Introduction
The high incidence of breast cancer in modern
society is a serious threat to women’s health. Approximately 30% of
early stage breast cancer patients eventually develop recurrence
and metastasis (1). Therefore,
targeted breast cancer cell research and treatment is of great
importance. Mesenchymal stem cells (MSCs) are non-hematopoietic
multipotent cells that may be isolated and expanded from a number
of different sources, including bone marrow and adipose tissue
(2,3). They are defined by a series of
specific cell surface antigens (4)
and an inborn ability to differentiate along multiple lineages,
including into osteoblasts, chondrocytes and adipocytes (5–8).
Although MSCs have a primary role in tissue regeneration (9,10),
they also possess the ability to migrate to the site of numerous
tumor types in vivo(11,12). A
recent study strengthened the link between MSCs and carcinoma
(13). The interaction between
cancer cells and the tumor microenvironment is increasingly being
regarded as an important regulator of malignant progression. It is
well-known that tumor cells secrete chemokines, cytokines and
growth factors that are able to recruit and activate a number of
MSCs. In turn, MSCs, as a part of the tumor microenvironment
secrete cytokines and chemokines to affect the growth and
metastasis of tumor cells (14,15).
MSCs are able to selectively target tumor sites. Indeed, actively
growing tumors recruit MSCs in their environment where they promote
tumor growth and metastasis to distant organs (16,17).
There is ample evidence that MSCs may be isolated from tumors such
as lipoma (18), bone sarcoma
(19) and uterine cervix cancer
(20). Cao et al(21) identified that MSC-like cells in
human gastric cancer tissues were similar to bone marrow (BM)-MSCs
in their morphology, surface antigens, specific gene expression and
differentiation potential. Lis et al(22) successfully isolated ovarian
cancer-associated MSCs and found that they could protect ovarian
cancer cells from hyperthermia by secreting CXCL12. Another study
showed that BM-MSCs could facilitate breast cancer cell metastasis
through the secretion of CC chemokine ligand 5 (CCL5) (14). These results indicate that the
interaction between MSCs and cancer cells may represent an
important therapeutic target for the prevention of cancer
progression. Overall, these studies show that MSCs in tumor tissues
may be important in modulating cancer cell proliferation and
metastasis. Relatively little is known regarding whether MSCs are
located in primary breast cancer tissue, and how they participate
in breast cancer proliferation and migration.
In the present study, we successfully isolated MSCs
from human breast cancer tissue and investigated the effect of
breast cancer MSCs (BC-MSCs) on the MCF-7 breast cancer cell line.
The results confirm the existence of MSCs in human breast cancer
tissue, and indicate that BC-MSCs may promote the proliferation and
migration of the MCF-7 breast cancer cell line in vitro.
Materials and methods
Cell culture
Cells were cultured as described previously
(23,24). Cells were maintained in Dulbecco’s
modified Eagle’s medium with low glucose (L-DMEM) supplemented with
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 U/ml
streptomycin, under mycoplasma-free conditions at 37°C in a
humidified atmosphere of 5% CO2 and 95% air.
Isolation and culture of BC-MSCs
Cancer tissue was obtained from patients (n=9) with
breast carcinoma who had undergone mastectomies at The Second
People’s Hospital of Kunshan (Kunshan, China). All patients agreed
voluntarily to participate in the study subject to the terms agreed
by the Ethics Committee of Jiangsu University. Fresh tissue
specimens were collected and washed with phosphate-buffered saline
(PBS). The tissue specimens were cut into 1 mm3-sized
pieces and floated in L-DMEM containing 10% FBS, 100 U/ml
penicillin and 100 U/ml streptomycin. The specimens were
subsequently incubated at 37°C in humid air with 5% CO2.
The medium was replaced every three days after the initial plating.
When adherent fibroblast-like cells appeared after 10 days of
culture, the cells were trypsinized and passaged (without dilution)
into a new flask for further expansion. The cells in passage 3 were
used for the evaluation of the experimental results.
Flow cytometry
Flow cytometric analyses were performed at passages
3 and 4. BC-MSCs (1.0×106 cells) were trypsinized,
washed twice in PBS and stained with monoclonal antibodies against
CD13, CD29 and CD71 (PE-conjugated); and CD4, CD10, CD14, CD34,
CD38, CD44, CD105, HLA-DR and HLA-I (FITC-conjugated)
(Becton-Dickinson, San Jose, CA, USA) for 30 min on ice. Labeled
cells were analyzed using a FACSCalibur flow cytometer
(Becton-Dickinson). PE-IgG1 and FITC-IgG1 were used in the
control group.
Osteogenic and adipogenic differentiation
in vitro
BC-MSCs were seeded at 5,000 cells/cm2 in
35-mm plates and cultured in L-DMEM with 10% FBS, and either
osteogenic [0.1 μM dexamethasone, 10 mM b-glycerophosphate, 50 mg/l
ascorbic acid and 4 μg/ml basic fibroblast growth factor (bFGF)
(Sigma-Aldrich, St. Louis, MO, USA)] or adipogenic (10−6
M dexamethasone, 0.5 μM isobutylmethylxanthine, 5 ng/ml linsulin,
60 μM indomethacin and 10−4 M hydrocortisone)
supplements (Cyagen Biosciences, Sunnyvale, CA, USA). The medium
was changed three times a week and the cells were induced for two
weeks. At the end of induction, the cells were subjected to
alkaline phosphatase staining and Oil Red O staining followed by
hematoxylin counterstaining.
Reverse transcription polymerase chain
reaction (RT-PCR)
RNA was extracted from ~1.0×106 MSCs,
differentiating cells or differentiated cells using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). RNA (1 μg) was processed for cDNA
synthesis with Superscript II reverse transcriptase, using
Oligo(dT) primer (Toyobo, Osaka, Japan) according to the
manufacturer’s instructions. PCR was performed using 1 μg of cDNA
sample with 0.3 units of Taq polymerase (CinnaGen, Tehran, Iran),
200 μM dNTPs, 10 pM of each primer, reaction buffer and
MgCl2 (Takara, Shiga, Japan) in a 25 μl PCR tube. The
PCR amplification was performed for 35 cycles using an ABI 2720
thermal cycler (Applied Biosystems, Foster City, CA, USA). The
cycling conditions were as follows: 94°C for 30 sec, 60°C (primer)
for 30 sec and 72°C for 30 sec, with a final extension at 72°C for
10 min, respectively. PCR products were separated on a 1.5% agarose
gel, stained with ethidium bromide and visualized under UV light.
For PCR, the forward and reverse primers were as follows: BMP-3,
5′-GACCCTCCAATCCAACCA-3′ (forward) and 5′-ACGCTTTCAGGCTCACAA-3′
(reverse); peroxisome proliferator-activated receptor γ-2
(PPARγ-2), 5′-GCCCAGGTTTGCTGAATG-3′ (forward) and 5′-TGAAG
ACTCATGTCTCTC-3′ (reverse); glyceraldehyde 3-phosphate
dehydrogenase, 5′-GGATTTGGTCGTATTGGG-3′ (forward) and
5′-GGAAGATGGTGATGGGATT-3′ (reverse).
Generation of conditioned media
Conditioned media was generated based on previously
published methods (25,26). BC-MSCs were plated to 70% confluency
in 35-mm plates with 10% FBS L-DMEM and allowed to adhere overnight
at 37°C and 5% CO2. The following day, the media was
removed and the cells were washed twice with PBS. Cells were then
re-incubated with non-serum culture media. After 24 h, the media
was collected, spun down to remove cell debris (698 × g for 5 min)
and passed through a 0.45 μm filter (Sigma-Aldrich). CM aliquots
were frozen at −20°C until required (not exceeding two weeks). To
prepare different concentrations of BC-MSC-CM (10 and 20%), the
near 100% BC-MSC-CM was diluted accordingly in freshly prepared
L-DMEM with 10% FBS.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were plated at a density of 2.5×103
cells/well in a 96-well plate in 200 μl 10% FBS L-DMEM, and allowed
to attach overnight. Cells were then treated with 10 and 20%
BC-MSC-CM for 48 h. MTT (20 μl) was added to each well for the last
4 h. When the reaction was terminated, all the solution was
discarded and 150 μl of dimethyl sulfoxide was added to each well.
The 96-well plate was shaken to ensure complete solubilization of
the purple formazan crystals. Absorbance at 490 nm was measured by
an enzyme-linked immunosorbent assay reader.
Scratch wound assay
Cells were grown to confluence and then scratched
with a cell scraper (Nunc, Inc., Naperville, IL, USA). The
resulting debris was removed by gentle washing with medium. The
cells were subsequently placed in an incubator. Cells were
maintained for up to 24 h with or without CM. The images of the
closing wound were acquired by inverted microscopy and analyzed
using ImageJ software (National Institute of Health, Bethesda, MD,
USA).
Transwell migration assay
Migration assays were performed based on the study
by Karnoub et al(14) and
the manufacturer’s instructions (Corning Inc., Corning, NY, USA).
There was conditioned medium (0, 10 and 20%) in the bottom of the
transwell. A volume of 5×104 MCF-7 cells was plated in
100 μl of serum-free L-DMEM in the top of the chamber and incubated
for 10 h at 37°C. The cells on the top side of the filter were
removed by scrubbing twice with a cotton-tipped swab. Migrating
cells were fixed in formaldehyde and stained with crystal violet.
Four lower power fields (magnification, ×100) were randomly
selected in each chamber to observe the cells and Cell Counter
software (Borland Software Corporation, Scotts Valley, CA, USA) was
used to count the stained migrated cells. Each experimental group
was repeated three times.
Statistical analysis
Studies involving more than two groups were analyzed
by one-way ANOVA with Newman-Keuls multiple comparison test, using
the GraphPad Prism V.5 software program. The results were expressed
as the means ± SD from three different replicates for individual
assays. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphological characterization and
identification of BC-MSCs
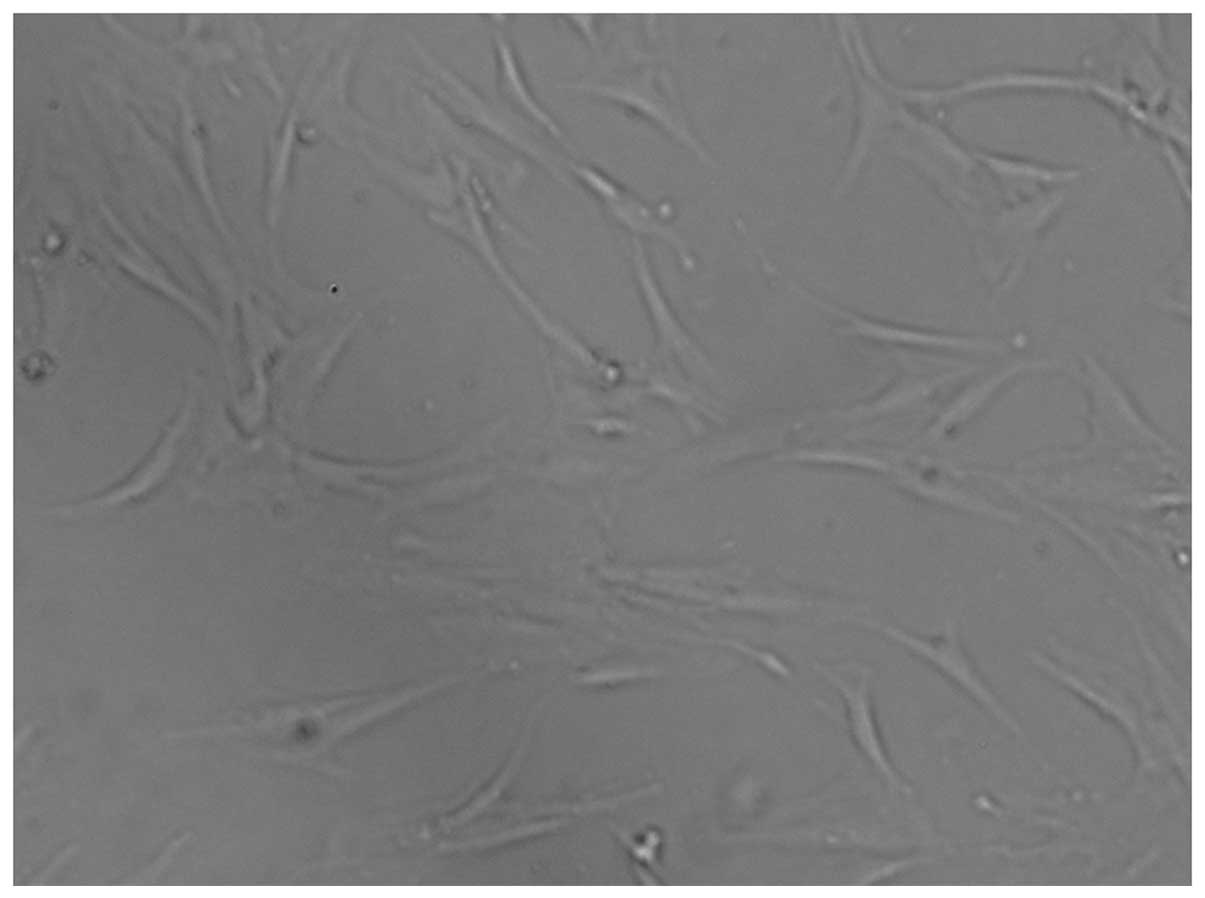
After the initial 3–5 days of primary culture, a
small population of single cells with a spindle shape were
observed, which had adhered to the plastic surfaces. On days 7–10
after the initial plating, the cells were displayed as long
spindle-shaped or polygonal fibroblastic cells (Fig. 1). Flow cytometry analysis
demonstrated that the BC-MSCs possessed uniform surface markers.
The BC-MSCs expressed the same surface antigens as human BM-MSCs,
and they were positive for CD13, CD29, CD44, CD105 and HLA-I, but
negative for CD4, CD10, CD14, CD31, CD34, CD38 and HLA-DR. MSCs
isolated from the bone marrow of healthy adult donors were used as
a positive control (Fig. 2).
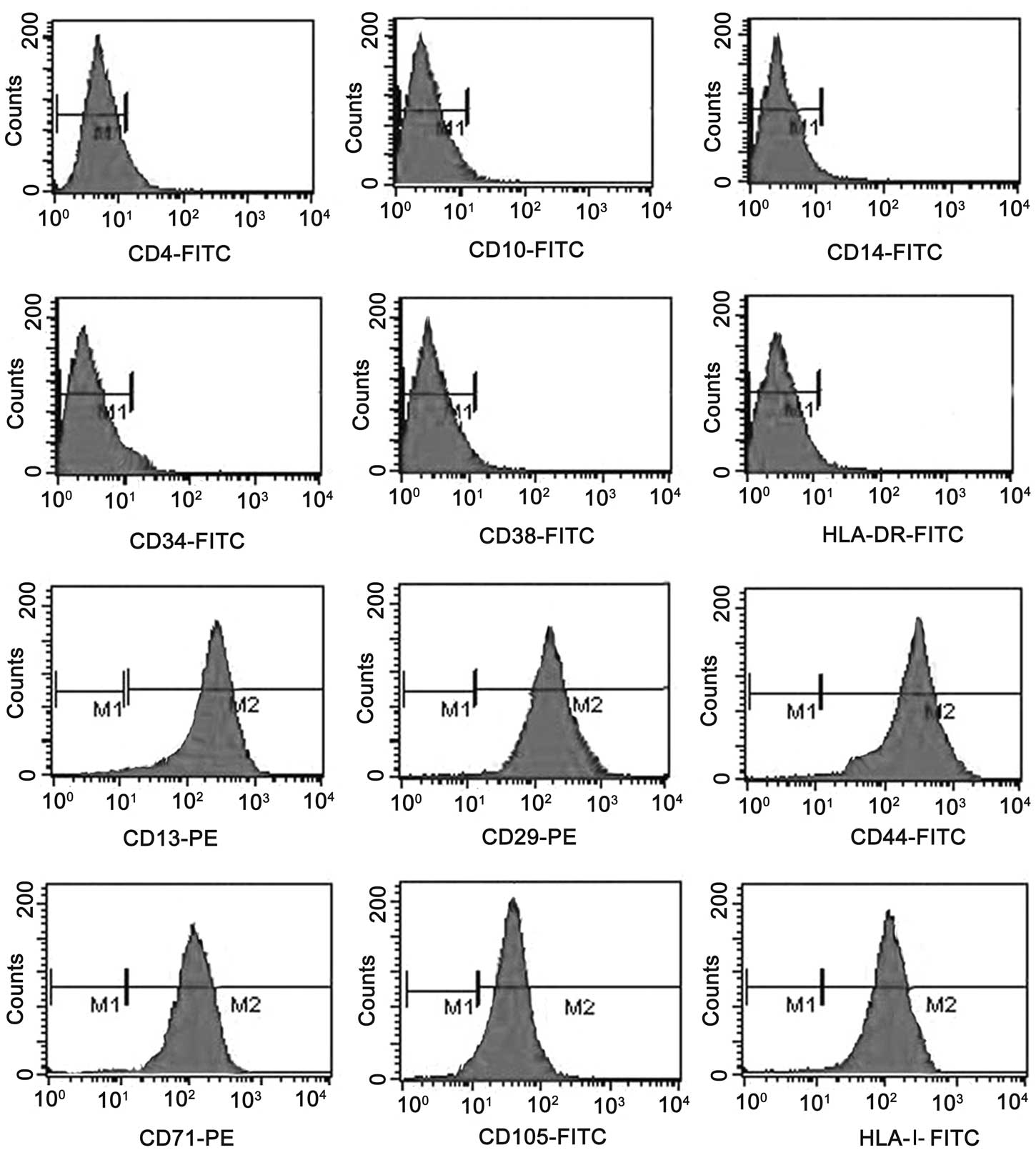 | Figure 2Surface antigens of BC-MSCs. BC-MSCs
were positive for CD13, CD29, CD44, CD71, CD105 and HLA-I, but
negative for CD4, CD10, CD14, CD34, CD38 and HLA-DR. BC-MSCs,
breast cancer mesenchymal stem cells. |
Multilineage differentiation potential of
BC-MSCs
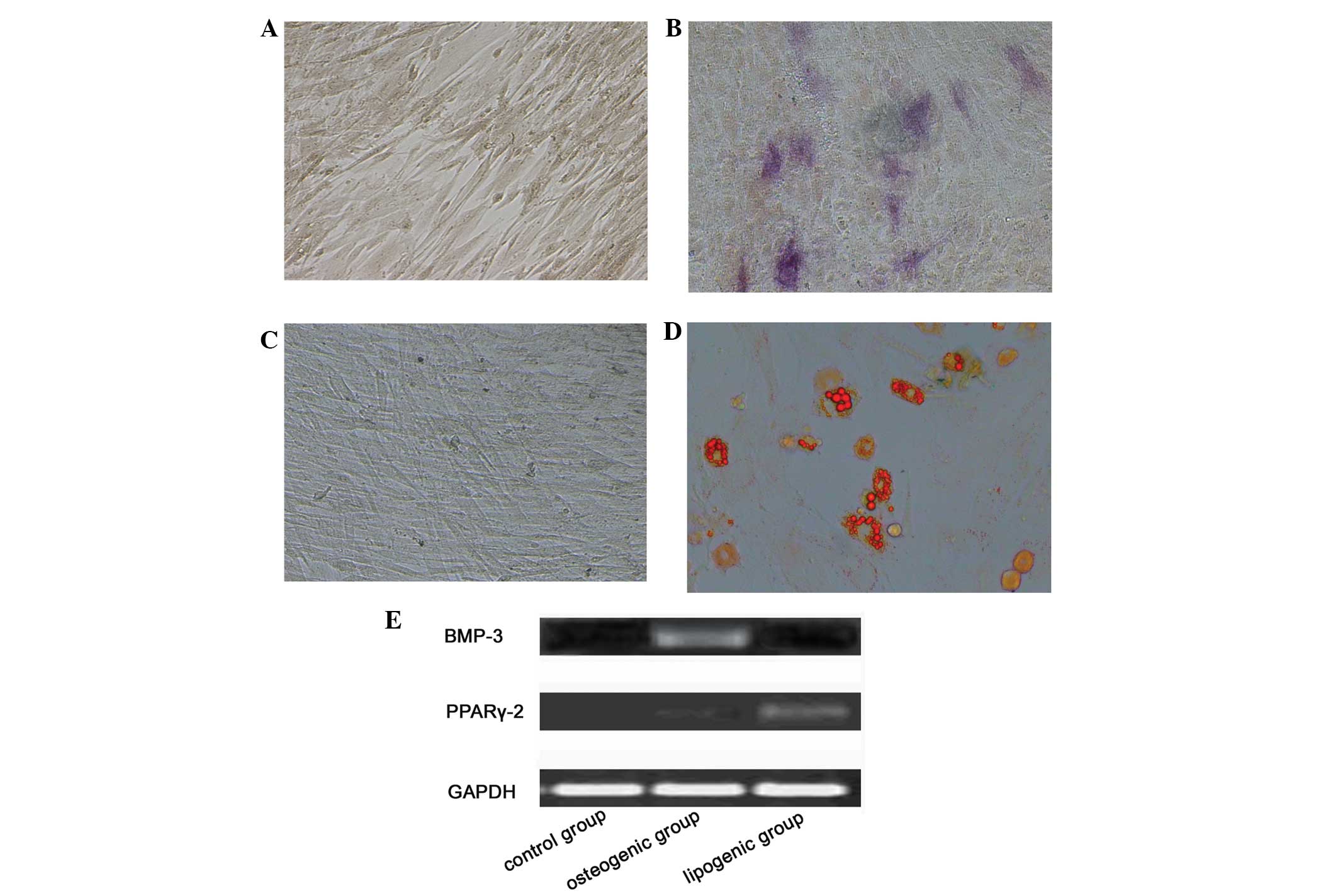
In vitro multilineage differentiation
potential is the functional standard for verifying the identity of
MSCs. Differentiation of BC-MSCs was apparent after two weeks of
induction in the special medium. At the end of the second week,
both BC-MSCs were capable of differentiation into either osteocytes
or adipocytes, shown by the positive staining of alkaline
phosphatase (Fig. 3A and B) and Oil
Red O (Fig. 3C and D). With
osteogenic supplementation, the differentiation was apparent after
incubation. RT-PCR results showed that these cells highly expressed
BMP-3 (Fig. 3E). Similarly, after
adipogenic induction, the cells expressed PPARγ-2 (Fig. 3E). Non-treated control cultures did
not notably express BMP-3 and PPARγ-2.
BC-MSC-CM enhances the proliferation of
MCF-7 breast cancer cells in vitro
For the MTT assay, MCF-7 cancer cell lines cultured
in BC-MSC-CM (10 and 20%) showed an increase in cell proliferation.
Compared with the control group, MCF-7 cells were increased by 164
and 137%, respectively (Fig. 4).
The increase in cell proliferation observed for MCF-7 was
statistically significant compared with that of the control
group.
BC-MSCs promote the migration of MCF-7
breast cancer cells in vitro
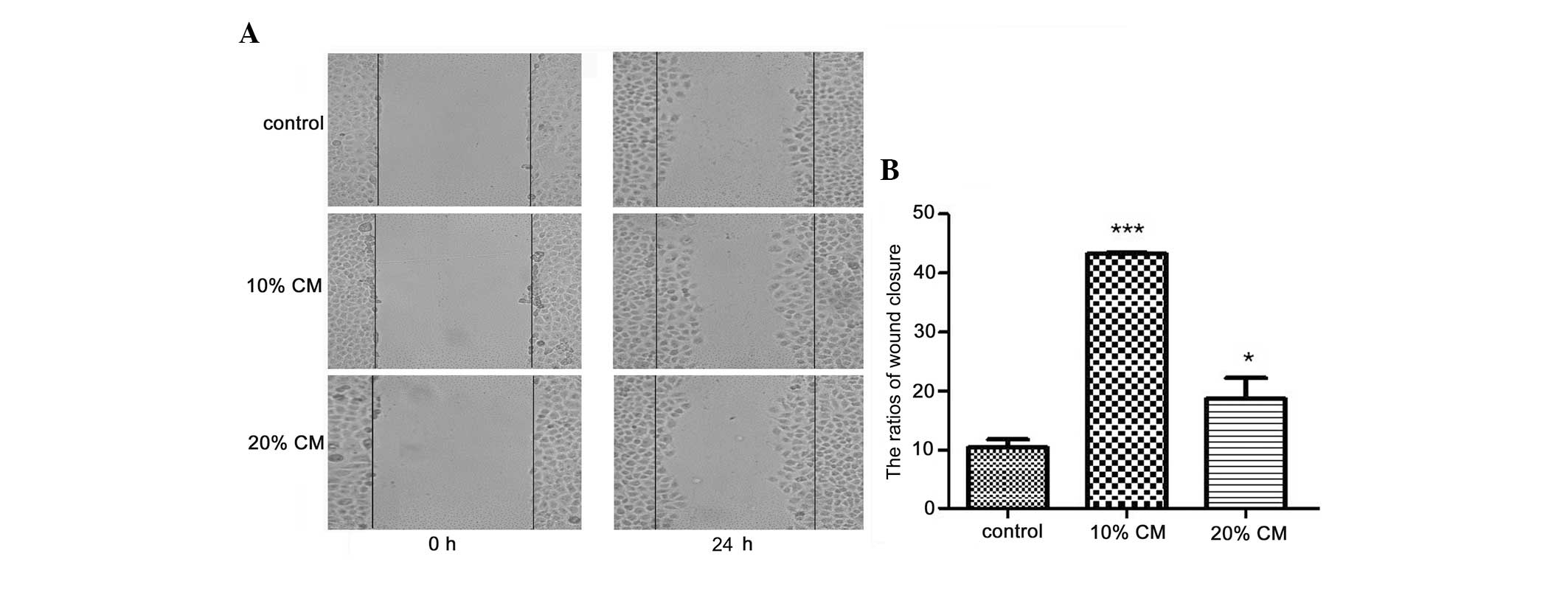
Scratch wounds were inflicted on cells pre-treated
with or without BC-MSC-CM for 24 h. The surface area of the wounds
generated did not differ between the groups at 0 h, while
differences were observed between the groups after 24 h of
treatment (Fig. 5A). The wound
closure ratios were 10.2±1.3, 44.0±0.3 and 18.5±3.1% for MCF-7
cells in the control group, 10% BC-MSC-CM group and 20% BC-MSC-CM
group, respectively, with the data showing statistical significance
(Fig. 5B). In this study, we set
out to determine whether BC-MSCs affect the migration potential of
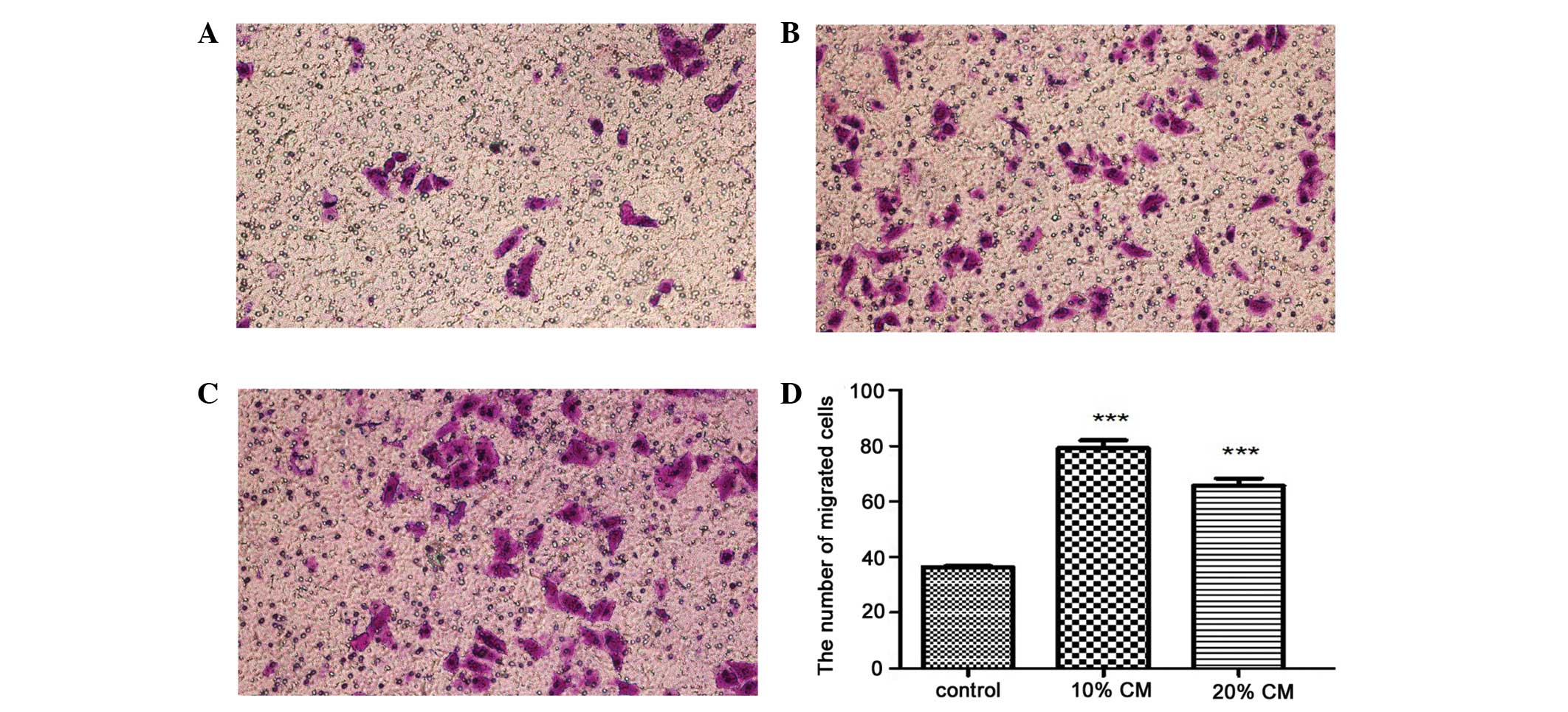
the normally non-metastatic MCF-7 cell line. In the first 24 h of
culture, the cell migration assay showed cell migration in the
MCF-7 cancer cell line stimulated with 10 and 20% BC-MSC-CM after
overnight starvation in serum-free medium. After 8 h of culture,
the migration of MCF-7 cells was enhanced by BC-MSC-CM (10 and 20%)
compared with that of the control group (Fig. 6A–C). A greater number of viable
cells migrated into the lower chamber of the transwell following
treatment with BC-MSC-CM (10 and 20%) compared with that of the
controls, as indicated by the viability assay. The mean numbers of
migrated cells per lower power fields were 38.3±0.6, 81.7±2.1 and
62.3±1.5, respectively. There were significant differences between
the control group and the BC-MSC-CM groups (Fig. 6D). The results showed that treatment
with BC-MSC-CM greatly increased the ability of the MCF-7 cells to
migrate to the lower side of the well.
Discussion
In contrast to research on cancer-associated MSCs
obtained from the bone marrow of hematological malignancies
(27), MSCs derived from solid
tumors have not been studied in detail and their role in cancer
progression remains poorly defined. A detailed characterization of
their role in human cancer progression would help to clarify the
potential targets for cancer therapy.
Previous studies that have detected the effects of
human MSCs (hMSCs) on primary carcinoma cells have resulted in
conflicting findings (23,28–30),
and no apparent effects of MSCs on cancer progression have been
reported. It is possible that MSCs derived from cancer tissue may
be affected by the tumor microenvironment and, in turn, affect the
tumor cells (31). Although it has
been reported that MSCs may support tumor growth (15) and promote cancer metastasis
(14), further study on the
detailed role of MSCs in tumor progression and its mechanisms is
still required in various models.
In this study, BC-MSCs from human breast cancer
tissues showed a homogenous immunophenotype and a multi-lineage
differentiation potential (osteoblast and adipocyte) under
appropriate conditions. BC-MSCs grew rapidly and showed
fibroblastic morphology. We demonstrated that they were
homogeneously positive for the mesenchymal cell markers CD13, CD29,
CD44, CD71, CD105 and HLA-I, but negative for CD4, CD10, CD14,
CD34, CD38 and HLA-DR.
To investigate the differentiation potential of
BC-MSCs, we used the third passage from BC-MSCs for culturing in
the conditions that favored the osteogenic and adipogenic
differentiation of MSCs. The results showed that BC-MSCs were
alkaline phosphatase- and Oil Red O-positive after being induced.
Furthermore, RT-PCR results showed that these cells highly
expressed osteogenic and adipogenic marker genes, such as BMP-3 and
PPARγ-2. Therefore, our experiments revealed that BC-MSCs may be
induced to differentiate into bone and fat in vitro. The
results demonstrated the successful isolation and identification of
canonical MSCs in primary breast cancer tissue.
Furthermore, we observed the effects of BC-MSCs on
the proliferation and migration of the human breast cancer cell
line, MCF-7, in vitro. We selected the CM from the BC-MSCs
to culture MCF-7 rather than create a co-culture. Cell co-culture
studies are occasionally unreliable due to possible artificial
growth advantages of one cell type over the other induced by the
culture environment rather than by a genuine anticancer effect
(32). To rule out this
possibility, we focused on the role of the BC-MSC-CM rather than of
the direct cells in the present study. The effect of BC-MSC-CM on
MCF-7 in vitro was examined using MTT cell proliferation.
The results of MTT cell proliferation showed that BC-MSC-CM
significantly stimulated cancer cell proliferation. Therefore, this
indicates that BC-MSC-CM may have certain increased effects on the
growth of breast cancer in vitro.
A scratch wound assay and a transwell assay were
conducted to investigate MCF-7 migration. From the statistical
data, we hypothesized that 10 and 20% CM may significantly promote
MCF-7 cancer cell migration. Our results are consistent with the
study by Zhu et al(33),
which showed that hMSC-CM enhanced tumor growth is sustainable in
serial transplantation, indicating that MSC-secreted factors have
profound effects on the ‘reprogramming’ of tumor growth.
However, controversies exist concerning the
correlations between MSCs and tumors. We speculated that this may
be related to the source of the MSCs, individual variations in the
physiological immune status of the donors, differences in culture
and experimental methods, the type and site of carcinoma, or a
combination of these factors. Therefore, studies are required
before MSCs may be widely used in clinical cancer therapy, and the
mechanism between MSCs, tumorigenesis and tumor progression
requires further research. MSCs may provide a new approach for
cancer therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81270214) and the Science
Foundation of Kunshan (grant no. KS1331).
References
|
1
|
Lv YG, Yu F, Yao Q, Chen JH and Wang L:
The role of survivin in diagnosis, prognosis and treatment of
breast cancer. J Thorac Dis. 2:100–110. 2010.PubMed/NCBI
|
|
2
|
Bieback K, Kern S, Kocaömer A, Ferlik K
and Bugert P: Comparing mesenchymal stromal cells from different
human tissues: bone marrow, adipose tissue and umbilical cord
blood. Biomed Mater Eng. 18(Suppl 1): S71–S76. 2008.PubMed/NCBI
|
|
3
|
Digirolamo CM, Stokes D, Colter D, Phinney
DG, Class R and Prockop DJ: Propagation and senescence of human
marrow stromal cells in culture: a simple colony-forming assay
identifies samples with the greatest potential to propagate and
differentiate. Br J Haematol. 107:275–281. 1999. View Article : Google Scholar
|
|
4
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
5
|
Haynesworth SE, Baber MA and Caplan AI:
Cell surface antigens on human marrow-derived mesenchymal cells are
detected by monoclonal antibodies. Bone. 13:69–80. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mackay AM, Beck SC, Murphy JM, Barry FP,
Chichester CO and Pittenger MF: Chondrogenic differentiation of
cultured human mesenchymal stem cells from marrow. Tissue Eng.
4:415–428. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiao C, Xu W, Zhu W, et al: Human
mesenchymal stem cells isolated from the umbilical cord. Cell Biol
Int. 32:8–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Chen L, Scott PG and Tredget EE:
Mesenchymal stem cells enhance wound healing through
differentiation and angiogenesis. Stem Cells. 25:2648–2659. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Y, Wang J, Scott PG and Tredget EE:
Bone marrow-derived stem cells in wound healing: a review. Wound
Repair Regen. 15(Suppl 1): S18–S26. 2007. View Article : Google Scholar
|
|
11
|
Dwyer RM, Khan S, Barry FP, O’Brien T and
Kerin MJ: Advances in mesenchymal stem cell-mediated gene therapy
for cancer. Stem Cell Res Ther. 1:252010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roorda BD, ter Elst A, Kamps WA and de
Bont ES: Bone marrow-derived cells and tumor growth: contribution
of bone marrow-derived cells to tumor micro-environments with
special focus on mesenchymal stem cells. Crit Rev Oncol Hematol.
69:187–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu W, Xu W, Jiang R, et al: Mesenchymal
stem cells derived from bone marrow favor tumor cell growth in
vivo. Exp Mol Pathol. 80:267–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hall B, Andreeff M and Marini F: The
participation of mesenchymal stem cells in tumor stroma formation
and their application as targeted-gene delivery vehicles. Handb Exp
Pharmacol. 180:263–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin TM, Chang HW, Wang KH, et al:
Isolation and identification of mesenchymal stem cells from human
lipoma tissue. Biochem Biophys Res Commun. 361:883–889. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Cai H, Qian H, et al: Mesenchymal
stem cells isolated from human uterine cervix cancer tissues. Cell
Biol Int. 35:119–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao H, Xu W, Qian H, et al: Mesenchymal
stem cell-like cells derived from human gastric cancer tissues.
Cancer Lett. 274:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lis R, Touboul C, Mirshahi P, et al: Tumor
associated mesenchymal stem cells protects ovarian cancer cells
from hyperthermia through CXCL12. Int J Cancer. 128:715–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rhodes LV, Muir SE, Elliott S, et al:
Adult human mesenchymal stem cells enhance breast tumorigenesis and
promote hormone independence. Breast Cancer Res Treat. 121:293–300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salvo VA, Boué SM, Fonseca JP, et al:
Antiestrogenic glyceollins suppress human breast and ovarian
carcinoma tumorigenesis. Clin Cancer Res. 12:7159–7164. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gnecchi M and Melo LG: Bone marrow-derived
mesenchymal stem cells: isolation, expansion, characterization,
viral transduction, and production of conditioned medium. Methods
Mol Biol. 482:281–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Molloy AP, Martin FT, Dwyer RM, et al:
Mesenchymal stem cell secretion of chemokines during
differentiation into osteoblasts, and their potential role in
mediating interactions with breast cancer cells. Int J Cancer.
124:326–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwamoto S, Mihara K, Downing JR, Pui CH
and Campana D: Mesenchymal cells regulate the response of acute
lymphoblastic leukemia cells to asparaginase. J Clin Invest.
117:1049–1057. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giordano A, Galderisi U and Marino IR:
From the laboratory bench to the patient’s bedside: an update on
clinical trials with mesenchymal stem cells. J Cell Physiol.
211:27–35. 2007.
|
|
29
|
Mishra PJ, Mishra PJ, Glod JW and Banerjee
D: Mesenchymal stem cells: flip side of the coin. Cancer Res.
69:1255–1258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spaeth EL, Dembinski JL, Sasser AK, et al:
Mesenchymal stem cell transition to tumor-associated fibroblasts
contributes to fibrovascular network expansion and tumor
progression. PLoS One. 4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Hu T, Shen J, et al: Separation,
cultivation and biological characteristics of oral
carcinoma-associated fibroblasts. Oral Dis. 12:375–380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gauthaman K, Yee FC, Cheyyatraivendran S,
Biswas A, Choolani M and Bongso A: Human umbilical cord Wharton’s
jelly stem cell (hWJSC) extracts inhibit cancer cell growth in
vitro. J Cell Biochem. 113:2027–2039. 2012.
|
|
33
|
Zhu W, Huang L, Li Y, et al: Mesenchymal
stem cell-secreted soluble signaling molecules potentiate tumor
growth. Cell Cycle. 10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|