Introduction
Ovarian cancer is the leading cause of mortality
from gynecological malignancy and the fifth most common cause of
cancer-related mortality in females (1). Currently the standard treatment for
advanced-stage ovarian cancer is primary cytoreductive surgery,
followed by platinum and paclitaxel combination chemotherapy.
Although there have been improvements, the long-term survival rate
remains poor due to the emergence of drug resistance. The majority
of ovarian cancer patients who are initially sensitive to
chemotherapeutic agents will eventually relapse, and in a number of
cases, acquired resistance leaves no available curative treatments
(2). Emerging evidence indicates
that a deregulated apoptosis pathway is a major contributor to
tumor initiation and the progression and development of acquired
resistance to anticancer therapies (3).
microRNAs (miRNAs/miRs) are a class of non-coding
RNAs that regulate gene expression (4) by repressing mRNA translation or
cleaving target mRNA. miRNAs play a role in growth control, and an
association between miRNAs and cancer is anticipated. Furthermore,
miRNAs that are involved in specific networks, including apoptosis,
proliferation or receptor-driven pathways, may affect the response
to targeted therapies or to chemotherapy. miRNAs are differentially
expressed in chemosensitive and chemoresistant cells (5). miR-98, -21 and -125b have been shown
to potentiate chemoresistance (6–8).
Sorrentino et al investigated the role of miRNAs in
drug-resistant ovarian cancer cells (9). miR-125b was shown to be downregulated
in the A2780/TAX cells and upregulated in the other resistant cell
lines. Yang et al investigated miRNA expression profiles in
cisplatin (CDDP)-resistant ovarian cancer cells and identified that
miR-106a was upregulated in CDDP-resistant ovarian cancer cells
(10). However, to the best of our
knowledge, there have been no studies with regard to the mechanism
of miR-106a modulating the sensitivity of ovarian cancer cells to
CDDP.
The present study investigated miR-106a expression
in CDDP-resistant ovarian cancer cells and the effect of miR-106a
downregulation on CDDP chemosensitivity in an ovarian cancer cell
line by inducing apoptosis enhancement. miR-106a may be used as a
valid therapeutic target in strategies that employ novel
multimodality therapies for patients with ovarian cancer.
Materials and methods
Cell culture
The human ovarian cancer OVCAR3 cell lines were
purchased from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The CDDP-resistant ovarian cancer
OVCAR3/CIS cell line was induced using progressive concentrations
of CDDP as described previously (11). The OVCAR3 and CDDP-resistant
OVCAR3/CIS cell lines were cultured in PRMI-1640 medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified incubator with 5% CO2 at 37°C. The OVCAR3/CIS
cells were alternately fed with medium containing 7.5 μg/ml CDDP
and were regularly tested for the maintenance of drug-resistance.
The CDDP-resistant cell line was maintained in drug-free medium for
1 week prior to the follow-up experiments.
Quantitative reverse transcription PCR
(qRT-PCR)
To analyze the miR-106a expression levels, RNA was
extracted from the cells. The stem-loop qRT-PCR assay was used to
quantify the miRNA expression levels as described previously
(12). The qRT-PCR primers were as
follows: miR-106a RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTACCT-3′; miR-106a
PCR primer sense, 5′-CGCAAAAGTGCTTACAGTGCA-3′ and antisense,
5′-GTGCAGGGTCCGAGGT-3′; U6 RT primer, 5-CTCAACTGGTGTCGTGGAGTCGGCAA
TTCAGTTGAGGGGACAAA-3′; and U6 PCR primer sense,
5′-CTCGCTTCGGCAGCACA-3′ and antisense, 5′-AACGCT TCACGAATTTGCGT-3′.
The SYBR Premix Ex Taq™ kit (Takara, Dalian, China) was used
according to the manufacturer’s instructions, and qRT-PCR was
performed and analyzed using the CFX-96 Real-Time PCR Detection
System (Bio-Rad, Hercules, CA, USA). The PCR conditions were 95°C
for 3 min, followed by 40 cycles of 95°C for 30 sec, 58°C for 30
sec and 72°C for 30 sec. The expression levels of miR-106a were
normalized with reference to the expression levels of U6 snRNA, and
the fold changes were calculated by relative quantification
(2−ΔΔCt) (13). For
PDCD4 mRNA detection, qRT-PCR was performed as described previously
(14). Each sample was run in
triplicate.
Cell transfection
The cells were seeded in six-well plates to ensure
that they would reach 30% confluence the following day. The
transfection of the miR-106a mimic, miR-106a antisense
oligonucleotide (ASO) or negative control (NC) oligonucleotide was
performed using the Lipofectamine 2000 reagent (Invitrogen,
Carlsbad, CA, USA) in antibiotic-free Opti-MEM (Invitrogen)
according to the manufacturer’s instructions. Following 48 h of
transfection, the cells were harvested and processed for further
analysis.
Luciferase reporter assays
The full-length 3′ UTR of PDCD4 was amplified and
cloned into the Xba1-site of pGL3 (Promega, Madison, WI,
USA), checked for orientation, sequenced and named Luc-PDCD4Wt. The
site-directed mutagenesis of the miR-21 target site in the
PDCD4-3′-UTR was performed using the QuikChange Mutagenesis kit
(Stratagene, Heidelberg, Germany), with Luc-PDCD4Wt as a template.
For the reporter assays, the OVCAR3 cells were transfected with
wild-type (WT) or mutant reporter plasmids and with miR-106a mimics
using lipofectamine 2000 (Invitrogen). The reporter assays were
performed at 48 h post-transfection using the Dual-luciferase
assay-system (Promega) and normalized for transfection efficiency
by cotransfected Renilla-luciferase.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cells were seeded into 96-well plates at
5×103 cells/well, allowed to grow overnight and then
treated with various concentrations of CDDP (QiLu Pharmaceutical,
Jinan, China). Following 24 h of treatment, 20 μl 5 mg/ml MTT
reagent (Sigma-Aldrich, St. Louis, MO, USA) was added and incubated
in the dark for 4 h. The viability of the treated cells was
calculated from the average OD 490 values compared with that of the
untreated cells. Each treatment was carried out in triplicate.
Western blot assay
The extraction and detection of the proteins was
performed as described previously (15). The protein samples were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The membranes were then blocked with 1% BSA in
TBST containing 0.1% Tween-20 for 1 h. The filters were then
incubated overnight at 4°C with rabbit mAb against PDCD4, cleaved
caspase-3, -8 and -9 (1:1,000; Cell Signaling Technology, Beverly,
MA, USA) and mouse mAb GAPDH (1:2,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Immunoblotting was performed by
incubating the membranes at 4°C overnight followed by the goat
anti-mouse secondary antibodies (Zhongshan Biotechnology Ltd., Co.,
Beijing, China) conjugated for 1 h at room temperature. Subsequent
to washing the membranes, antibody binding was detected using an
enhanced chemoluminescence kit (Pierce, Rockford, IL, USA). All
western blot experiments were repeated at least three times.
Knockdown of PDCD4 expression mediated by
siRNA
The siRNAs targeting PDCD4 and non-specific NC were
purchased from Ambion (Austin, TX, USA). The siRNAs were
transfected into the OVCAR3 cells using Lipofectamine 2000 reagent
(Invitrogen) according to the manufacturer’s instructions.
Following the treatment, the cells were harvested for the
subsequent experiments. The experiment was repeated three
times.
Apoptosis assay
To quantify CDDP-induced apoptosis, annexin
V/propidium iodide (PI) staining was performed and apoptosis was
evaluated using flow cytometry (FCM) analysis. Briefly, following
the treatment with PDCD4 siRNA and CDDP, floating and attached
cells were collected and subjected to annexin V/PI staining using
an annexin V-FITC Apoptosis Detection kit (Keygene, Nanjing,
China), according to the manufacturer’s instructions. The resulting
fluorescence was measured by FCM using the Becton Dickinson
FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
All the quantitative data were analyzed using
Student’s t-tests. All the tests that were performed were
two-sided. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of PDCD4 correlates with the
cytotoxic activity of CDDP in ovarian cancer cell lines
To determine the effect of PDCD4 expression on the
sensitivity of ovarian cancer cells to CDDP, the expression of
PDCD4 was measured in the OVCAR3 and OVCAR3/CIS cells using qRT-PCR
and western blot analysis. As shown in Fig. 1A–C, PDCD4 expression was low in the
OVCAR3/CIS cells and high in the OVCAR3 cells. The results from the
MTT assays revealed that the OVCAR3 cells with relatively high
levels of PDCD4 expression were more sensitive to CDDP compared
with the OVCAR3/CIS cells (Fig.
1D). These results indicate that PDCD4 expression may be
associated with a high sensitivity to CDDP in ovarian cancer cell
lines. The expression levels of miR-106a in the OVCAR3 and
OVCAR3/CIS cells were detected using stem-loop qRT-PCR. It was
shown that miR-106a had an average 2.63-fold higher expression
level in the OVCAR3/CIS cells compared with the OVCAR3 cells
(P<0.05; Fig. 1E). These results
indicated that miR-106a may play a crucial role in the development
of CDDP resistance in epithelial ovarian cancer. TargetScan
(http://www.targetscan.org/) was used to
computationally predict the targets of miR-106a. The tumor
suppressor, PDCD4, was predicted to be one such potential target
(Fig. 1F).
Correlation between miR-106a and CDDP
resistance in ovarian cancer cells
To directly test the correlation between miR-106a
and CDDP resistance in the ovarian cancer cells, miR-106a
expression was functionally changed using mimics and inhibitors
in vitro, and subsequently, the resulting alterations of the
drug sensitivity were evaluated by the MTT assay. In response to
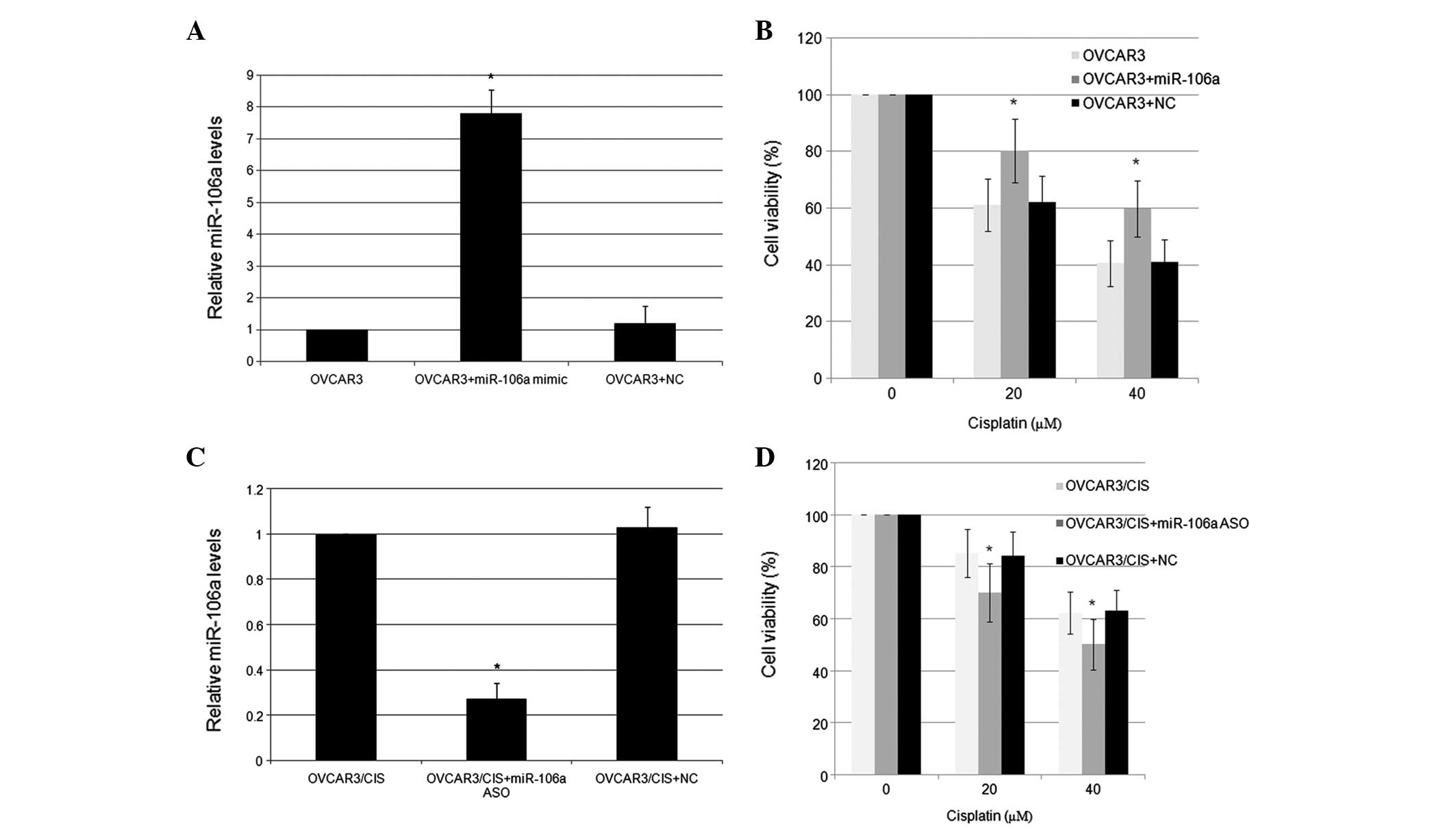
transfection with 100 pmol miR-106a mimics, the expression level of
miR-106a in the OVCAR3 cells was increased 7.8-fold compared with
the NC (Fig. 2A). The
overexpression of miR-106a was associated with the significantly
increased survival rate of the OVCAR3 cells (Fig. 2B). The OVCAR3/CIS cells were
transfected with either the miR-106a ASO or an NC, and were
subsequently incubated with various doses of CDDP. Conversely,
transfection with 100 pmol miR-106a inhibitors effectively reduced
miR-106a expression and resulted in a significantly lower survival
rate in the OVCAR3/CIS cell lines (Fig.
2C and D).
PDCD4 is a target of miR-106a
PDCD4 protein expression was significantly
downregulated in the OVCAR3/CIS cells compared with the parental
OVCAR3 cells. However, it remains unclear whether the
downregulation of PDCD4 induced by the overexpression of miR-106a
is involved in the resistance of the OVCAR3 cells to CDDP.
Computer-aided algorithms were obtained from TargetScan (http://www.targetscan.org/) and the potential binding
site of miR-106a (position 854–860) was predicted to be the PDCD4
3′-UTR. As shown in Fig. 3A,
transfection of the miR-106a mimics in the OVCAR3 cells with the WT
3′-UTR (pLuc-PDCD4 3′-UTR-wild) vector significantly decreased the
luciferase activity compared with the control inhibitor
(P<0.05). However, transfection of the miR-106a mimics in the
OVCAR3 cells with the mutant 3′-UTR (pLuc-PDCD4 3′-UTR-mut) vector
showed no effect on luciferase activity compared with the control
inhibitor (P>0.05). Furthermore, the expression levels of the
PDCD4 protein in the OVCAR3/CIS cells that were transfected with
the miR-106a inhibitor were significantly increased compared with
that of the OVCAR3/CIS cells that were transfected with the control
inhibitor (P<0.05; Fig. 3B and
C). Conversely, the expression levels of the PDCD4 protein in
the OVCAR3 cells that were transfected with the miR-106a mimic were
significantly increased compared with that of the OVCAR3 cells that
were transfected with the control inhibitor (P<0.05; Fig. 3D and E). All these data indicated
that PDCD4 was post-transcriptionally regulated by miR-106a in the
OVCAR3 cells.
PDCD4 is a key signaling molecule in
induced CDDP resistance in OVCAR3 cells
To further confirm the effect of PDCD4 on the
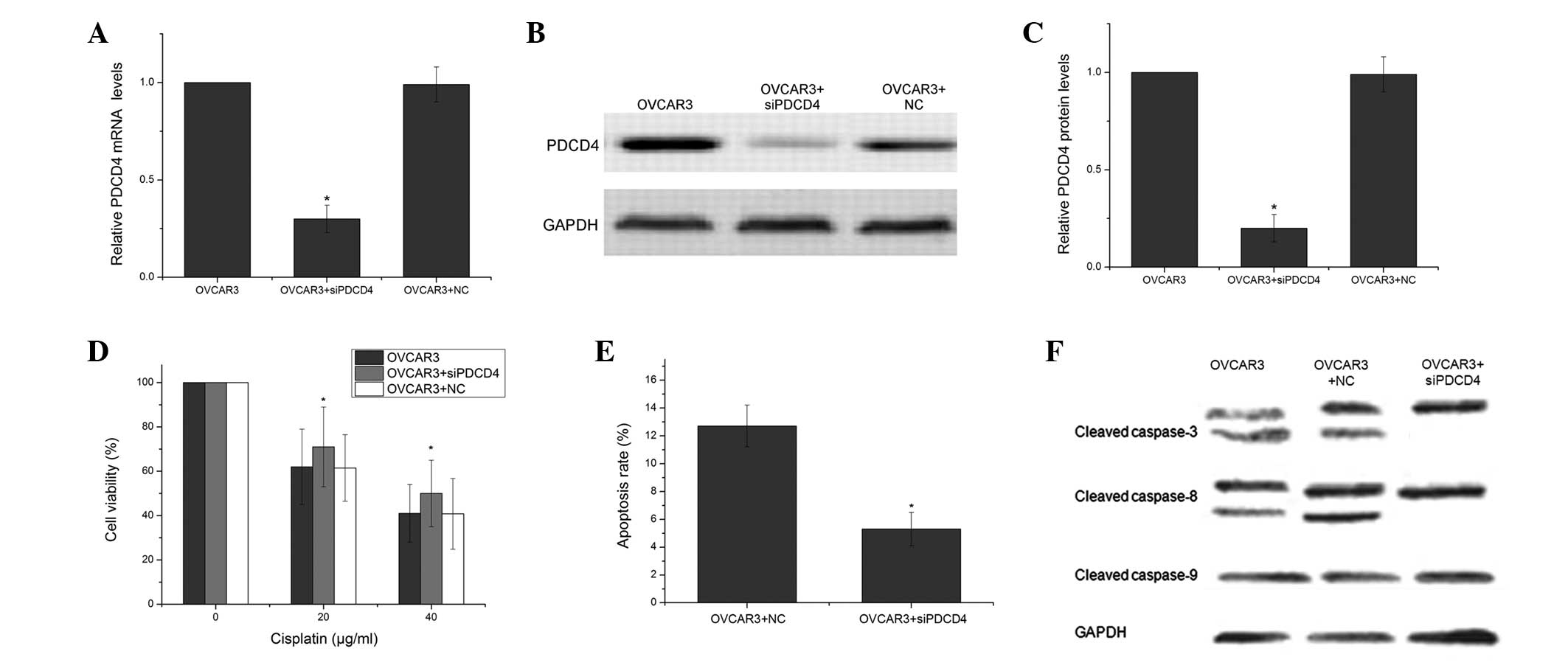
chemosensitivity of ovarian cancer cells, PDCD4 expression was
knocked down using PDCD4-specific siRNAs in the OVCAR3 cells. As
shown in Fig. 4A–C, the
PDCD4-specific siRNAs markedly inhibited the expression of the
PDCD4 mRNA by 70% and the PDCD4 protein by 80%, whereas the NC had
no significant effect on PDCD4 expression. The PDCD4 siRNAs
significantly increased cell viability compared with the cells that
were treated with the NC (P<0.05; Fig. 4D). This indicated that the
downregulation of PDCD4 enhanced the resistance to CDDP. The PDCD4
siRNAs and the scrambled siRNA-transfected OVCAR3 cells were
analyzed using FCM to determine cell apoptosis. The PDCD4 siRNAs
were identified to decrease the level of CDDP-induced apoptosis in
the OVCAR3 cells. (P<0.05; Fig.
4E) To further examine the particular apoptotic pathways by
which PDCD4 promotes CDDP-induced apoptosis, the expression of
several apoptosis-related proteins was measured. As shown in
Fig. 4F, treatment with the PDCD4
siRNAs decreased the expression of cleaved caspase-3 and caspase-8
in the OVCAR3 cells compared with the OVCAR3 cells that were
treated with the NC.
Discussion
Platinum-based combination chemotherapy is the most
widely used method in the treatment of ovarian cancer (16). However, due to resistance, the
method often fails to cure patients. Therefore, the reversal of
platinum resistance in ovarian cancer and increased sensitivity to
platinum-based chemotherapy drugs are crucial issues.
miRNAs are a growing class of small, non-coding RNAs
that regulate gene expression by targeting mRNAs to cause
translational repression and/or degradation. A large number of
miRNAs have been identified as deregulated in various types of
human malignancy. Increasing evidence indicates that the
deregulation of miRNAs has been frequently observed in cell
proliferation, differentiation, apoptosis, metastasis and drug
resistance (17–20). The mechanisms responsible for the
chemotherapy resistance by miRNAs have not been clearly identified.
To date, several miRNAs, including miR-451, -21, -214, -23a and
-141, have been reported to be involved in the process of CDDP
resistance in various tumors (21–23).
Based on these findings, Fu et al(11) performed global miRNA expression
profiling in human ovarian CDDP-resistance and parental cancer
cells, and identified that miR-15a, -19a, -21, -204, -93 and -96
were upregulated and that miR-22 and -489 were downregulated. The
present study identified that miR-106a was overexpressed 2.7-fold
in the CDDP-resistant OVCR3/CIS cells compared with the
corresponding CDDP-sensitive parental cell line, and the subsequent
qRT-PCR experiment confirmed this result. Knockdown of miR-106a
enhanced CDDP chemosensitivity in the CDDP-resistant OVCR3/CDDP
cells, while the ectopic expression of miR-106a caused the OVCR3
cells to be resistant to CDDP-induced apoptosis.
Numerous miR-106a targets have been predicted by
TargetScan, including PDCD4. The overexpression of PDCD4 has been
reported to increase the sensitivity to CDDP and paclitaxel, but
not to etoposide or 5-fluorouracil in human prostate cancer PC3
cells (24). Consistent with this
finding, the present study demonstrated that PDCD4 is a target of
miR-106a and that it plays a role in CDDP resistance in the OVCR3
cell line. Furthermore, knockdown of PDCD4 significantly increased
the cell survival rate and had an overall effect that was similar
to miR-106a overexpression. To the best of our knowledge, this is
the first study to describe an association between miR-106a, PDCD4
expression and drug resistance in CDDP-treated OVCR3 cells. The
loss or reduction of PDCD4 expression may be a reason for
chemoresistance in ovarian cancer, and the restoration of PDCD4
expression may reverse the resistance of ovarian cancer to
chemotherapy. To date, the mechanism by which PDCD4 enhances
chemosensitivity remains unclear. Apoptosis in hepatocellular
carcinoma cells was induced by PDCD4 through mitochondrial events
and the caspase cascade, including increases in cytosolic
cytochrome c and mitochondrial Bax and a reduction in
procaspase-3, -8, and -9. The present results revealed that the
combination of PDCD4 with CDDP markedly elevated the expression of
cleaved caspase-3 and -8. Taken together, the data indicated that
PDCD4 promoted CDDP-induced apoptosis mainly through the death
receptor-mediated pathway.
In summary, the present study demonstrated that the
enhancement of miR-106a expression contributes to the generation of
CDDP-resistant ovarian cancer cells, partly by targeting PDCD4.
PDCD4 promoted CDDP-induced apoptosis mainly through the death
receptor-mediated pathway. The results provide evidence that
miR-106a may potentially be used as a predictor of the chemotherapy
response in ovarian cancer and is a promising therapeutic target in
the treatment of this disease.
References
|
1
|
Ehlén A, Brennan DJ, Nodin B, et al:
Expression of the RNA-binding protein RBM3 is associated with a
favourable prognosis and cisplatin sensitivity in epithelial
ovarian cancer. J Transl Med. 8:782010.PubMed/NCBI
|
|
2
|
Weinberg LE, Rodriguez G and Hurteau JA:
The role of neoadjuvant chemotherapy in treating advanced
epithelial ovarian cancer. J Surg Oncol. 101:334–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrocca F and Lieberman J:
Micromanipulating cancer: microRNA-based therapeutics? RNA Biol.
6:335–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boren T, Xiong Y, Hakam A, et al:
MicroRNAs and their target messenger RNAs associated with ovarian
cancer response to chemotherapy. Gynecol Oncol. 113:249–255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hebert C, Norris K, Scheper MA, Nikitakis
N and Sauk JJ: High mobility group A2 is a target for miRNA-98 in
head and neck squamous cell carcinoma. Mol Cancer. 6:52007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moriyama T, Ohuchida K, Mizumoto K, et al:
MicroRNA-21 modulates biological functions of pancreatic cancer
cells including their proliferation, invasion, and chemoresistance.
Mol Cancer Ther. 8:1067–1074. 2009. View Article : Google Scholar
|
|
8
|
Zhou M, Liu Z, Zhao Y, et al:
MicroRNA-125b confers the resistance of breast cancer cells to
paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist
killer 1 (Bak1) expression. J Biol Chem. 285:21496–21507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Li N, Wang H, Jia X, Wang X and
Luo J: Altered microRNA expression in cisplatin-resistant ovarian
cancer cells and upregulation of miR-130a associated with
MDR1/P-glyco protein-mediated drug resistance. Oncol Rep.
28:592–600. 2012.
|
|
11
|
Fu X, Tian J, Zhang L, Chen Y and Hao Q:
Involvement of microRNA-93, a new regulator of PTEN/Akt signaling
pathway, in regulation of chemotherapeutic drug cisplatin
chemosensitivity in ovarian cancer cells. FEBS Lett. 586:1279–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acid Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
14
|
Li J, Fu H, Xu C, et al: miR-183 inhibits
TGF-beta1-induced apoptosis by downregulation of PDCD4 expression
in human hepatocellular carcinoma cells. BMC Cancer. 10:3542010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei ZT, Zhang X, Wang XY, et al: PDCD4
inhibits the malignant phenotype of ovarian cancer cells. Cancer
Sci. 100:1408–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams G, Zekri J, Wong H, Walking J and
Green JA: Platinum-based adjuvant chemotherapy for early-stage
epithelial ovarian cancer: single or combination chemotherapy?
BJOG. 117:1459–1467. 2010. View Article : Google Scholar
|
|
17
|
Amaral JD, Xavier JM, Steer CJ and
Rodrigues CM: Targeting the p53 pathway of apoptosis. Curr Pharm
Des. 16:2493–2503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dykxhoorn DM: MicroRNAs and metastasis:
little RNAs go a long way. Cancer Res. 70:6401–6406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garg M: MicroRNAs, stem cells and cancer
stem cells. World J Stem Cells. 4:62–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hummel R, Hussey DJ and Haier J:
MicroRNAs: predictors and modifiers of chemo- and radiotherapy in
different tumour types. Eur J Cancer. 46:298–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT
and Zhang CP: MicroRNAs contribute to the chemoresistance of
cisplatin in tongue squamous cell carcinoma lines. Oral Oncol.
46:317–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hummel R, Watson DI, Smith C, et al:
Mir-148a improves response to chemotherapy in sensitive and
resistant oesophageal adenocarcinoma and squamous cell carcinoma
cells. J Gastrointest Surg. 15:429–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamano R, Miyata H, Yamasaki M, et al:
Overexpression of miR-200c induces chemoresistance in esophageal
cancers mediated through activation of the Akt signaling pathway.
Clin Cancer Res. 17:3029–3038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jansen AP, Camalier CE, Stark C and
Colburn NH: Characterization of programmed cell death 4 in multiple
human cancers reveals a novel enhancer of drug sensitivity. Mol
Cancer Ther. 3:103–110. 2004.PubMed/NCBI
|