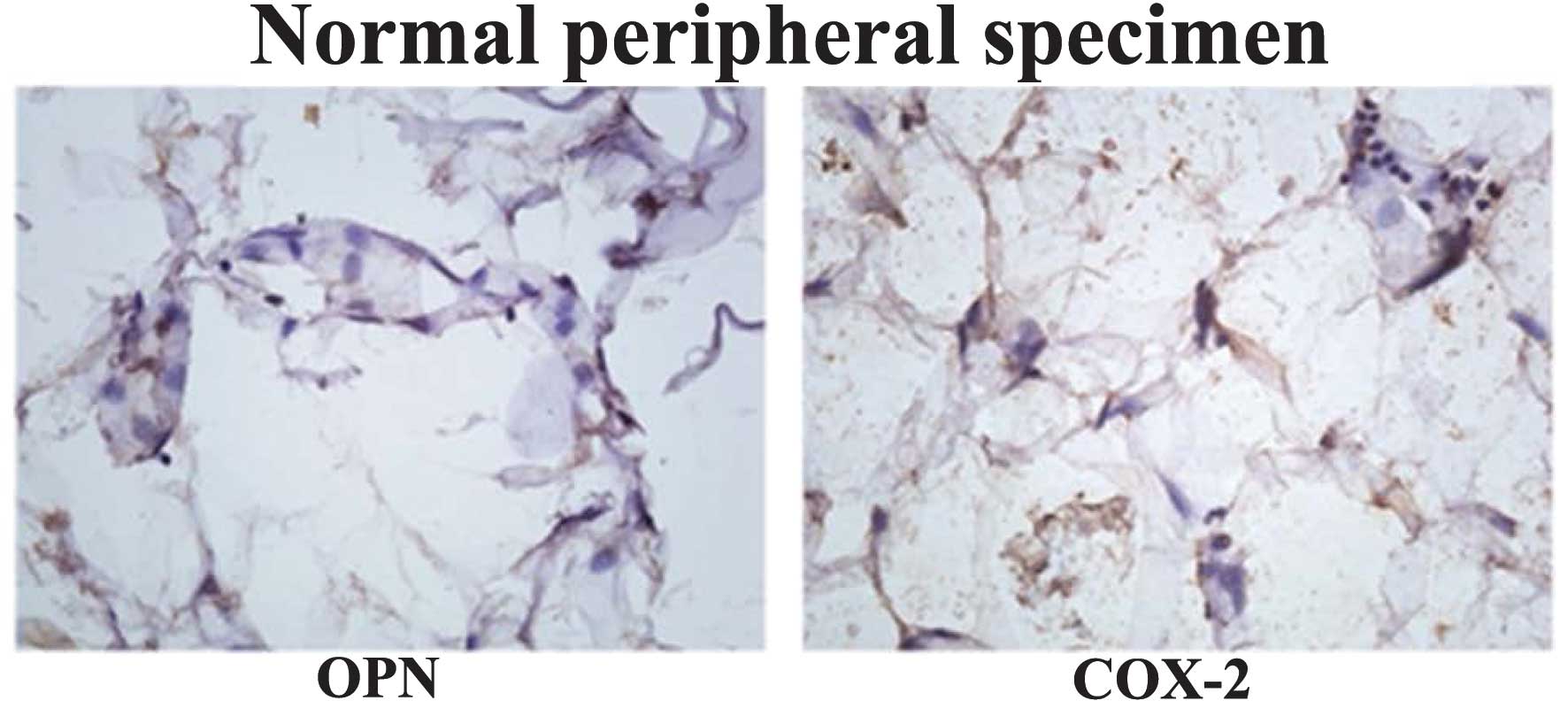
COX-2 was examined in the peripheral normal specimens and
negligible expression of these proteins was identified compared
with the tumor specimens of the multiple subtypes (Fig. 3). Furthermore, fibroadenoma
specimens were analyzed and the results indicated that there was
weak expression of OPN and COX-2 (data not shown).
 | Table IIICorrelation of OPN and COX-2 with the
tumor subtypes and clinicopathological parameters. |
Table III
Correlation of OPN and COX-2 with the
tumor subtypes and clinicopathological parameters.
| OPN expression | COX-2
expression |
|---|
|
|
|
|---|
| Clinicopathological
features | n | Scorea | P-value | n | Score | P-value |
|---|
| HER2
overexpression | 15 | 6.20±0.94 | | 15 | 5.80±1.20 | |
| Non-HER2
overexpression | 52 | 4.56±2.68 | 0.043 | 51 | 4.63±2.20 | 0.101 |
| Tumor stage |
| 1 | 12 | 5.92±2.10 | | 12 | 5.42±1.50 | |
| 2 | 46 | 4.59±2.58 | | 45 | 4.64±2.32 | |
| 3 | 6 | 6.00±1.41 | | 6 | 5.67±1.03 | |
| 4 | 2 | 3.00±4.24 | 0.261 | 2 | 5.00±1.41 | 0.898 |
| Tumor grade |
| 1 | 4 | 3.25±3.77 | | 4 | 3.25±3.77 | |
| 2 | 47 | 5.02±2.49 | | 46 | 4.87±1.98 | |
| 3 | 15 | 4.93±2.15 | 0.455 | 15 | 5.33±1.79 | 0.708 |
| Nodal status |
| − | 32 | 4.84±2.78 | | 32 | 4.53±2.44 | |
| + | 32 | 4.87±2.29 | 0.432 | 31 | 5.23±1.68 | 0.566 |
Association of COX-2 expression with
tumor subtypes and clinicopathological features
The expression of COX-2 in the 66 primary tumors (18
luminal A, 17 luminal B, 15 HER2-overexpressing and 16 triple
negative tumors) was analyzed by IHC and it revealed no significant
correlation between COX-2 expression and the clinicopathological
features. The mean COX-2 level was higher in the
HER2-overexpressing subtype than in the luminal A, luminal B or
triple negative groups. However, the correlation was not identified
to be statistically significant when the tumor subtypes were
divided into HER2-overexpressing and non-HER2-overexpressing groups
(P=0.101; Table III).
Discussion
A total of 1,638,910 new cancer cases and 577,190
mortalities from cancer were predicted to occur in the USA in 2012,
which accounted for ~23% of the total mortalities (30). However, over the last few decades,
there have been significant advances in breast cancer management,
leading to the early detection of the disease and the development
of more effective treatment modalities, which has resulted in a
significant decline in breast cancer mortalities and improved
outcomes of females with the disease (31,32).
Breast cancer is no longer considered to be a single disease, but
rather a multifaceted disease comprised of distinct biological
subtypes and a diverse natural history, thus presenting a varied
spectrum of clinical, pathological and molecular features with
various prognostic and therapeutic implications.
A previous study showed that the new molecular
classification of breast cancer is of significant prognostic value
(33). The subtyping of breast
cancer using microarrays is an efficient method to perform a
molecular classification. However, the majority of the archived
clinical specimens are not amenable to such an analysis. These
assays are also limited to research laboratories and therefore are
not advantageous for clinical practice. The IHC-based
classification systems remain of use in clinical practice,
particularly when fresh tissue is not available, and has been shown
to correlate well with the intrinsic classification using gene
expression by microarrays: ER/PR+ and HER2−
with luminal A; ER/PR+ and HER2+ with luminal
B; ER−, PR− and HER2+ with the
HER2-overexpressing group; and ER−, PR− and
HER2− with triple negative breast cancer (34–39).
Early relapse and mortality were more frequent among the
HER2-overexpressing and triple negative subtypes. Several studies
have shown a trend towards a poor outcome for patients with cancer
belonging to these groups (40–42).
The data of the present study indicated that the
HER2-overexpressing and triple negative subtypes were associated
with higher nuclear and histological grades of tumor, while only
the triple negative subtype was associated with a higher
pathological T-stage. The present study aimed to establish the
level of expression and clinical significance of OPN and COX-2 in
patients presenting with various subtypes of breast cancer. It was
observed that the HER2-overexpressing subtype of breast cancer was
significantly associated with OPN overexpression. The mean OPN and
COX-2 levels were significantly higher in the HER2-overexpressing
breast cancer group. The HER2 oncoprotein is a transmembrane
receptor, belonging to the epidermal growth factor receptor family,
with tyrosine kinase activity, resulting in intracellular signaling
and the activation of genes that are involved in cell growth, which
is associated with shortened survival rates, enhanced
aggressiveness and a poor prognosis. Therefore, abnormal OPN and
COX-2 expression may contribute to the aggressive behavior and poor
prognosis in patients with the HER2-overexpressing subtype.
Additional prospective and molecular level studies are required for
an improved understanding of the role of OPN and COX-2 in the
HER2-overexpressing subtype.
Acknowledgements
The authors would like to thank Dr Smita Kale and
Deepti Tomar for critically reading the manuscript, and the Indian
Academy of Science, Bangalore for providing the Summer Research
Fellowship. This study was supported by the University Grant
Commission (UGC).
References
|
1
|
Murthy NS, Chaudhry K, Nadayil D, Agarwal
UK and Saxena S: Changing trends in incidence of breast cancer:
Indian scenario. Indian J Cancer. 46:73–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sengupta A: The emergence of the menopause
in India. Climacteric. 6:92–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapur P, Sinha B and Pereira BM: Measuring
climacteric symptoms and age at natural menopause in an Indian
population using the Greene Climacteric Scale. Menopause.
16:378–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liaw L, Birk DE, Ballas CB, Whitsitt JS,
Davidson JM and Hogan BL: Altered wound healing in mice lacking a
functional osteopontin gene (spp1). J Clin Invest. 101:1468–1478.
1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sodek J, Ganss B and McKee MD:
Osteopontin. Crit Rev Oral Biol Med. 11:279–303. 2000. View Article : Google Scholar
|
|
7
|
Jain S, Chakraborty G, Bulbule A, Kaur R
and Kundu GC: Osteopontin: an emerging therapeutic target for
anticancer therapy. Expert Opin Ther Targets. 11:81–90. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed M, Behera R, Chakraborty G, et al:
Osteopontin: a potentially important therapeutic target in cancer.
Expert Opin Ther Targets. 15:1113–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rudland PS, Platt-Higgins A, El-Tanani M,
et al: Prognostic significance of the metastasis-associated protein
osteopontin in human breast cancer. Cancer Res. 62:3417–3427.
2002.PubMed/NCBI
|
|
10
|
Chakraborty G, Jain S, Behera R, Ahmed M,
Sharma P, Kumar V and Kundu GC: The multifaceted roles of
osteopontin in cell signaling, tumor progression and angiogenesis.
Curr Mol Med. 6:819–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mirza M, Shaughnessy E, Hurley JK,
Vanpatten KA, Pestano GA, He B and Weber GF: Osteopontin-c is a
selective marker for breast cancer. Int J Cancer. 122:889–897.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henry NL and Hayes DF: Uses and abuses of
tumor markers in the diagnosis, monitoring and treatment of primary
and metastatic breast cancer. Oncologist. 11:541–552. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clark GM: Prognostic and predictive
factors. Diseases of the Breast. Harris JR, Lippman ME, Morrow M
and Osborne CK: Lippinscott, Williams and Wilkins; Philadelphia:
pp. 489–514. 2000
|
|
14
|
Andre F and Pusztai L: Molecular
classification of breast cancer: implications for selection of
adjuvant chemotherapy. Nat Clin Pract Oncol. 3:621–632. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandrasekharan NV and Simmons DL: The
cyclooxygenases. Genome Biol. 5:2412004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramsay RG, Ciznadija D, Vanevski M and
Mantamadiotis T: Transcriptional regulation of cyclo-oxygenase
expression: three pillars of control. Int J Immunopathol Pharmacol.
16:S59–S67. 2003.PubMed/NCBI
|
|
17
|
Sarkar FH, Adsule S, Li Y and Padhye S:
Back to the future: COX-2 inhibitors for chemoprevention and cancer
therapy. Mini Rev Med Chem. 7:599–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kis B, Snipes JA, Isse T, Nagy K and
Busija DW: Putative cyclooxygenase-3 expression in rat brain cells.
J Cereb Blood Flow Metab. 23:1287–1292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ristimäki A, Sivula A, Lundin J, et al:
Prognostic significance of elevated cyclooxygenase-2 expression in
breast cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
20
|
Boland GP, Butt IS, Prasad R, Knox WF and
Bundred NJ: COX-2 expression is associated with an aggressive
phenotype in ductal carcinoma in situ. Br J Cancer. 90:423–429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shim V, Gauthier ML, Sudilovsky D, et al:
Cyclooxygenase-2 expression is related to nuclear grade in ductal
carcinoma in situ and is increased in its normal adjacent
epithelium. Cancer Res. 63:2347–2350. 2003.PubMed/NCBI
|
|
22
|
Costa C, Soares R, Reis-Filho JS, Leitão
D, Amendoeira I and Schmitt FC: Cyclo-oxygenase 2 expression is
associated with angiogenesis and lymph node metastasis in human
breast cancer. J Clin Pathol. 55:429–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davies G, Salter J, Hills M, Martin LA,
Sacks N and Dowsett M: Correlation between cyclooxygenase-2
expression and angiogenesis in human breast cancer. Clin Cancer
Res. 9:2651–2656. 2003.PubMed/NCBI
|
|
24
|
Denkert C, Winzer KJ, Müller BM, et al:
Elevated expression of cyclooxygenase-2 is a negative prognostic
factor for disease free survival and overall survival in patients
with breast carcinoma. Cancer. 97:2978–2987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shim JY, An HJ, Lee YH, Kim SK, Lee KP and
Lee KS: Overexpression of cyclooxygenase-2 is associated with
breast carcinoma and its poor prognostic factors. Mod Pathol.
16:1199–1204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan KB, Yong WP and Putti TC:
Cyclooxygenase-2 expression: a potential prognostic and predictive
marker for high-grade ductal carcinoma in situ of the breast.
Histopathology. 44:24–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Doussal V, Tubiana-Hulin M, Friedman S,
Hacene K, Spyratos F and Brunet M: Prognostic value of histologic
grade nuclear components of Scarff-Bloom-Richardson (SBR). An
improved score modification based on a multivariate analysis of
1262 invasive ductal breast carcinomas. Cancer. 64:1914–1921.
1989.
|
|
28
|
Greene FL, Page DL and Fleming ID: AJCC
Cancer Staging Manual. 6th edition. Springer; New York, NY: 2002,
View Article : Google Scholar
|
|
29
|
Allred DC, Clark GM, Elledge R, et al:
Association of p53 protein expression with tumor cell proliferation
rate and clinical outcome in node-negative breast cancer. J Natl
Cancer Inst. 85:200–206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
31
|
Glass AG, Lacey JV Jr, Carreon JD and
Hoover RN: Breast cancer incidence, 1980–2006: combined roles of
menopausal hormone therapy, screening mammography, and estrogen
receptor status. J Natl Cancer Inst. 99:1152–1161. 2007.
|
|
32
|
Ravdin PM, Cronin KA, Howlader N, et al:
The decrease in breast-cancer incidence in 2003 in the United
States. N Engl J Med. 356:1670–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dolled-Filhart M, Rydén L, Cregger M,
Jirstrom K, Harigopal M, Camp RL and Rimm DL: Classification of
breast cancer using genetic algorithms and tissue microarrays. Clin
Cancer Res. 12:6459–6468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu R, Wang X, Chen GY, et al: The
prognostic role of a gene signature from tumorigenic breast-cancer
cells. N Engl J Med. 356:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sorlie T, Tibshirani R, Parker J, et al:
Repeated observation of breast tumor subtypes in independent gene
expression data sets. Proc Natl Acad Sci USA. 100:8418–8423. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sørlie T, Perou CM, Fan C, et al: Gene
expression profiles do not consistently predict the clinical
treatment response in locally advanced breast cancer. Mol Cancer
Ther. 5:2914–2918. 2006.
|
|
38
|
Van de Vijver MJ, He YD, van’t Veer LJ, et
al: A gene-expression signature as a predictor of survival in
breast cancer. N Engl J Med. 347:1999–2009. 2002.
|
|
39
|
Wang Y, Klijn JG, Zhang Y, et al: Gene
expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kreike B, van Kouwenhove M, Horlings H,
Weigelt B, Peterse H, Bartelink H and van de Vijver MJ: Gene
expression profiling and histopathological characterization of
triple-negative/basal-like breast carcinomas. Breast Cancer Res.
9:R652007. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsuda H, Hirohashi S, Shimosato Y, et al:
Correlation between long-term survival in breast cancer patients
and amplification of two putative oncogene-coamplification units:
hst-1/int-2 and c-erbB-2/ear-1. Cancer Res. 49:3104–3108.
1989.PubMed/NCBI
|