Introduction
Oral squamous cell carcinoma (OSCC) is the most
frequently occurring malignant tumor of the oral cavity worldwide
(1) and continues to be a serious
public health issue. In spite of significant advances in
therapeutics and early diagnosis, the prognosis of patients with
OSCC remains poor (1–3). OSCC tumorigenesis is a complex and
multistep process determined by multiple genetic factors (4–8), and
the current tumor-node-metastasis (TNM: T, tumor size; N, lymph
node status; M, metastasis) classification and histopathological
factors, including tumor depth and grade, do not fully predict the
clinical outcome in all cases (9).
Thus, it is crucial to search for potential molecular markers
underlying the development of OSCC and the ability to predict its
prognosis, which may be clinically useful for guiding personalized
therapy for patients.
The p53 tumor suppressor gene, located on
chromosome 17p13.1, encodes a critical stress response protein that
functions primarily as a transcription factor, regulating a large
number of genes in response to a variety of cellular insults,
including oncogene activation and DNA damage (10). The p53 protein suppresses cellular
transformation by inducing growth arrest, apoptosis, DNA repair and
differentiation in damaged cells (11). Mutations and alterations in the
p53 gene have been implicated in almost all human cancers,
and p53 status is, therefore, one of the most important biomarkers
for a variety of cancer types (12,13).
Wild-type p53 protein (encoded by the wild-type p53 gene)
has an extremely short half-life and is usually undetectable by
immunohistochemistry (IHC) (14).
By contrast, mutant p53 protein (encoded by the mutant p53
gene) is often stabilized by mutations, accumulated at extremely
high levels and detectable in tumors by IHC (14). Thus, the immunohistochemical
expression of p53 is often used to identify p53 gene status.
At present, the immunohistochemical expression of p53 and its
effect on prognosis in OSCC has been investigated in a number of
previously published studies, but the data show conflicting results
(15–18). Furthermore, the prognostic value of
p53 status may vary among patients with different treatment
plans.
In the present study, p53 immunohistochemical
staining was performed using pre-operative biopsy samples from 44
OSCC patients, and prognostic value was evaluated together with
other factors, including lymph node metastasis and tumor size.
Materials and methods
Patients and tumor samples
Archival pathological specimens for
immunohistochemical study were obtained from the Fuchu Metropolitan
Hospital (Tokyo, Japan) and Sendai National Hospital (Sendai,
Japan). The specimens consisted of 44 cases of OSCC. The present
study was approved by the Ethics Committee of these two hospitals
and the Graduate School of Medicine, Tohoku University (Sendai,
Miyagi, Japan). The experiments were undertaken with the informed
written consent of each patient and the study conformed to the Code
of Ethics of the World Medical Association (Declaration of
Helsinki). Diagnostic verification and tumor subtyping and grading
were independently performed by two certified pathologists.
Patients with distant metastases at diagnosis were excluded from
this study. T- and N-classification was assigned according to the
staging of the Union for International Cancer Control (19). All patients had undergone surgical
resection of the tumors and were classified into two groups
consisting of 24 patients who had been treated with neoadjuvant
chemotherapy and 20 who had been treated with surgery alone.
Chemotherapy consisted of ~70 mg/m2 cisplatinum and 550
mg/m2 5-fluorouracil. Surgery was performed four to six
weeks after neoadjuvant therapy. The clinicopathological
characteristics of the patients are shown in Table I. The mean follow-up period was 35.6
months (range, 5–95.3 months).
 | Table IClinicopathological characteristics of
44 OSCC patients. |
Table I
Clinicopathological characteristics of
44 OSCC patients.
| Parameters | Patients, n |
|---|
| Gender |
| Male | 28 |
| Female | 16 |
| Age, years |
| 37–83
(64.6±11.1)a | 44 |
| Primary lesion |
| Tongue | 22 |
| Lower gingiva | 10 |
| Floor of the
mouth | 5 |
| Buccal mucosa | 3 |
| Upper gingiva | 4 |
| T |
| T1 | 5 |
| T2 | 18 |
| T3 | 8 |
| T4 | 13 |
| N |
| N0 | 25 |
| N+ | 19 |
| Neoadjuvant
treatment |
| Chemotherapy | 24 |
| None | 20 |
| Total | 44 |
IHC
Histological specimens for diagnosis that were
obtained from a diagnostic biopsy underwent standard
immunohistochemical staining for the p53 protein. Briefly,
formalin-fixed, paraffin-embedded archived tissue blocks were
sectioned at 4 μm and transferred to microscope slides. The
sections were deparaffinized in xylene and rehydrated in ethanol
solution. Antigen retrieval was performed in 10 mM citrate buffer
(pH 6.0) using a microwave (15 min; 100°C) and cooled to room
temperature. Endogenous peroxidase was blocked with 0.3%
H2O2 in methanol for 30 min. Non-specific
binding was blocked with 2.5% skimmed milk for 20 min at room
temperature. Following rinsing with wash buffer, sections were
incubated overnight at 4°C with anti-human p53 mouse monoclonal
antibody (Clone DO-7; Dako, Carpinteria, CA, USA) at a 1:400
dilution. Subsequently, biotinylated goat anti-mouse antibody and
an ABC kit (both Dako) were used for detection. The sections were
developed with diaminobenzidine tetrahydrochloride (Dojin,
Kumamoto, Japan) and counterstained with hematoxylin. Negative
controls were employed in which the primary antibody was replaced
by phosphate-buffered saline. Positively stained cells were counted
under a microscope using ×200 magnification in a minimum of five
selected areas with frequent positive staining. A minimum of 2,000
cells were counted in each section. The tumor was considered
p53-positive if ≥10% of the nuclei of the tumor cells were
positively stained.
Statistical analysis
For the statistical analyses, SPSS software (SPPS
for Windows, version 12.0; SPSS, Inc., Chicago, IL, USA) was
utilized. The Kaplan-Meier method was used to assess actual 5-year
survival rates and the differences between groups were analyzed by
a log-rank test. For all analyses, P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of p53 protein in OSCC
Immunohistochemical staining showed that 21 of the
44 specimens (47.7%) examined were p53-positive. p53 protein was
exclusively expressed in the nuclei and not in the cytoplasm of the
cancer cells (Fig. 1). p53 was
mainly expressed in the invasive front of the cancer cell nest.
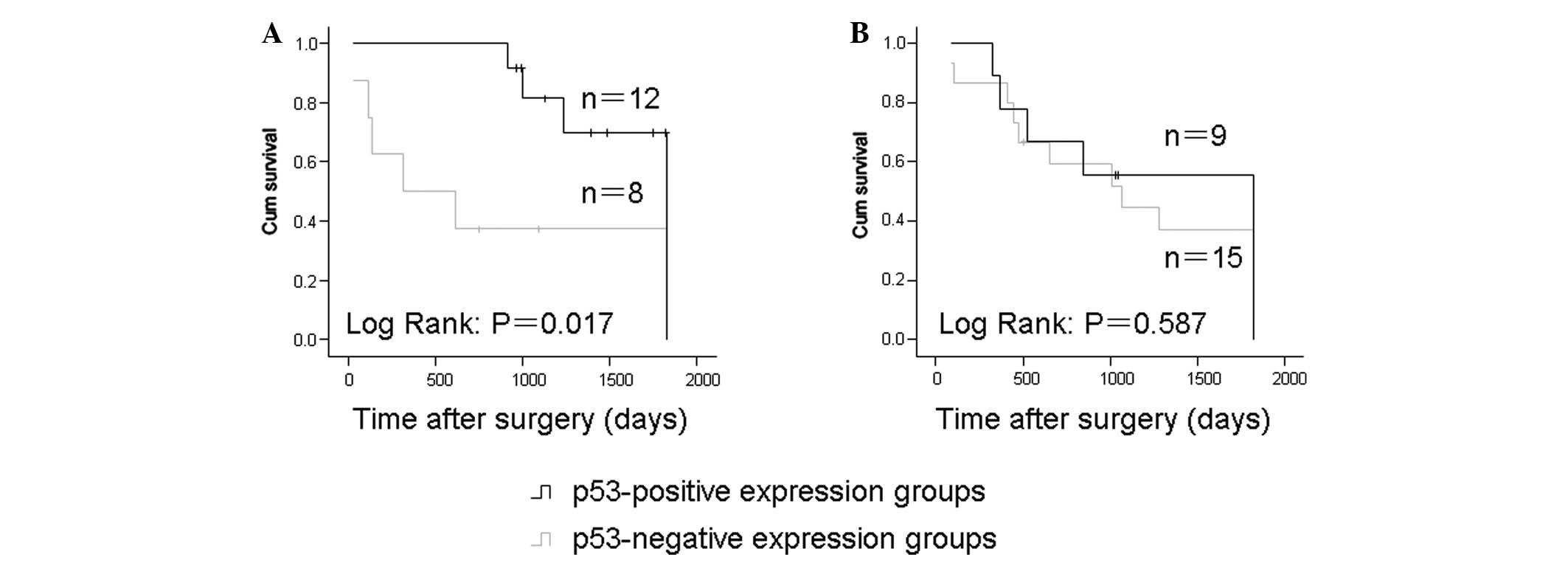
p53 expression in OSCC and its prognostic
significance
Among the cases treated with surgery alone, the
five-year survival rates were 25 and 58.3% for the p53-negative and
-positive expression groups, respectively. In addition, the
p53-positive expression group showed a significantly higher
survival rate compared with the p53-negative expression group
(P=0.017; Fig. 2A). No significant
correlation between p53 expression and patient survival was
observed in the neoadjuvant chemotherapy group (P=0.385; Fig. 2B).
N-classification and prognosis
Analysis of Kaplan-Meier survival curves showed that
OSCC patients with positive lymph node metastasis had significantly
shorter overall survival times compared with others among the cases
receiving surgery alone (P=0.03). Similar to the p53 expression
status, the N status was not significantly correlated with survival
in the chemotherapy group (Fig.
3).
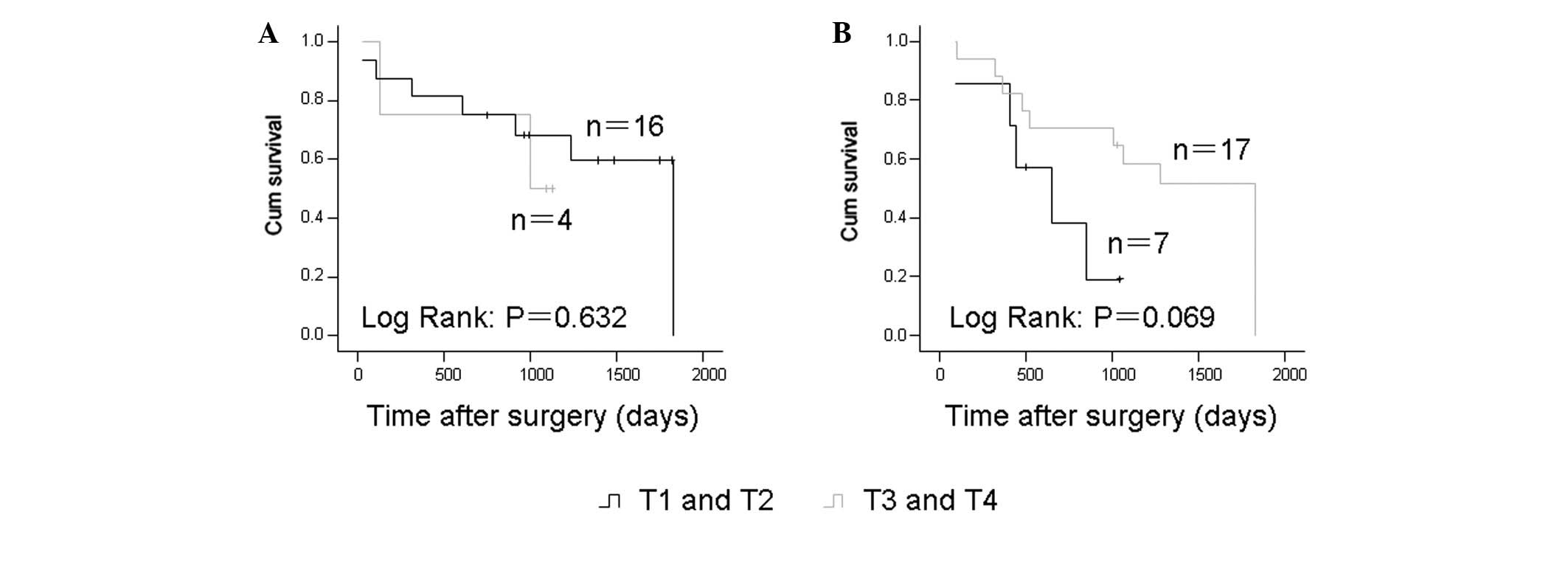
T-classification and prognosis
The T-classification and prognosis were further
evaluated in these samples. In the cases receiving surgery alone
and in the cases who underwent neoadjuvant chemotherapy, the
T-classification showed no correlation with the five-year survival
rate (Fig. 4).
Discussion
In the present study, biopsy samples were selected
instead of surgical resection samples for examination. This was as
clinical doctors are able to obtain biopsy samples earlier than
surgical resection samples and an early prognostic evaluation is
useful for guiding treatment. However, p53 expression showed no
significant difference between the biopsy and surgical resection
samples in the present study (data not shown).
TNM staging is used as a standard system for the
prediction of the prognosis of OSCC, however, certain studies have
shown that even among patients of the same stage, patient prognoses
are discordant (20). In the
present study, superior patient survival was observed with surgery
alone with N0 compared with N+, which is consistent with
previous studies (21,22). Nevertheless, certain studies have
reported that the N-classification does not correlate with the
survival of the patients (23,24).
No significant correlation was identified between the
T-classification and patient survival. Similar to the
N-classification, the correlation between the T-classification and
survival remains controversial (3,9). Such
conflicting results may be due to the fact that the TNM system only
considers the anatomical characteristics of the tumors without
considering the biological and molecular characteristics.
In the present study, the level of positive p53
expression in the OSCC patient samples was 47.7%, a similar level
to that observed in previous studies (18,25).
Additionally, the present study showed that p53 was often observed
to be expressed in the outer layer of the cancer cell nest, the
location of the aggressive tumor invasion, which is consistent with
the results of a previous study (17). Contrary to our predictions, the
results of the present study showed that p53-positive expression in
the biopsy of OSCC patients undergoing surgery only is an indicator
of an improved survival time. To the best of our knowledge, only
two types of results have been reported concerning the association
between the immunohistochemical expression of p53 and the prognosis
of OSCC, namely, the negative expression of p53 indicating an
improved prognosis or no correlation between p53 expression and
prognosis (15–18). p53 mutation results in not only the
loss of tumor suppressor function, but also in the gain of new
oncogenic properties, including increasing the tumor formation
ability and drug resistance (13).
The results of the present study, therefore, do not appear to be
consistent with the concept of p53 mutation. There are several
possible reasons for this inconsistency.
Firstly, p53 immunohistochemical staining does not
always indicate the p53 mutation status. Certain p53 mutations are
IHC-null/negative (14), while
specific tumors under continuous stress stimulation lead to
accumulation of functional wild-type p53 protein (26). Secondly, studies of tumor cell lines
and mouse tumor models have shown that oncogene activation and
abnormal proliferation are able to trigger apoptosis through the
coupling of the signal transduction pathway of apoptosis and cell
proliferation through p53-dependent and -independent mechanisms,
and the p53 mutation may lead to higher cell apoptosis levels
(27,28). When these apoptosis levels reach a
certain threshold, it may affect survival. As an example, Zheng
et al(29) reported that
intestinal-type gastric carcinomas with a more favorable prognosis
frequently exhibited elevated levels of proliferation and apoptosis
accompanied by a higher expression level of mutant p53 compared
with diffuse-type carcinomas, with a higher degree of malignancy.
Notably, one recent study reported that mutant p53 dictated an
improved chemotherapy response in a mouse mammary gland tumor model
compared with wild-type p53 due to the mechanism whereby wild-type
p53-mediated senescence impairs the apoptotic response to
chemotherapy and the clinical outcome in breast cancer (30). Therefore, p53 status may be a good
factor for the evaluation of OSCC prognosis when considered
together with other factors, such as apoptosis and the senescence
level. In addition, in the present study, p53-positive expression
and N0 were statistically correlated with a good prognosis in OSCC
patients receiving surgery alone, but not in the chemotherapy
group, which may be due to the fact that chemotherapy completely
disrupted the inherent apoptosis mechanisms of the tumor cells.
In summary, the results of the present study showed
that p53-positive immunoexpression and N0 status indicates an
improved prognosis in OSCC patients receiving surgery alone, but
not in patients undergoing surgery and neoadjuvant chemotherapy.
p53 status may serve as a good prognostic factor for the survival
of OSCC patients when combined with other factors, such as
apoptosis and senescence. Considering the inherent limitations of
IHC studies, it may be necessary to combine the IHC study with
p53 gene exon sequencing to confirm the p53 mutation status
in future studies.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Khuri FR, Lee JJ, Lippman SM, et al:
Randomized phase III trial of low-dose isotretinoin for prevention
of second primary tumors in stage I and II head and neck cancer
patients. J Natl Cancer Inst. 98:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gasparotto D and Maestro R: Molecular
approaches to the staging of head and neck carcinomas (review). Int
J Oncol. 31:175–180. 2007.PubMed/NCBI
|
|
4
|
Shibata M, Kodani I, Osaki M, et al:
Cyclo-oxygenase-1 and -2 expression in human oral mucosa,
dysplasias and squamous cell carcinomas and their pathological
significance. Oral Oncol. 41:304–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kodani I, Shomori K, Osaki M, Kuratate I,
Ryoke K and Ito H: Expression of minichromosome maintenance 2
(MCM2), Ki-67, and cell-cycle-related molecules, and apoptosis in
the normal-dysplasia-carcinoma sequence of the oral mucosa.
Pathobiology. 69:150–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lumerman H, Freedman P and Kerpel S: Oral
epithelial dysplasia and the development of invasive squamous cell
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
79:321–329. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silverman S Jr, Gorsky M and Lozada F:
Oral leukoplakia and malignant transformation. A follow-up study of
257 patients. Cancer. 53:563–568. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banoczy J and Csiba A: Occurrence of
epithelial dysplasia in oral leukoplakia. Analysis and follow-up
study of 12 cases. Oral Surg Oral Med Oral Pathol. 42:766–774.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalavrezos N and Bhandari R: Current
trends and future perspectives in the surgical management of oral
cancer. Oral Oncol. 46:429–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guimaraes DP and Hainaut P: TP53: a key
gene in human cancer. Biochimie. 84:83–93. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oren M: Decision making by p53: life,
death and cancer. Cell Death Differ. 10:431–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gasco M and Crook T: The p53 network in
head and neck cancer. Oral Oncol. 39:222–231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brosh R and Rotter V: When mutants gain
new powers: news from the mutant p53 field. Nat Rev Cancer.
9:701–713. 2009.PubMed/NCBI
|
|
14
|
Soussi T and Béroud C: Assessing TP53
status in human tumours to evaluate clinical outcome. Nat Rev
Cancer. 1:233–240. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perisanidis C, Perisanidis B, Wrba F, et
al: Evaluation of immunohistochemical expression of p53, p21, p27,
cyclin D1, and Ki67 in oral and oropharyngeal squamous cell
carcinoma. J Oral Pathol Med. 41:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coutinho-Camillo CM, Lourenco SV,
Nishimoto IN, Kowalski LP and Soares FA: Nucleophosmin, p53, and
Ki-67 expression patterns on an oral squamous cell carcinoma tissue
microarray. Hum Pathol. 41:1079–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato K, Kawashiri S, Tanaka A, et al:
Predictive value of measuring p53 labeling index at the invasive
front of oral squamous cell carcinomas. Pathol Oncol Res. 14:57–61.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oliveira LR, Ribeiro-Silva A, Costa JP,
Simões AL, Matteo MA and Zucoloto S: Prognostic factors and
survival analysis in a sample of oral squamous cell carcinoma
patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
106:685–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
International Union Against Cancer. TNM
Classification of Malignant Tumours. Wiley-Liss; New York, NY:
2000
|
|
20
|
Howaldt HP, Kainz M, Euler B and Vorast H:
Proposal for modification of the TNM staging classification for
cancer of the oral cavity. DOSAK J Craniomaxillofac Surg.
27:275–288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kreppel M, Drebber U, Eich HT, et al:
Combined-modality treatment in advanced oral squamous cell
carcinoma: Primary surgery followed by adjuvant concomitant
radiochemotherapy. Strahlenther Onkol. 187:555–560. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SY, Nam SY, Choi SH, Cho KJ and Roh
JL: Prognostic value of lymph node density in node-positive
patients with oral squamous cell carcinoma. Ann Surg Oncol.
18:2310–2317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pillai KR, Sujathan K, Madhavan J and
Abraham EK: Significance of silver-stained nucleolar organizer
regions in early diagnosis and prognosis of oral squamous cell
carcinoma: a multivariate analysis. In Vivo. 19:807–812.
2005.PubMed/NCBI
|
|
24
|
Iype EM, Pandey M, Mathew A, Thomas G and
Nair MK: Squamous cell cancer of the buccal mucosa in young adults.
Br J Oral Maxillofac Surg. 42:185–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Girod SC, Krueger G and Pape HD: p53 and
Ki 67 expression in preneoplastic and neoplastic lesions of the
oral mucosa. Int J Oral Maxillofac Surg. 22:285–288. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olivier M, Hainaut P and Borresen-Dale A:
Prognostic and predictive value of TP53 mutations in human cancer.
25 Years of p53 Research. Hainaut P and Wiman K: Springer;
Dordrecht, the Netherlands: pp. 321–338. 2005, View Article : Google Scholar
|
|
27
|
Jäättelä M: Multiple cell death pathways
as regulators of tumour initiation and progression. Oncogene.
23:2746–2756. 2004.PubMed/NCBI
|
|
28
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng H, Takahashi H, Murai Y, et al:
Pathobiological characteristics of intestinal and diffuse-type
gastric carcinoma in Japan: an immunostaining study on the tissue
microarray. J Clin Pathol. 60:273–277. 2007. View Article : Google Scholar
|
|
30
|
Jackson JG, Pant V, Li Q, et al:
p53-mediated senescence impairs the apoptotic response to
chemotherapy and clinical outcome in breast cancer. Cancer Cell.
21:793–806. 2012. View Article : Google Scholar : PubMed/NCBI
|