Introduction
Acute promyelocytic leukemia (APL) is a relatively
rare subtype of acute myelogenous leukemia that occurs in 8–15% of
all acute non-lymphoblastic leukemia patients, with a mean
incidence of two to three cases per million members of the global
population each year (1). APL is
characterized by pathological coagulation (coagulopathy) involving
abnormal accumulation of immature granulocytes, particularly
promyelocytes, leading to fibrinolysis and hemostatic failure
(1,2). Unlike other leukemia subtypes, optimal
treatment of APL requires rapid initiation of all-trans
retinoic acid (ATRA) therapy and targeted supportive care for
APL-specific complications, including bleeding disorders, APL
differentiation syndrome, QT prolongation and other ATRA-related
toxicities (3). The wide-spread
clinical employment of combined ATRA regimens, including ATRA and
arsenic compounds, has reduced relapse from ~50% to <10% in
adult patients with APL over the past two decades (4,5).
However, increased knowledge of the outcomes in this remaining
group of treated patients with APL that exhibit relapse is crucial
to understanding APL pathophysiology and to improving survival in
this patient subpopulation.
APL is caused by the cumulative effects of somatic
mutations, ultimately resulting in the development of
mutagen-induced carcinogenesis, and often occurs with advanced age
(1). Cytogenetically, between 95
and 100% of APL cases have been reported to be associated with
karyotypic abnormalities involving pathognomonic translocations at
t(15;17)(q22–24;q11–21) that juxtapose the retinoic acid
receptor α (RARα) gene with the promyelocytic leukemia (PML) gene
(6,7). This translocation has been implicated
in the blockage of normal differentiation of immature myeloid cells
into mature granulocytes (8,9) and
the inhibition of programmed cell death in myeloid cells (7). Furthermore, 10–50% of all patients
with APL exhibit FLT3 mutations, either as internal tandem
duplications or kinase domain mutations (10), and FLT3 mutations generally
correlate with high white blood cell (WBC) counts
(>10×109/l), which are indicative of higher patient
risk of relapse (6).
ATRA treatments for APL are unique in that they act
by dissociating the nuclear hormone receptor complex NCOR-HDAC from
RARα. This then initiates the maturation of leukemic promyelocytes,
rather than inducing cell death (11). While ATRA monotherapies have
demonstrated relatively high relapse rates, combined therapies
involving anthracyclines and other active agents are able to
markedly reduce relapse rates (12). Arsenic compounds, such as arsenic
trioxide (ATO) and arsenic tetrasulfide (ATS), are the most active
single agents in refractory APL treatment due to their ability to
induce partial myeloid differentiation and caspase-specific
apoptosis (13), with a relapse
rate of <20% following monotherapy (14). Combined treatment regimens involving
both ATRA and ATO have been reported to eradicate leukemic
progenitor cells and reduce the relapse rate to <10% in even
high-risk patients with APL (15).
Furthermore, combined ATRA- and arsenic compound-based (ATRA +
arsenic compound) salvage therapies have been demonstrated to
induce complete remission in 50–80% of refractory or relapsed
patients with APL (16).
Though contemporary combined ATRA + arsenic compound
therapies are effective in inducing complete remission (CR) in the
majority of patients, a notable patient group still exhibits
relapse, although the characteristics of this group are relatively
undocumented. For these patients, the risks and benefits of ATRA +
arsenic compound retreatment versus retreatment with other
modalities remain controversial, as few evidence-based studies have
specifically examined cohorts of relapsed patients with APL. The
current study examines the characteristics and effectiveness of the
treatment of first-time relapsed patients with APL that were
originally treated with combined ATRA + arsenic compound therapies.
The results provide a unique insight into the outcomes of these
patients that is useful for the evaluation and selection of
treatment strategies.
Materials and methods
Study design
A total of 25 first-time relapse patients with APL,
who were previously treated with first-line ATRA + arsenic compound
therapy and were subsequently treated in the Hematological Unit of
Peking University People’s Hospital (Beijing, China) between
January 1994 and December 2010, were included in this
retrospective, observational analysis. The study protocol was
approved by the Institutional Review Board at Peking University
People’s Hospital. All patients provided written informed consent,
prior to their treatment, for the use of their data in the
subsequent research.
Patients
Patients were included that: i) were diagnosed with
APL in accordance with the morphological criteria (M0–M7) of the
French-American-British classification system for myelocytic
leukemias (17); ii) exhibited APL
confirmed by both cytogenetic assay for t(15;17)(q24;q21)
and reverse transcription polymerase chain reaction (PCR) analysis
for PML-RARα, as previously described by de Botton et
al(18); iii) underwent initial
induction therapy with first-line ATRA (25 mg/m2/day)
and low-dose cytotoxic agents, with or without adjuvant treatment
with ATO (10 mg/kg/day) or up-titrated ATS (2250–60 mg/kg/day); iv)
exhibited relapse, defined as any disease recurrence following CR,
including morphological, molecular and extra-medullary relapses;
and v) were treated with consolidation chemotherapy involving
cytarabine (Ara-C)- or anthracycline-based therapies with
alternating maintenance ATRA (25 mg/m2/day) for two
weeks, once every three months, and ATO (10 mg/kg/day) or ATS (60
mg/kg/day) for two weeks, twice every three months. WBC and
platelet counts were further used to classify patients as low risk
(<10×109/l; >40×109/l), intermediate
risk (<10×109/l; <40×109/l) or high
risk (≥10×109/l; <40×109/l), respectively,
as previously described (18).
Relapse and re-induction therapy
Following confirmation of APL relapse, appropriate
re-induction regimens were immediately administered using one of
six therapeutic regimens: i) ATRA + arsenic compound combination
therapy (Ruijin Pharmaceuticals Co., Ltd., Shanghai, China);
salvage chemotherapy with ii) mitoxantrone (Shenghe Pharmaceuticals
Co., Ltd., Sichuan, China) + Ara-C (Pfizer, Inc., New York, NY,
USA) (MA), iii) homoharringtonin (Minsheng Pharmaceutical Group
Co., Ltd., Zhejiang, China) + Ara-C (HA), or iv) homoharringtonin +
Ara-C + daunomycin (Pfizer, Inc.) (HAD); v) gemtuzumab ozogamicin
treatment (Wyeth Pharmaceuticals, Philadelphia, PA, USA); or vi)
intrathecal chemotherapy with cytarabine and dexamethasone (CSPC
Zhongnuo Pharmaceutical Co., Ltd., Shijiazhuang, China). Relapse
types were classified as morphological relapse (≥5% blasts per
abnormal promyelocytes in the bone marrow or per leukemic cells in
the peripheral blood), molecular relapse (PML/RARα gene
conversion from PCR-negative to -positive in patients without
morphological abnormalities in two successive four-week bone marrow
samples) or extramedullary relapse (abnormal promyelocytes in the
cerebrospinal fluid or extramedullary granulocytic sarcoma).
Laboratory monitoring and
assessments
Follow-up bone marrow aspiration was repeated at
three-month intervals during maintenance therapy (ATRA + arsenic
compounds with alternating maintenance chemotherapy)
administration. Patient tolerance, based on gastrointestinal
reactions and hepatotoxicity (reduced drug dose when hepatotoxicity
grade ≥3 and drug withdrawal when hepatotoxicity grade 4), and
urine arsenic compounds were closely monitored, and the doses of
arsenic compounds were adjusted in accordance with standards
published by the National Cancer Institute (19).
Outcome assessments
The patients were followed up for a minimum of six
months after relapse treatment. The outcome of post-retreatment
remission rates, duration of remission and toxic effects were
recorded. CR was defined as <5% blasts or abnormal promyelocytes
in the bone marrow, coupled with peripheral blood absolute
neutrophil count ≥1.5×109/l, untransfused hemoglobin
levels ≥100 g/l and platelet count ≥100×109/l. Molecular
remission was defined as a negative bone marrow PCR for the
PML/RARα gene at a sensitivity of 10−4.
Treatment with reconsolidation therapies and other therapies, such
as allogeneic and autologous hematopoietic stem cell
transplantation (allo-HSCT and auto-HSCT, respectively), were
recorded.
Statistical analysis
This was a retrospective, observational analysis and
only descriptive statistics are provided. Data are presented as the
mean ± standard deviation, the mean ± interquartile range or the
percentile [n (%)], as appropriate.
Results
Clinical characteristics of patients
initially diagnosed with APL
A total of 25 patients initially diagnosed with APL,
17 males and 8 females (mean age, 36.4±10.3 years; range, 19–64
years; Table I), were included in
the study. Patients were followed up for a median of four years
(range, 0.5–13 years) following their initial treatment (data not
shown). According to the classification system by Sanz et
al(4), four patients (16.0%)
were at low risk, 12 (48.0%) were at intermediate risk and nine
(36.0%) were at high risk of ALP relapse (Table I). All patients were previously
administered with ATRA and low-dose cytotoxic agents during initial
induction therapy, and 16 patients (64.0%) received adjuvant
treatment with ATO or up-titrated ATS. Thirteen patients (52.0%)
received intrathecal chemotherapy for the treatment of central
nervous system (CNS) involvement at the time of initial diagnosis,
and all patients received prophylactic intrathecal chemotherapy
following CR (data not shown).
 | Table IDemographic and clinical
characteristics of patients with acute promyelocytic leukemia at
the time of initial diagnosis and first relapse. |
Table I
Demographic and clinical
characteristics of patients with acute promyelocytic leukemia at
the time of initial diagnosis and first relapse.
| Value |
|---|
|
|
|---|
| Characteristics | Initial
diagnosis | Relapse |
|---|
| Gender, n (%) |
| Male | 17 (68.0) | |
| Female | 8 (32.0) | |
| Age, years | 36.4±10.3 | |
| WBC count,
109/la | 7.8±16.6 | 4.8±2.7 |
| Hemoglobin,
g/dla | 99.0±52.0 | 125.9±29.4 |
| DIC, n (%) | 12 (48.0) | 9 (36.0) |
| CNS leukemia, n
(%) | 13 (52.0) | 11 (44.0) |
| Sanz risk
categoryb, n (%) |
| Low risk | 4 (16.0) | |
| Intermediate
risk | 12 (48.0) | |
| High risk | 9 (36.0) | |
| Platelet count,
109/la | 28.2±33.0 | 140.0±139.0 |
| Chromosome
aberration, n (%) | 7 (28.0) | |
| Equiarm 17q | 1 (4.0) | |
| +2p−, −4, inv(14),
22p+, +mar | 1 (4.0) | |
| +8 | 1 (4.0) | |
| 1p+, 16q+ | 1 (4.0) | |
| 7q− | 1 (4.0) | |
| 16p+, 14q− | 1 (4.0) | |
| 11p+, 17p−, −14,
−20, acea2 | 1 (4.0) | |
| CD56 expression, n
(%) |
| Negative | 6 (24.0) | |
| Positive | 3 (12.0) | |
| Unknown | 16 (64.0) | |
| CD117 expression, n
(%) |
| Negative | 1 (4.0) | |
| Positive | 11 (44.0) | |
| Unknown | 13 (52.0) | |
| Time to relapse,
monthsa | 17.0±17.0 | 15.0±17.0 |
Clinical characteristics of relapsed
patients with APL
The first relapse occurred at a median of 17 months
(range, 5–84 months) following initial treatment (Table I). Relapses involved bone marrow in
19 patients (76.0%), the CNS alone in one patient (4.0%), molecular
relapse in one patient (4.0%) and bone marrow/CNS (complete
relapse) in four patients (16.0%) (Table II). The relapsed patients with APL
showed a median WBC count of 4.8×109/l (range,
1.3–144.2×109/l), a median hemoglobin level of
125.9±29.4 g/l (range, 63.9–182 g/l) and a median platelet count of
140.0×109/l (range, 9.0–266.0×109/l)
(Table I). First relapse data is
presented in comparison with initial clinical values in Table I and in full detail in Table II.
 | Table IIClinical characteristics of patients
with acute promyelocytic leukemia at the time of first relapse. |
Table II
Clinical characteristics of patients
with acute promyelocytic leukemia at the time of first relapse.
| Characteristics | n (%) |
|---|
| Relapse type |
| Bone marrow | 19 (76.0) |
| CNS alone | 1 (4.0) |
| Molecular | 1 (4.0) |
| Bone
marrow/CNS | 4 (16.0) |
| First relapse
induced |
| ATRA + arsenic
compounds | 16 (64.0) |
| ED | 4 (16.0) |
| GO | 1 (4.0) |
| Salvage
chemotherapy | 3 (12.0) |
| Intrathecal
chemotherapy | 1 (4.0) |
| Induced side
effects |
| ED | 6 (24.0) |
| Grade 1
hepatotoxicity | 2 (8.0) |
| Grade 2
hepatotoxicity | 4 (16.0) |
| Grade 3 bone
marrow suppression | 4 (16.0) |
| Grade 4 bone
marrow suppression | 1 (4.0) |
| None | 8 (32.0) |
| First recurrence
consolidate |
| ATRA + arsenic
compounds |
| with alternating
chemotherapy | 9 (36.0) |
| ED | 6 (24.0) |
| GO | 1 (4.0) |
| Chemotherapy
alone | 4 (16.0) |
| Arsenic
compounds | 2 (8.0) |
| None | 3 (12.0) |
| First relapse
treatment efficacy |
| CR | 15 (60.0) |
| ED | 6 (24.0) |
| NR | 4 (16.0) |
| Second relapse |
| ED | 6 (24.0) |
| Non-relapse | 4 (16.0) |
| Relapse | 15 (60.0) |
| Third relapse |
| ED | 11 (44.0) |
| Non-relapse | 6 (24.0) |
| Died of
allo-HSCT | 2 (8.0) |
| Relapse | 6 (24.0) |
| Current Status |
| CR | 8 (32.0) |
| Death | 17 (68.0) |
Re-induction therapy selection and
efficacy
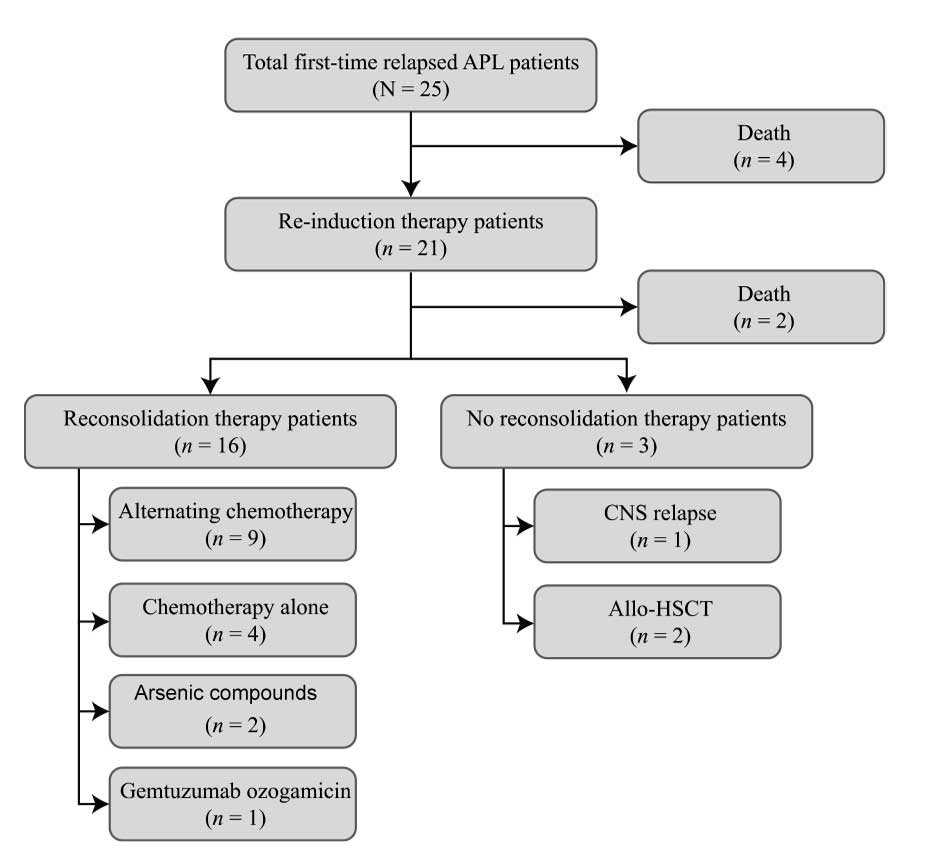
Four relapsed patients with APL succumbed to the
disease before re-induction therapy. For re-induction therapy,
patients were treated with either ATRA + arsenic compound
combination therapy (16/25, 64.0%), salvage chemotherapy with HA
(2/25, 8.0%), salvage chemotherapy with HAD (1/25, 4.0%),
gemtuzumab ozogamicin (for the single case of molecular relapse;
1/25, 4.0%) or intrathecal chemotherapy (for the single case of
isolated CNS relapse; 1/25, 4.0%) (Table II). Two of those who did not
survive (8.0%) were treated with ATRA + arsenic compound
re-induction therapy. Only one (4.0%) patient recovered completely
from isolated CNS relapse following intrathecal chemotherapy,
remaining disease-free for 13 years. CR was also observed following
ATRA + arsenic compound therapy (10/25, 40.0%), chemotherapy (3/25,
12.0%)and targeted therapy (1/25, 4.0%); and non-remission (NR)
following ATRA + arsenic compounds (4/25, 16%) (Table II). Fig. 1 details the treatment and outcomes
of the patients.
Reconsolidation therapy and survival
Among the 19 surviving patients (76.0%), complete
recovery from CNS relapse following intrathecal chemotherapy
occurred in one patient (1/19, 5.3%); one patient (5.3%) treated
with allo-HSCT following secondary CR remained disease-free at the
end of the study period; two patients (10.5%) remained in CR
following remission re-induction; secondary bone marrow and CNS
relapse occurred in 14 patients (73.7%) and one patient (5.3%),
respectively. The rate of secondary relapse was 78.9% (15/19)
(Table II).
Among the 19 surviving patients (76.0%), complete
recovery from CNS relapse following intrathecal chemotherapy
occurred in one patient (1/25, 4.0%); one patient (4.0%) treated
with allo-HSCT following secondary CR remained disease-free at the
end of the study period; two patients (8.0%) remained in CR
following remission re-induction; and secondary bone marrow and CNS
relapse occurred in 14 patients (56.0%) and one patient (4.0%),
respectively. The rate of secondary relapse was 78.9% (15/19)
(Table II).
Toxic effects of ATRA + arsenic compound
re-induction therapy
Adverse events were observed in all ATRA + arsenic
compound re-induction therapy patients. Of the 16 surviving
patients, six (6/16, 37.5%) exhibited no treatment-emergent
toxicity, six (6/16, 37.5%) exhibited grade 1–2 hepatotoxicity, two
(2/16, 12.5%) exhibited grade 3–4 bone marrow suppression and two
(2/16, 12.5%) exhibited treatment-related mortality. Salvage
chemotherapy was poorly tolerated in all three patients receiving
chemotherapy for re-induction, with grades 3–4 bone marrow
suppression. No toxicity was reported in the two patients that
received isolated intrathecal chemotherapy or gemtuzumab ozogamicin
(Table II).
Discussion
The present study demonstrated that ATRA + arsenic
compound-based combination therapy was effective in re-inducing
morphological remission in relapsed patients with APL with previous
exposure to ATRA + arsenic compounds; however, these patients
remained subject to low molecular remission rates and at high risk
of secondary relapse. Notably, allo-HSCT yielded good re-induction
results, suggesting that early allo-HSCT should be more carefully
explored as a therapeutic option for re-inducing morphological
remission in relapsed patients with APL with previous exposure to
ATRA + arsenic compounds.
Combinations of ATRA and chemotherapy have been
widely accepted as front-line treatments for the majority of
relapsed patients with APL (12).
In the present study, the overall good results produced by ATRA +
arsenic compounds, leading to remission in the majority of patients
with APL, are consistent with a previous study that recommends this
treatment for salvage patients with APL or those that have
previously received ATRA-based combination therapies (16). Furthermore, arsenic compound
monotherapy has been reported to effectively re-induce molecular
remission in 80–90% of relapsed patients with APL, making it useful
as both an initial induction and consolidation treatment in
high-risk patients (16). Breccia
et al(13) reported that
prolonged ATO-based salvage therapy achieved a high remission rate
in relapsed ATO-naïve patients without requiring HSCT.
Cumulatively, the results of the current study and the findings of
these previous studies are in agreement and indicate that
morphological remission in relapsed patients with APL with previous
exposure to ATRA + arsenic compounds may be efficiently obtained
using ATRA + arsenic compound-based combination therapy. Notably,
these findings are generally consistent with the broader guidelines
published by the National Comprehensive Cancer Network in 2011
(20).
In the patient cohort of the present study, APL
relapse occurred at a median of 17 months after initial reports of
CR, a relapse time significantly earlier than that reported by
Thirugnanam et al (20.3 months) (14). A high remission re-induction rate
was achieved in patients in the current study following ATRA +
arsenic compound-based salvage treatment with or without
chemotherapy, although these patients had previous exposure to ATRA
+ arsenic compounds. Furthermore, alternative chemotherapy was
generally effective; however, these patients often experienced
secondary relapse and the duration of remission was relatively
short (median, 15 months). By contrast, Thirugnanam et
al(14) reported a CR rate of
93% in relapsed patients with APL with previous exposure to ATO
monotherapy following ATRA + arsenic compound-based salvage
treatment with or without anthracycline-based chemotherapy.
Furthermore, these patients with APL only received ATRA as salvage
treatment for relapse, rather than induction or maintenance therapy
for initial treatment (14);
whereas, the majority of patients in the current study received
arsenic compounds induction and maintenance therapies as well. This
may contribute to discrepancies between the findings of the current
study and those of Thirugnanam et al(14).
The current results indicated a high frequency of
extra-medullary relapse, with the CNS involved in the relapse of
approximately one fifth of current patients, and this was
inconsistent with a previous study in which only 8% of patients
exhibited relapse in the CNS (14).
Furthermore, the majority of patients in the present study with CNS
relapse also exhibited relapse in the bone marrow. As ATRA
upregulates the expression of adhesion molecules, such as CD11b,
CD13 and CD56, on ALP cell surfaces (21), and concomitantly stimulates the
secretion of interleukin-1 (22),
ATRA promotes endothelial expression of vascular cell adhesion
molecule 1 and intercellular adhesion molecule 1 (23). As a result, patients with APL
exposed to ATRA are more likely to exhibit relapse involving the
CNS or even the pseudotumor cerebri (4), which may explain the occurrence of
relapse in the CNS in the patients of the present study.
Additionally, granulocytosis may independently contribute to CNS
involvement during ALP relapse (24), further raising the risk of relapse
involving the CNS.
Only a small percentage of the patients in the
current study achieved molecular remission, suggesting that
relapsed patients with APL previously exposed to ATRA + arsenic
compound-based combination treatments were at very high risk of
secondary relapse. Notably, all the patients previously treated
with ATRA + arsenic compounds combined with chemotherapy exhibited
relapse following auto-HSCT. Therefore, auto-HSCT may not be
suitable for these patients, despite general recommendations that
auto-HSCT rather than allo-HSCT is optimal for use in patients with
APL who initially achieve CR following primary relapse (25). By contrast, allo-HSCT should be
considered in relapsed patients that exhibit a relatively short
duration of remission or do not achieve molecular remission,
preferably following the secondary rather than tertiary
morphological CR episode (26).
As all the patients included in the current study
were exposed to ATRA, further comparative studies are required to
identify whether ATRA is involved in the development of CNS relapse
and by which mechanisms this action may occur. Additionally,
variations in dosage and treatment duration must be considered,
which may contribute to relapse occurrence based on yet
undetermined risk factors. Furthermore, the sample size of the
present study is relatively small and, thus, may not be fully
representative of broader APL patient populations.
ATRA + arsenic compound-based salvage treatments
with or without chemotherapy are effective agents for re-inducing
complete remission in relapsed patients with APL previously exposed
to combined ATRA + arsenic compound therapies. Molecular remission,
however, is relatively rare following such salvage treatment in
relapsed patients with APL, and the vast majority of these patients
will exhibit secondary relapse. Furthermore, the findings of the
present study suggest that auto-HSCT may be unsuitable for use in
relapsed patients with APL who are at high risk of secondary
relapse, with early allo-HSCT yielding a more notable beneficial
survival benefit. Large-scale comparative studies, however, will be
required to fully elucidate this correlation.
References
|
1
|
Douer D, Preston-Martin S, Chang E,
Nichols PW, Watkins KJ and Levine AM: High frequency of acute
promyelocytic leukemia among Latinos with acute myeloid leukemia.
Blood. 87:308–313. 1996.
|
|
2
|
Vickers M, Jackson G and Taylor P: The
incidence of acute promyelocytic leukemia appears constant over
most of a human lifespan, implying only one rate limiting mutation.
Leukemia. 14:722–726. 2000. View Article : Google Scholar
|
|
3
|
Kelaidi C, Chevret S, De Botton S, et al:
Improved outcome of acute promyelocytic leukemia with high WBC
counts over the last 15 years: the European APL Group experience. J
Clin Oncol. 27:2668–2676. 2009.PubMed/NCBI
|
|
4
|
Sanz MA, Grimwade D, Tallman MS, et al:
Management of acute promyelocytic leukemia: recommendations from an
expert panel on behalf of the European LeukemiaNet. Blood.
113:1875–1891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Degos L and Zhen YW: All trans retinoic
acid in acute promyelocytic leukemia. Oncogene. 20:7140–7145. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lo-Coco F, Avvisati G, Vignetti M, et al:
Front-line treatment of acute promyelocytic leukemia with AIDA
induction followed by risk-adapted consolidation for adults younger
than 61 years: results of the AIDA-2000 trial of the GIMEMA Group.
Blood. 116:3171–3179. 2010.
|
|
7
|
Fu S, Consoli U, Hanania EG, et al:
PML/RARalpha, a fusion protein in acute promyelocytic leukemia,
prevents growth factor withdrawal-induced apoptosis in TF-1 cells.
Clin Cancer Res. 1:583–590. 1995.
|
|
8
|
de The H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15;17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990.
|
|
9
|
Fenaux P, Chastang C, Chevret S, et al: A
randomized comparison of all transretinoic acid (ATRA) followed by
chemotherapy and ATRA plus chemotherapy and the role of maintenance
therapy in newly diagnosed acute promyelocytic leukemia. The
European APL Group. Blood. 94:1192–1200. 1999.
|
|
10
|
Callens C, Chevret S, Cayuela JM, et al:
Prognostic implication of FLT3 and Ras gene mutations in patients
with acute promyelocytic leukemia (APL): a retrospective study from
the European APL Group. Leukemia. 19:1153–1160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tallman MS, Andersen JW, Schiffer CA, et
al: All-trans retinoic acid in acute promyelocytic leukemia:
long-term outcome and prognostic factor analysis from the North
American Intergroup protocol. Blood. 100:4298–4302. 2002.
View Article : Google Scholar
|
|
12
|
Asou N, Kishimoto Y, Kiyoi H, et al: A
randomized study with or without intensified maintenance
chemotherapy in patients with acute promyelocytic leukemia who have
become negative for PML-RARalpha transcript after consolidation
therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study.
Blood. 110:59–66. 2007. View Article : Google Scholar
|
|
13
|
Breccia M, Cicconi L, Minotti C, et al:
Efficacy of prolonged therapy with combined arsenic trioxide and
ATRA for relapse of acute promyelocytic leukemia. Haematologica.
96:1390–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thirugnanam R, George B, Chendamarai E, et
al: Comparison of clinical outcomes of patients with relapsed acute
promyelocytic leukemia induced with arsenic trioxide and
consolidated with either an autologous stem cell transplant or an
arsenic trioxide-based regimen. Biol Blood Marrow Transplant.
15:1479–1484. 2009. View Article : Google Scholar
|
|
15
|
Ghavamzadeh A, Alimoghaddam K, Ghaffari
SH, et al: Treatment of acute promyelocytic leukemia with arsenic
trioxide without ATRA and/or chemotherapy. Ann Oncol. 17:131–134.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ravandi F, Estey E, Jones D, et al:
Effective treatment of acute promyelocytic leukemia with
all-trans-retinoic acid, arsenic trioxide, and gemtuzumab
ozogamicin. J Clin Oncol. 27:504–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
American Cancer Society Publications.
Leukemia-Acute Myeloid (Myelogenous): How is it classified?
http://www.cancer.org/cancer/leukemia-acutemyeloidaml/detailedguide/leukemia-acute-myeloid-myelogenous-classified.
Accessed April 15, 2012
|
|
18
|
de Botton S, Sanz MA, Chevret S, et al:
Extramedullary relapse in acute promyelocytic leukemia treated with
all-trans retinoic acid and chemotherapy. Leukemia. 20:35–41.
2006.
|
|
19
|
National Cancer Institute. Cancer therapy
evaluation program: Common toxicity criteria manual. Version 2.0.
1999.
|
|
20
|
National Comprehensive Cancer Network.
Clinical practice guidelines in oncology: Acute myeloid leukemia.
2011.
|
|
21
|
Nagai S, Takahashi T and Kurokawa M:
Beneficial and adverse effects of molecularly targeted therapies
for acute promyelocytic leukemia in central nervous system. CNS
Neurol Disord Drug Targets. 8:387–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Machner B, Neppert B, Paulsen M, Hofmann
C, Sander T and Helmchen C: Pseudotumor cerebri as a reversible
side effect of all-trans retinoic acid treatment in acute
promyelocytic leukaemia. Eur J Neurol. 15:e68–e69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Botton S, Fawaz A, Chevret S, et al:
Autologous and allogeneic stem-cell transplantation as salvage
treatment of acute promyelocytic leukemia initially treated with
all-trans-retinoic acid: a retrospective analysis of the European
acute promyelocytic leukemia group. J Clin Oncol. 23:120–126.
2005.
|
|
24
|
O’Brien S, Kantarjian HM, Keating M, et
al: Association of granulocytosis with poor prognosis in patients
with acute myelogenous leukemia and translocation of chromosomes 8
and 21. J Clin Oncol. 7:1081–1086. 1989.PubMed/NCBI
|
|
25
|
Kohno A, Morishita Y, Iida H, et al:
Hematopoietic stem cell transplantation for acute promyelocytic
leukemia in second or third complete remission: a retrospective
analysis in the Nagoya Blood and Marrow Transplantation Group. Int
J Hematol. 87:210–216. 2008. View Article : Google Scholar
|
|
26
|
Bourquin JP, Thornley I, Neuberg D, et al:
Favorable outcome of allogeneic hematopoietic stem cell
transplantation for relapsed or refractory acute promyelocytic
leukemia in childhood. Bone Marrow Transplant. 34:795–798. 2004.
View Article : Google Scholar
|