Introduction
Juemingzi is the seed of the Cassia tora L.
(Leguminosae) plant and has been used as a laxative and a tonic, as
well as being a popular health drink (1). Pharmaceutical research has
concentrated on the beneficial activities of Juemingzi, including
its anti-aging, anticancer and antioxidant effects (2–5).
Juemingzi contains anthraquinones, naphtho-pyrones, fatty acids,
amino acids and inorganic elements (6). Types of Juemingzi with a high
anthraquinone content, including chrysophanol, physcion and
obtusin, may aid in cancer prevention (7).
Metastasis is a multistep process that begins when a
primary tumor acquires mutations and becomes invasive. The tumor
cells eventually enter into the blood or lymph (8). Metastases arise most commonly in the
lung, liver, brain and bone. Notably, the lung is the most common
site for systemic sarcoma metastases due to the substantial
vasculature that feeds into this organ, in addition to particular
trophic factors (9).
The sarcoma 180 mouse cell line is derived from a
sarcoma that was carried in Swiss Webster mice and has been
described to grow in multiple inbred mouse strains due to
β2-microglobulin deficiency, major histocompatibility complex (MHC)
class I destabilization and a lack of recognition by host cytotoxic
T lymphocytes. An injection of these cells into mice results in
mortality due to the accumulation of ascites fluid (10). BALB/c mice are distributed globally
and are among the most widely used inbred strains that are used for
animal experimentation. Balbc/c mice are often used for in
vivo cancer research. The sarcoma 180 tumor-bearing mouse model
was a staple research animal model that was used for the tumor and
metastasis study (11).
The present study investigated the antitumor effect
of Juemingzi in sarcoma 180-transplanted mice using a mouse model.
The effects of Juemingzi at different concentrations were
determined. Additionally, the serum levels and splenocyte cell
proliferation were assessed.
Materials and methods
Preparations of Juemingzi (Cassia tora
L)
Juemingzi was purchased from Yunnan Baiyao Group Co.
Ltd. (Kunming, China), stored at −80°C and freeze-dried to produce
a powder. A 20-fold volume of methanol was added to the powdered
sample and extracted twice by stirring overnight. The methanol
extract was evaporated using a rotary evaporator (N-1100; Eywla,
Tokyo, Japan), concentrated and dissolved in dimethylsulfoxide
(Amresco, Solon, OH, USA) to adjust to the stock concentration
(20%, w/v).
Animals
Female six-week-old Balb/c mice (n=50) were
purchased from Chongqing Medical University (Chongqing, China). The
mice were maintained in a temperature controlled (25±2°C; relative
humidity, 50±5%) facility with a 12-h light/dark cycle and free
access to a standard mouse diet and water. This study followed a
protocol approved by the Animal Ethics Committee of Chongqing
Medical University (Chongqing, China).
Cell preparation
Mouse sarcoma 180 cells were purchased from the
Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). The sarcoma 180 cell line was cultured for 7–10 days in the
abdominal cavity of a Balbc/c mouse and the cultured cells were
harvested with the peritoneal fluid and centrifuged at 300 × g for
10 min in phosphate-buffered saline (PBS). The separated sarcoma
cells were suspended in PBS, centrifuged at 1,800 × g for 5 min and
their concentration was adjusted to 1.0×106 cells/ml by
diluting in Dulbecco’s modified Eagle’s medium.
In vivo antitumor activity assay
The sarcoma 180 cells (0.2 ml; concentration,
1.0×106 cells/ml) were implanted subcutaneously in the
left groin of the mice in the control and sample groups (11). The mice from the normal and control
groups were fed with a normal diet and water. The sample group mice
were administered 50, 100 or 200 mg/kg b.w. intragastric Juemingzi
for 28 days. The mice were sacrificed using CO2. The
tumors were then removed and weighed. The tumor growth inhibition
ratio (I.R.) was calculated using the following formula: I.R. (%) =
(Cw−Tw)/Cw × 100; where Cw and Tw represent the average tumor
weight of the control and experimental groups, respectively.
Serum aspartate aminotransferase (AST),
alanine transaminase (ALT) and blood urea nitrogen (BUN)
levels
The AST, ALT and BUN levels in the serum were
determined using enzyme-linked immunosorbent assay (ELISA) kits
(Shanghai Institute of Biological Products Co., Ltd., Shanghai,
China).
Splenocyte proliferation assay
Splenocytes were obtained by gentle disruption of
the spleen of the Balb/c female mice and filtration via a 40-μm
Nylon cell strainer (Falcon, NJ, USA). The erythrocytes were lysed
with 0.38% NH4Cl-Tris buffer (pH 7.4), while the
remaining cells were resuspended in RPMI-1640 with 10 mM Hepes, 10%
fetal bovine serum, 100 mg/l streptomycin and 100 IU/ml
penicillin.
The splenocytes (1×106 cells/ml) were
treated with mitogens, including lipopolysaccharide (LPS) and
concanavalin A (Con A) (Grand Island Biological Co., Grand Island,
NY, USA), at 10 μg/ml, and co-cultured with the test samples in
24-well plates for 24, 48 and 72 h at 37°C in a humidified
atmosphere of 5% CO2. The proliferation of the
splenocytes was measured using a
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide assay
(12).
ELISA analysis of serum
inflammation-related cytokines
For the serum cytokine assay, blood from the
inferior vena cava was collected into a tube and centrifuged at
1,800 × g for 10 min at 4°C. The serum was aspirated and assayed as
follows. The serum concentrations of the inflammatory-related
cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-1β
(Biolegend, San Diego, CA, USA), were measured by ELISA, according
to the manufacturer’s instructions (Biolegend, CA, USA). Briefly,
subsequent to adding the biotinylated antibody reagent to the
96-well plates, the supernatants of the homogenized serum were
incubated at 37°C in CO2 for 2 h. Subsequent to being
washed with PBS, streptavidin-horseradish peroxidase solution was
added and the plate was incubated for 30 min at room temperature.
The absorbance was measured at 450 nm using a microplate reader
(model 680, Bio-Rad, Hercules, CA, USA) (13).
Statistical analysis
Analysis of variance (ANOVA) was performed and the
results are presented as the mean ± standard deviation. The
differences between the mean values of the individual groups were
assessed using one-way ANOVA and Duncan’s multiple range tests.
P<0.05 was considered to indicate a statistically significant
difference. The SAS v9.1 statistical software package (SAS
Institute Inc., Cary, NC, USA) was used to perform all the
statistical analyses.
Results
Tumor growth inhibitory effects of
Juemingzi
The antitumor activity of Juemingzi was tested
against the sarcoma 180 tumor-bearing mice. The average tumor
weight of the sarcoma 180 cell-bearing mice receiving a normal diet
(control) was 4.8±0.4 g, whereas that of the mice that were treated
with 50 and 100 mg/kg b.w. Juemingzi was 4.1±0.5 and 3.3±0.5 g,
respectively and that of the mice that were treated with 200 mg/kg
b.w. Juemingzi was 2.9±0.4 g. Juemingzi extract (50, 100 and 200
mg/kg b.w.)-treated mice demonstrated tumor growth inhibitory rates
of 14.6, 31.3 and 39.6%, respectively. According to the results, a
good antitumor effect was observed in the tumors that were treated
with high concentrations of Juemingzi (Table I).
 | Table IAnti-tumor activities of 50, 100 and
200 mg/kg b.w. Juemingzi-treated sarcoma 180 tumor-injected
mice. |
Table I
Anti-tumor activities of 50, 100 and
200 mg/kg b.w. Juemingzi-treated sarcoma 180 tumor-injected
mice.
| Group | Tumor weight (g) | Inhibitory rate
(%) |
|---|
| Control | 4.8±0.4a | |
| Juemingzi |
| 50 mg/kg b.w. | 4.1±0.5b | 14.6 |
| 100 mg/kg b.w. | 3.3±0.5c | 31.3 |
| 200 mg/kg b.w. | 2.9±0.4d | 39.6 |
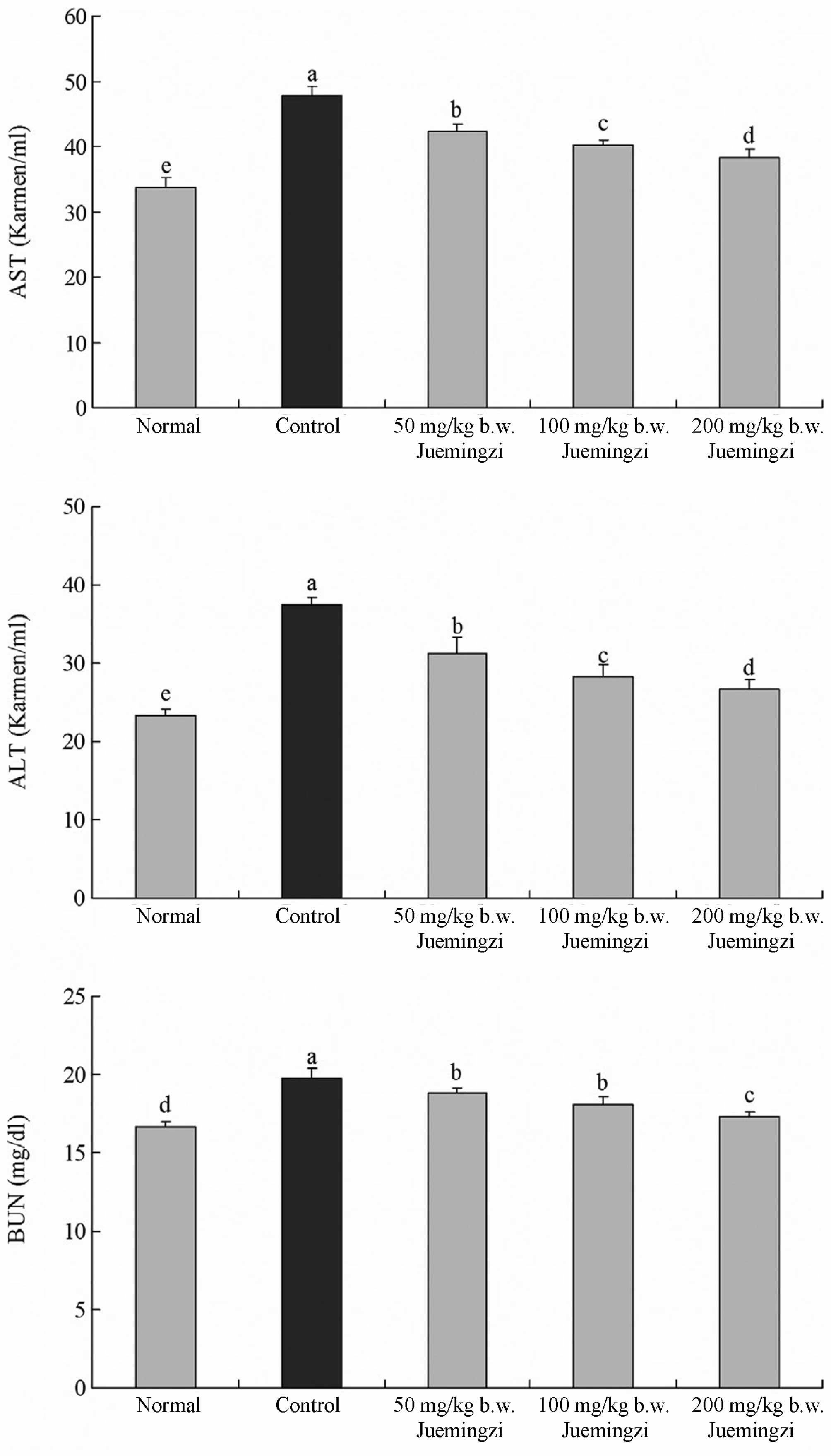
Effect of Juemingzi on serum AST, ALT and
BUN levels
The AST levels in the normal mice were 33.8±1.4
Karmen U/ml. However, that of the sarcoma 180 control mice was
significantly increased to 47.9±1.2 Karmen U/ml. The levels of AST
in the mice that were treated with 50, 100 and 200 mg/kg b.w.
Juemingzi were 42.2±1.2, 40.1±0.8 and 38.3±1.4 Karmen U/ml,
respectively (Fig. 1).
The ALT levels in the normal group were 23.3±0.8
Karmen U/ml, whereas those in the control group were 37.4±1.0
Karmen U/ml, reflecting a marked increase. The ALT levels in the
50-, 100- and 200-mg/kg b.w. Juemingzi groups decreased to
31.2±2.2, 28.3±1.6 and 26.6±1.4 Karmen U/ml, respectively (Fig. 1).
The levels of BUN in the 50-, 100- and 200-mg/kg
b.w. Juemingzi groups were 18.8±0.3, 18.1±0.5 and 17.3±0.3 mg/dl,
respectively, which were marginally lower than those of the control
group (19.8±0.6 mg/dl). However, the BUN levels in the 200 mg/kg
b.w. normal group were 16.7±0.3 mg/dl (Fig. 1). The AST, ALT and BUN levels in the
Juemingzi groups were lower than those in the control group and the
mice from the 200-mg/kg b.w. Juemingzi group demonstrated levels
that were similar to those of the normal group.
Effects of Juemingzi on lymphocyte
proliferation
The lymphocytes of the sarcoma 180 tumor-bearing
mice in each group were irritated by LPS or Con A. The irritated
lymphocytes were compared with the non-irritated PBS lymphocytes.
As shown in Fig. 2, after 24 h, the
proliferation rate of the Con A-treated lymphocytes was greater
than that of the LPS-treated lymphocytes. The Con A-treated
lymphocytes in the 200-mg/kg b.w. Juemingzi group (50.1%)
demonstrated a marginally lower lymphocyte proliferation rate than
that of the normal group (53.2%). The lymphocyte proliferation rate
in the 50- and 100-mg/kg b.w. Juemingzi groups was 42.7 and 43.4%,
respectively. By these results, the Con A-treated lymphocyte
proliferation rate was higher in the group that was treated with a
high concentration of Juemingzi compared with those that were
treated with a lower concentration.
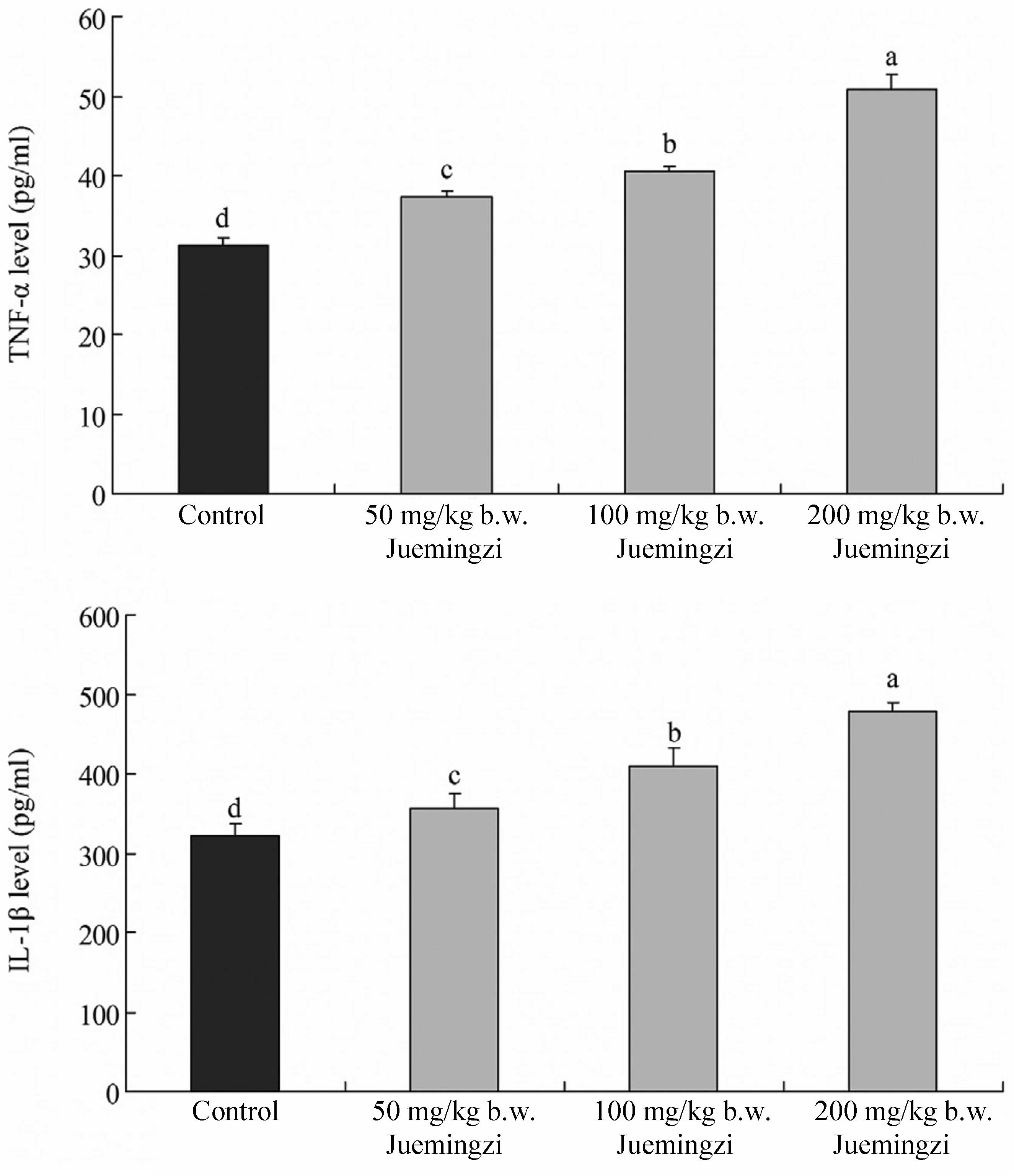
Effect of Juemingzi on serum TNF-α and
IL-1β levels
The serum TNF-α and IL-1β levels of the mice in the
Juemingzi-treated groups were significantly higher than those in
the control group (Fig. 3). The
increases in the TNF-α and IL-1β levels in the 200 mg/kg b.w.
Juemingzi-treated mice were 50.8±2.0 and 478.3±10.5 pg/ml,
respectively, compared with those of the control group (31.2±1.0
and 322.7±14.3 pg/ml). The TNF-α levels in the mice that were
treated with 50 and 100 mg/kg b.w. Juemingzi were 37.3±0.8 and
40.5±0.7, respectively and the IL-1β levels were 355.9±19.2 and
408.6±24.1, respectively.
Discussion
Although Juemingzi has been used in medicine, the
scientific data concerning its effects are limited. Juemingzi has
previously been demonstrated to have various therapeutic effects on
numerous pathological conditions, including inflammation, aging and
cancer (14).
Cell injury can release endogenous damage-associated
molecular patterns that activate innate immunity. Cell injury can
also increase the risk of cancer (15). AST and ALT are enzymes that are
located in cells that leak out into the general circulation when
cells are injured. AST is located in a number of body tissues,
including the heart, muscle, kidney, brain and lung tissues
(16). A decreased BUN:creatinine
ratio indicates malnutrition and syndrome of inappropriate
antidiuretic hormone secretion, which is occasionally observed with
lung diseases, cancer and diseases of the central nervous system
(17).
Lymphocytes are a type of white blood cell in the
vertebrate immune system. The three major types of lymphocyte are T
cells, B cells and natural killer (NK) cells (18). NK cells are a part of the innate
immune system and play a major role in defending the host from
tumors and virally infected cells. NK cells distinguish infected
cells and tumors from normal and uninfected cells by recognizing
changes in the surface MHC class I molecule. NK cells are activated
in response to a family of cytokines called interferons. Activated
NK cells release cytotoxic (cell-killing) granules, which then
destroy the altered cells (19).
TNF-α and Il-1β are significant regulators of host
defense against tumor cells (20).
The observed increase in the production of these cytokines may
suggest an enhanced ability of the host to combat the growth of
tumors. Macrophages are activated by β-glucans and other cell
mediators to kill tumor cells by the production of TNF-α and ILs.
The bioactivities of polysaccharides and polysaccharide-protein
complexes are dependent on the binding of the surface receptor of
immune cells. These receptors, known as pattern recognition
molecules, are able to recognize foreign ligands during the initial
phases of the immune response (21). At high levels of IL-1β, cancer cells
that receive genotoxic insults engage the apoptotic pathways.
Cotreatment with an inhibitor of IL-1β and TNF-α synthesis has been
demonstrated to prevent carcinogen-induced lesions (22). Oxidation is highly reactive and has
the potential to cause damage to cells, including damage that may
lead to cancer. Juemingzi demonstrated a preventive effect against
atherosclerosis by inhibiting LDL oxidation (23). Additionally, Juemingzi was found
have in vitro anticancer effects in cancer cells (24). The present study provided further
data indicating the in vivo anticancer effects of
Juemingzi.
The results from the present study have demonstrated
that Juemingzi is able to decrease sarcoma 180 tumors. Juemingzi
exhibited strong activity, as observed by the tumor weight count,
serum levels assay and lymphocyte proliferation rate. An increased
concentration of Juemingzi is significant for augmenting these
antitumor effects on sarcoma 180-treated tumors. The active
compounds obtained from Juemingzi (Cassia tora L.) need to
be identified and evaluated in future studies.
Acknowledgements
This study was supported by Chongqing Programs for
Science and Technology Development (CSTC2011AC5173), Chongqing
Scientific Programs of Higher Education (KJ121508) and Program for
Chongqing Innovative Research Team in University (KJTD201325).
References
|
1
|
Yen GC and Chung DY: Antioxidant effects
of extracts from Cassia tora L. prepared under different
degrees of roasting on the oxidative damage to biomolecules. J
Agric Food Chem. 47:1326–1332. 1999.PubMed/NCBI
|
|
2
|
Liu JZ, Lin X, Li XE, et al: Effect of
protein and anthraquinone glucosides from Semen Cassia on learning
and memory capacity and related substances of senile mice induced
by D-galactose. Zhongguo Zhong Yao Za Zhi. 32:516–519. 2007.(In
Chinese).
|
|
3
|
Lin DJ and Jin Z: Experimental study on
protective effect of Semen Cassiae extract against acute liver
injury. LiShiZhen Med Mater Med Res. 17:214–215. 2006.
|
|
4
|
Yen GC, Chen HW and Duh PD: Extraction and
identification of an antioxidative component from Jue Ming Zi
(Cassia tora L.). J Agric Food Chem. 46:820–824. 1998.
View Article : Google Scholar
|
|
5
|
Kim SY, Kim JH, Kim SK, et al: Antioxidant
activities of selected oriental herb extracts. J Am Oil Chem Soc.
71:633–640. 1994. View Article : Google Scholar
|
|
6
|
Hao YJ, Sang YL and Zhao YQ: Research
progress of Jue-ming-zi. Chinese Tradit Herbal Drugs. 32:858–859.
2001.
|
|
7
|
Qi GF: Cassia analysis of lipid-lowering
active ingredients. Guang Ming Zhong Yi. 26:1569–1570. 2011.(In
Chinese).
|
|
8
|
Wittekind C and Neid M: Cancer invasion
and metastasis. Oncology. 69:14–16. 2005. View Article : Google Scholar
|
|
9
|
Vikis HG, Jackson EN, Krupnick AS, et al:
Strain-specific susceptibility for pulmonary metastasis of sarcoma
180 cells in inbred mice. Cancer Res. 70:4859–4867. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tarnowski GS, Mountain IM and Stock CC:
Influence of genotype of host on regression of solid and ascitic
forms of sarcoma 180 and effect of chemotherapy on the solid form.
Cancer Res. 33:1885–1888. 1973.PubMed/NCBI
|
|
11
|
Jung KO, Lee KY, Rhee SK, et al: Effects
of various kinds of salt on the tumor formation, NK cell activicty
and lipid peroxidation in Sarcoma-180 cell transplanted mice. J
Korean Assoc Cancer Prev. 7:134–142. 2002.
|
|
12
|
Huang GC, Wu LS, Chen LG, et al:
Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine
model of cyclophosphamide-induced leucopenia. J Ethnopharmacol.
109:229–235. 2007. View Article : Google Scholar
|
|
13
|
Park HS, Park JY and Yu RN: Relationship
of obesity and visceral adiposity with serum concentrations of CRP,
TNF-alpha and IL-6. Diabetes Res Clin Pract. 69:29–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X, Wang Q, Qian Y and Pang L:
Cassia tora L. (Jue-ming-zi) has anticancer activity in
TCA8113 cells in vitro and exerts anti-metastatic effects in vivo.
Oncol Lett. 5:1036–1042. 2013.
|
|
15
|
Seong SY and Matzinger P: Hydrophobicity:
an ancient damage-associated molecular pattern that initiates
innate immune responses. Nat Rev Immunol. 4:469–478. 2004.
View Article : Google Scholar
|
|
16
|
Delić R and Stefanović M: Optimal
laboratory panel for predicting preeclampsia. J Matern Fetal
Neonatal Med. 23:96–102. 2010.PubMed/NCBI
|
|
17
|
Verbalis JG, Goldsmith SR, Greenberg A, et
al: Hyponatremia treatment guidelines 2007: expert panel
recommendations. Am J Med. 120(11 Suppl 1): S1–S21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caligiuri MA: Human natural killer cells.
Blood. 112:461–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jangam SR, Yamada DH, McFall SM and Kelso
DM: Rapid, point-of-care extraction of human immunodeficiency virus
type 1 proviral DNA from whole blood for detection by real-time
PCR. J Clin Microbiol. 47:2363–2368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wells SM, Kew S, Yaqoob P, et al: Dietary
glutamine enhances cytokine production by murine macrophages.
Nutrition. 15:881–884. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gordon S: Pattern recognition receptors:
doubling up for the innate immune response. Cell. 111:927–930.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia ZB, Tao F, Guo L, Tao GJ and Ding XL:
Antioxidant properties of extracts from juemingzi (Cassia
tora L.) evaluated in vitro. LWT - Food Sci Technol.
40:1072–1077. 2007. View Article : Google Scholar
|
|
24
|
Zhao X, Wang Q, Qian Y and Pang L:
Cassia tora L. (Jue-ming-zi) has anticancer activity in
TCA8113 cells in vitro and exerts anti-metastatic effects in vivo.
Oncol Lett. 5:1036–1042. 2013.
|