Introduction
Osteosarcoma (OS) is a highly malignant bone tumor
that affects children and adolescents. The availability of
neo-adjuvant chemotherapy and surgery have significantly increased
the five-year survival rate of patients. However, patients with
metastasis, particularly in the lung, show poor survival rates
(1). Therefore, elucidation of the
molecular events underlying the invasiveness of OS may aid in
identifying the new targets for an improved diagnosis and treatment
of patients with metastatic OS.
Metastasis of a tumor involves several processes,
including increased proliferation of cells, remodeling of tissues
and invasion (2). Consistently,
cell invasion and migration are carried out by matrix
metalloproteinases (MMPs) (3). Most
significantly, MMP2 and MMP9 have been reported to cause invasion
and metastasis in various cancers (4,5).
MMPs are zinc-dependent endopeptidases whose
expression is regulated by proteolytic activation and by selective
inhibitory proteins. The majority of the extracellular matrix (ECM)
components are the substrates of MMPs (1). Furthermore, MMPs have been reported to
process several bioactive factors, apoptotic chemokines and cell
signaling factors, which affect immune responses (8). Collagen I is the major ECM component
that contributes to the structural and mechanical function of bone
(6). MMPs have the capacity to
degrade collagen and enhance metastasis and invasion (7). A higher expression of MMPs in
malignant tissues compared with normal tissues has been implicated
in malignant tumors of the prostate, lung, colon and pancreas, and
has been correlated with poor survival rates in patients with such
diseases (7).
Extracellular signal-regulated kinase (ERK)-5
belongs to the effector kinase of a mitogen-activated protein
kinase (MAPK) signaling pathway. ERK5 has been known to regulate
the expression of MMP2 and MMP9 (9,10) and
the degradation of the ECM (10).
Furthermore, ERK knockdown has been reported to reduce cellular
migration and invasion in PC3 cells (10). These studies indicate that ERK may
have a major role in cancer cell migration and invasion.
The present study aimed to elucidate the role of
collagen in OS by examining morphological features, cellular
attachment, proliferation status, expression of MMP2 and MMP9 and
ERK-mediated function in migration and invasiveness in an OS cell
line.
Materials and methods
Cell culture
The human OS U2OS cell line was obtained from the
American Type Culture Collection (ATCC, Manassas, VA, USA) and
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Cambrex Bio
Science Walkersville, Inc., Walkersville, MD, USA) containing 10%
fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 1X
penicillin-streptomycin in a humidified incubator at 37°C and 5%
CO2. When confluent, the detachment of cells was
performed using 0.25% trypsin and 0.05% EDTA (trypsin-EDTA) for
5–10 min and subcultured at the ratio of 1:5 every three days.
Morphology
The cells were cultured (2×105 cells/ml)
on non-coated or collagen-coated dishes. Following 48 h, the cells
were analyzed on a light microscope. Subsequently, the cells that
were treated with PD98059 (Bionol, Plymouth Meeting, PA, USA) were
also visualized. The comparisons of collagen and/or PD98059-treated
cells were performed along with the collagen and/or PD98059-treated
or untreated cells.
Cell attachment assay
The U2OS cells (6×104) were cultured on
non-coated or collagen-coated 6-well plates with or without PD98059
for the indicated time-points. Following the adhesion time that was
specified for the experiment, the supernatant media and the cells
were removed. The adherent layers were then washed with
phosphate-buffered saline (PBS) three times, and the adherent cells
were harvested using trypsin-EDTA, centrifuged at 400 × g for 5
min, resuspended in a complete medium and their cell counts
recorded on a Neubauer hemacytometer (Erma Inc., Tokyo, Japan).
Cell proliferation assay
The cell proliferation assay was performed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit (Amresco, Solon, OH, USA). The cells were plated
(5×104/well) on the collagen-coated or non-coated
96-well plates. Subsequently, for the ERK inhibition assay, the
cells were either left untreated or were treated with PD98059 (20
μM). The cells were incubated for the indicated time-points and 10
μl MTT solution was added to each well. Further incubation was
carried out for 4 h. The plates were read on an ELISA reader at 450
nm.
Reverse-transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. cDNA synthesis was performed using a
cDNA synthesis kit (Invitrogen). The primers and cycling conditions
have been previously described elsewhere (11). The PCR products were run on a 1.2%
agarose gel and their images were captured.
Western blotting
To detect the protein expression of phosphorylated
or total ERK, MMP2 and MMP9, the cells were detached using 0.25%
trypsin-EDTA and washed with PBS. Lysis buffer (Intron, Sungnam,
Korea) was used to prepare the total cell lysates. The lysates were
electrophoresed using 8% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (Amersham Pharmacia Biotech, Inc., Piscataway,
NJ, USA). The membranes were incubated with blocking solution that
contained rabbit polyclonal phospho-ERK, rabbit polyclonal ERK
(Cell Signaling Technology, Beverly, MA, USA), goat polyclonal MMP2
or goat polyclonal MMP9 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) antibodies. The membranes were then incubated with
horseradish-conjugated secondary antibodies (Amersham Pharmacia
Biotech, Inc.). An enhanced chemiluminescence detection kit
(Amersham Pharmacia Biotech, Inc.) was used to detect the epitope
on the proteins that were recognized by the specific
antibodies.
Cell migration assay
The U2OS cells were plated in collagen-coated or
non-coated 12-well plates until confluence in order to study the
effect of collagen and ERK on the OS cell migration assay. A linear
wound was gently created in the monolayer using a sterile yellow
pipette tip, followed by washing with the complete medium to remove
the cellular debris in order to yield an acellular line per well.
The cells were incubated with ERK inhibitor for 48 h. Images of the
wounded areas were captured and the location on the dish was noted
in terms of the distance between the cells.
Results
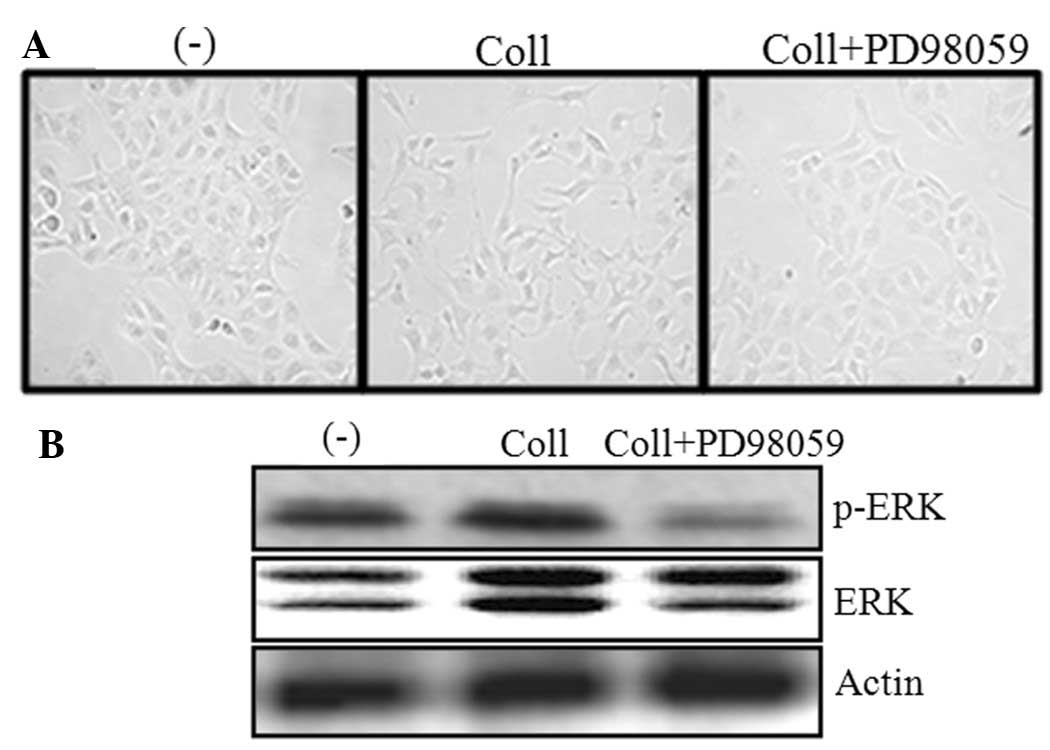
Collagen I induces changes in U2OS
cells
The U2OS cells were cultured on collagen-coated or
non-coated plates. Images were captured following 48 h using a 40X
objective lens. Fig. 1A shows that
the cells underwent morphological changes that were similar to the
epithelial to mesenchymal transition (EMT) when they were cultured
on the collagen-coated plate. The prominent feature was the
scattering of the cells that were plated on the collagen-coated
dishes. However, there was no significant difference in the
cellular morphology between the PD98059-treated or untreated cells
in the collagen-coated dishes. Furthermore, the effect of PD98059
on the phosphorylation of ERK was investigated. The data revealed
that PD98059 significantly inhibited the phosphorylation of ERK
(Fig. 1B).
Proliferation of U2OS is enhanced by
collagen I
An evaluation of the effect of collagen I on the
proliferation of the U2OS cells in the collagen-coated plates was
performed. Fig. 2A shows that the
U2OS cells proliferated significantly in the collagen-coated dishes
compared with the negative control. In order to elucidate the
effect of ERK inhibition on cellular proliferation, ERK expression
was inhibited in the cells using PD98059. However, there was no
significant difference in the proliferation of the U2OS cells
between the untreated or treated cells (Fig. 2B).
Adhesion of U2OS cells
The cell numbers that adhered to the collagen-coated
plates compared with the non-coated plates were determined within
the indicated time-points. Fig. 3A
shows that compared with the negative control, the U20S cell
numbers that adhered to the collagen I-coated plates was higher.
When the cells were treated with PD98059 and cultured on
collagen-coated dishes for up to 4 h, there was no significant
effect on the adhesion of the U2OS cells (Fig. 3B).
ERK inhibition suppresses the migratory
phenotype of U2OS cells
A wound healing assay was performed in order to
examine whether ERK inhibition played a role in the invasive
characteristics of the U2OS cells. The U2OS cells were treated with
or without ERK inhibitor on the collagen-coated dishes. Following
48 h, the migration of the cells along the scratched section was
measured. The number of cells that migrated along the wound area
was significantly higher in the collagen-coated dishes than in the
non-coated dishes (Fig. 4). When
compared with the PD98059-treated or untreated cells on the
collagen-coated dishes, it was observed that ERK inhibition
significantly suppressed the invasion of the U2OS cells in the
scratch area. These results illustrate that ERK pathway inhibition
suppresses the migratory capacities of U2OS cells.
ERK inhibition suppresses MMP9
expression
MMP2 and MMP9 expression was investigated in U2OS
cells in the presence or absence of collagen and/or ERK inhibitor.
MMP2 and MMP9 were selected as they have previously been implicated
in the invasive ability of cancer cells (11). Upon ERK inhibition, the
transcriptional activities of collagen-induced MMP9 was markedly
reduced. However, there was no reduction in MMP2 expression between
the PD98059-treated or untreated cells in the collagen-coated
dishes (Fig. 5A). Consistent with
these results, the protein expression of MMP9 was suppressed in the
ERK inhibitor-treated cells in the collagen-coated dishes. In
addition, no significant difference in MMP2 protein expression was
observed between the ERK inhibitor-treated and untreated cells on
the collagen-coated dishes (Fig.
5B). These results indicate that MMP9 is the target of ERK
signaling in U2OS cells. Further studies are required to elucidate
the role of MMP9 in the ERK-related invasive characteristics of
U2OS cells.
Discussion
The present study investigated the role of collagen
I in the morphology, adhesion, proliferation and invasive ability
of U2OS cells and the response of the ERK pathway in
collagen-treated cells. Collagen I was observed to cause the
EMT-like phenotype in the U2OS cells. In comparison with the cells
in the non-coated dishes, the cells in the collagen-coated dishes
demonstrated a scattering behavior, which is consistent with the
EMT-like phenotype (12).
Consistent with previously reported data (13), the present study observed that
collagen I upregulated the proliferation of the U2OS cells.
Treatment with PD98059 downregulated collagen-induced MMP9
expression and subsequently, the invasive phenotype of the U2OS
cells on the collagen-coated plates was diminished. However, no
significant differences in the adherence and proliferation of the
U2OS cells were observed between the collagen I-treated and
PD98059-treated cells in the collagen-coated plates.
Enhanced production of MMP9 correlates to the
invasive phenotype of cancer cells (14,15).
One study has reported that MMP9 overexpression in prostate cancer
is associated with ERK overexpression (9). The present study aimed to examine the
effect of MMP9 and ERK signaling on the invasive ability of U2OS
cells. Consistent with a previous study (9), it was observed that ERK upregulated
the expression of MMP9 and caused the upregulation of the invasive
capacity of the U2OS cells. Similar to the present results, another
previous study demonstrated that ERK silencing using siRNA
significantly downregulated MMP9, but not MMP2, in OS cells
(16).
The present study revealed that collagen caused an
EMT phenotype on the U2OS cells. Furthermore, collagen enhanced the
adhesion and proliferation of the U2OS cells. However, ERK-5
inhibition using PD98059 had no significant effect on the adhesion
and proliferation of the U2OS cells in the collagen-coated dishes.
Furthermore, ERK inhibition downregulated the invasive phenotype of
the U2OS cells through a suppressive effect on MMP9 expression.
Taken together, the data reveal that ERK may be a potent
therapeutic target for OS with invasive characteristics.
Acknowledgements
This study was financially supported by the Ministry
of Trade, Industry and Energy (MOTIE), the Korea Institute for
Advancement of Technology (KIAT) and the Honam Institute for
Regional Program Evaluation through the Leading Industry
Development for Economic Region (R000153801_00197752).
References
|
1
|
Husmann K, Arlt MJ, Muff R, Langsam B,
Bertz J, Born W and Fuchs B: Matrix Metalloproteinase 1 promotes
tumor formation and lung metastasis in an intratibial injection
osteosarcoma mouse model. Biochim Biophys Acta. 1832:347–354. 2013.
View Article : Google Scholar
|
|
2
|
Khanna C and Hunter K: Modeling metastasis
in vivo. Carcinogenesis. 26:513–523. 2004. View Article : Google Scholar
|
|
3
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cowan RW, Mak IW, Colterjohn N, Singh G
and Ghert M: Collagenase expression and activity in the stromal
cells from giant cell tumour of bone. Bone. 44:865–871. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005.PubMed/NCBI
|
|
8
|
Korpi JT, Hagström J, Lehtonen N,
Parkkinen J, Sorsa T, Salo T and Laitinen M: Expression of matrix
metalloproteinases-2, -8, -13, -26, and tissue inhibitors of
metalloproteinase-1 in human osteosarcoma. Surg Oncol. 20:e18–e22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta PB, Jenkins BL, McCarthy L, Thilak
L, Robson CN, Neal DE and Leung HY: MEK5 overexpression is
associated with metastatic prostate cancer, and stimulates
proliferation, MMP-9 expression and invasion. Oncogene.
22:1381–1389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramsay AK, McCracken SR, Soofi M, Fleming
J, Yu AX, Ahmad I, Morland R, Machesky L, Nixon C, Edwards DR,
Nuttall RK, Seywright M, Marquez R, Keller E and Leung HY: ERK5
signalling in prostate cancer promotes an invasive phenotype. Br J
Cancer. 104:664–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho HJ, Lee TS, Park JB, Park KK, Choe JY,
Sin DI, Park YY, Moon YS, Lee KG, Yeo JH, Han SM, Cho YS, Choi MR,
Park NG, Lee YS and Chang YC: Disulfiram suppresses invasive
ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9
expression. J Biochem Mol Biol. 40:1069–1076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shintani Y, Maeda M, Chaika N, Johnson KR
and Wheelock MJ: Collagen I promotes epithelial-to-mesenchymal
transition in lung cancer cells via transforming growth factor-beta
signaling. Am J Respir Cell Mol Biol. 38:95–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fallica B, Maffei JS, Villa S, Makin G and
Zaman M: Alteration of cellular behavior and response to PI3K
pathway inhibition by culture in 3D collagen gels. PLoS One.
7:e480242012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuchiya Y, Endo Y, Sato H, Okada Y, Mai
M, Sasaki T and Seiki M: Expression of type-IV collagenases in
human tumor cell lines that can form liver colonies in chick
embryos. Int J Cancer. 56:46–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SM, Lee H, Park YS, Lee Y and Seo SW:
ERK5 regulates invasiveness of osteosarcoma by inducing MMP-9. J
Orthop Res. 30:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|